New Insights into Nanoplastics Ecotoxicology: Effects of Long-Term Polystyrene Nanoparticles Exposure on Folsomia candida
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Species
2.2. Test Medium
2.3. Test Contaminant
2.4. Toxicity Tests
2.4.1. Soil Spiking Procedures
2.4.2. Standard Reproduction Test
2.4.3. Multigenerational Test (F1 to F3)
2.5. Biochemical Markers Analysis
2.6. Data Analysis
3. Results and Discussion
3.1. Characterization of Polystyrene Nanoparticles
3.2. Toxicity Tests
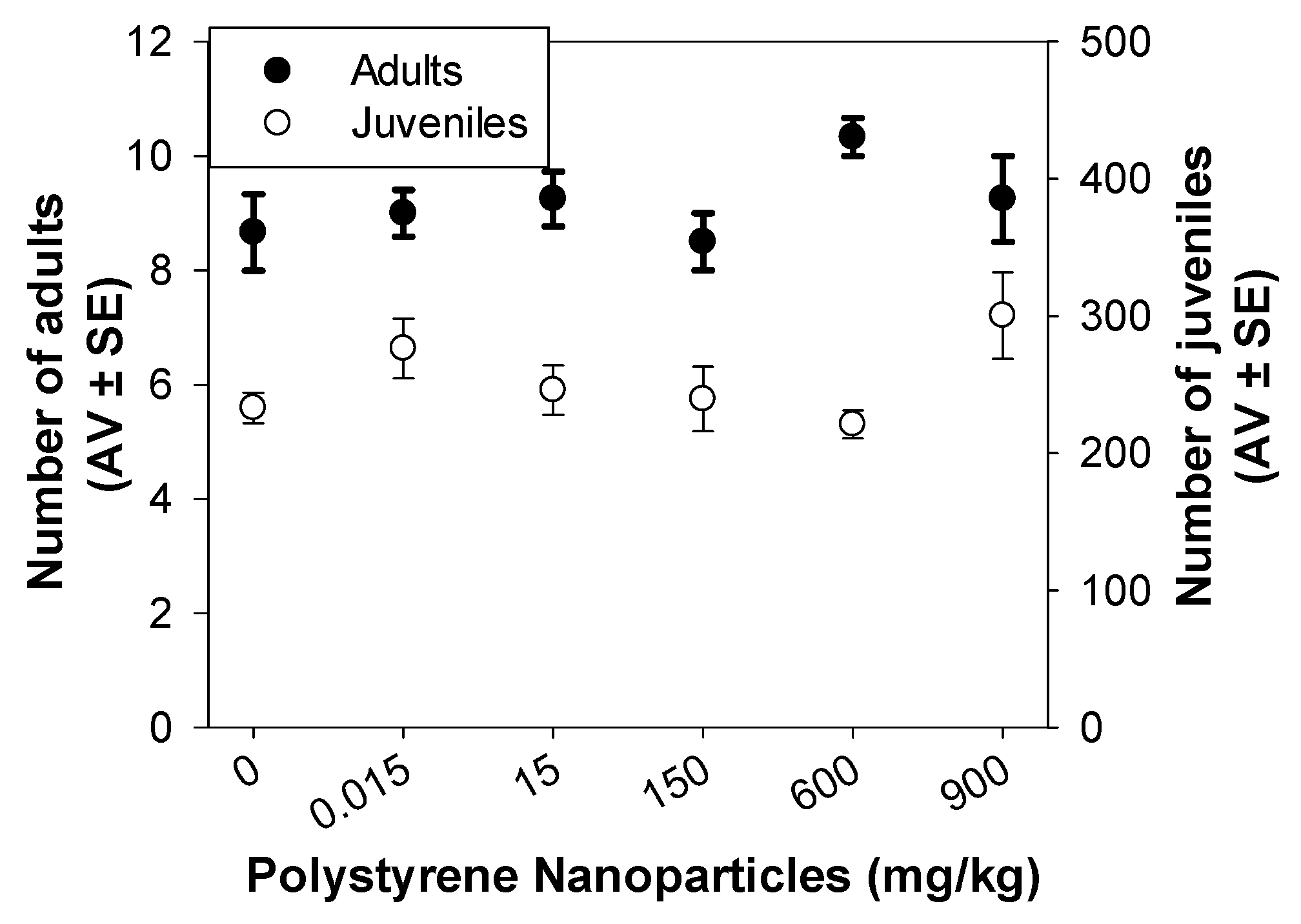
3.2.1. Standard Reproduction Test
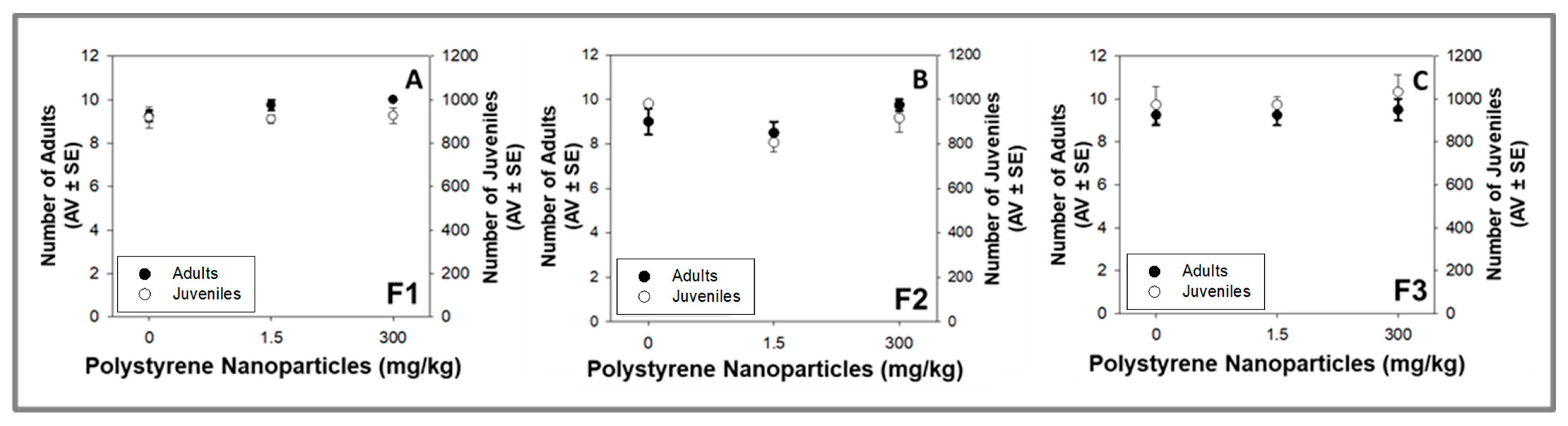
3.2.2. Multigenerational Test (F1 to F3)
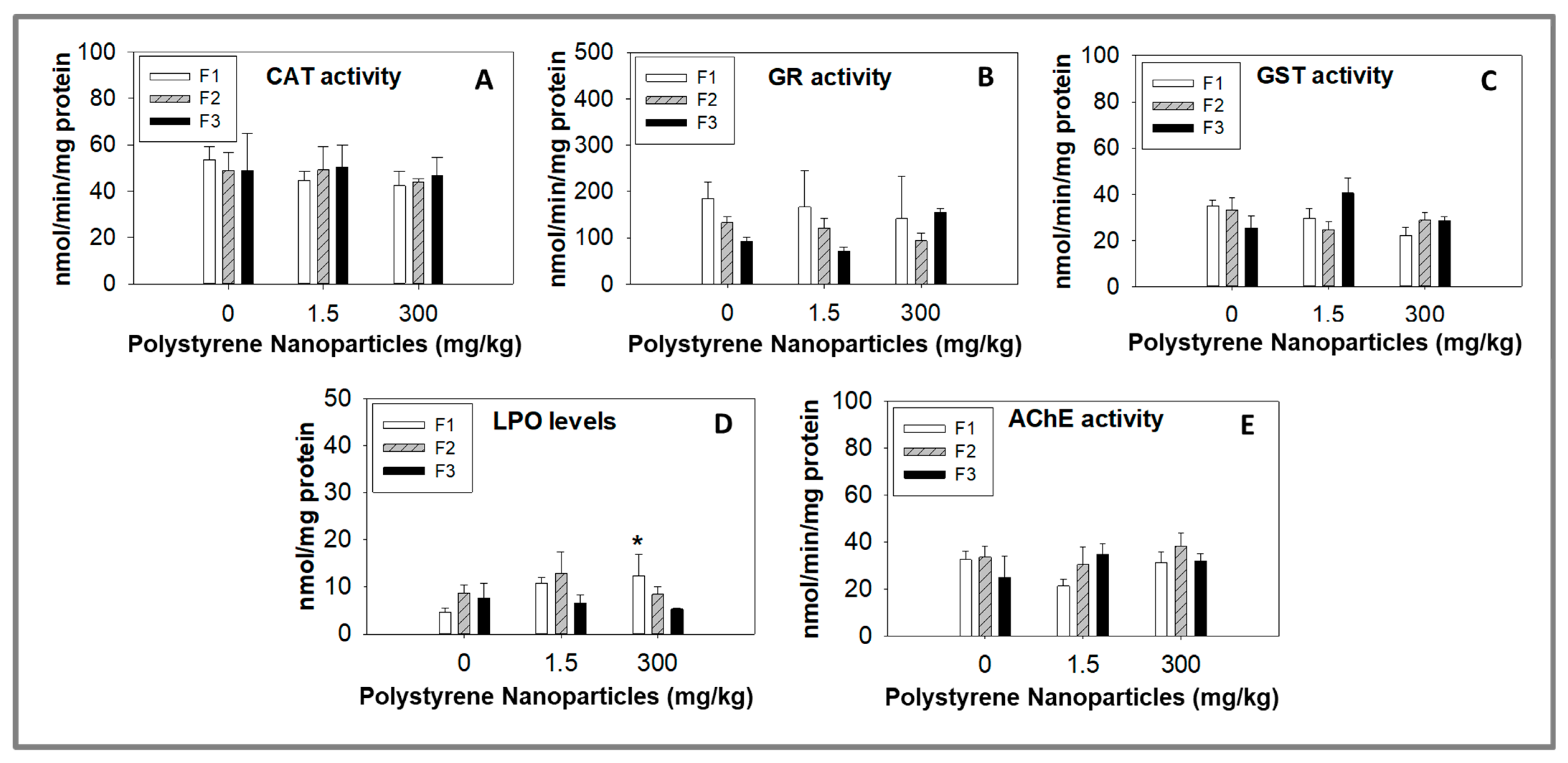
3.3. Biochemical Markers Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Surendran, U.; Jayakumar, M.; Raja, P.; Gopinath, G.; Chellam, P.V. Microplastics in terrestrial ecosystem: Sources and migration in soil environment. Chemosphere 2023, 318, 137946. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham Das, S. Impacts of Plastic Pollution on Ecosystem Services, Sustainable Development Goals, and Need to Focus on Circular Economy and Policy Interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Gigault, J.; Ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, E.; Singh, S.; Pandey, A.; Bhargava, P.C. Micro- and nano-plastics (MNPs) as emerging pollutant in ground water: Environmental impact, potential risks, limitations and way forward towards sustainable management. Chem. Eng. J. 2023, 459, 141568. [Google Scholar] [CrossRef]
- Ng, E.-L.; Huerta Lwanga, E.; Eldridge, S.M.; Johnston, P.; Hu, H.-W.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef]
- Pérez-Reverón, R.; Álvarez-Méndez, S.J.; González-Sálamo, J.; Socas-Hernández, C.; Díaz-Peña, F.J.; Hernández-Sánchez, C.; Hernández-Borges, J. Nanoplastics in the soil environment: Analytical methods, occurrence, fate and ecological implications. Environ. Pollut. 2023, 317, 120788. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Gomes, T.; Bour, A.; Coutris, C.; Almeida, A.C.; Bråte, I.L.; Wolf, R.; Bank, M.S.; Lusher, A.L. Ecotoxicological Impacts of Micro- and Nanoplastics in Terrestrial and Aquatic Environments. In Microplastic in the Environment: Pattern and Process; Bank, M.S., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 199–260. ISBN 978-3-030-78627-4. [Google Scholar]
- Guimarães, B.; Römbke, J.; Amorim, M.J.B. On the importance of longer-term exposure to stressors—A critical review and proposal for multigenerational testing in standard soil invertebrates. Sci. Total Environ. 2023, 854, 158680. [Google Scholar] [CrossRef]
- Barreto, A.; Santos, J.; Almeida, L.; Tavares, V.; Pinto, E.; Celeiro, M.; Garcia-Jares, C.; Maria, V.L. First approach to assess the effects of nanoplastics on the soil species Folsomia candida: A mixture design with bisphenol A and diphenhydramine. NanoImpact 2023, 29, 100450. [Google Scholar] [CrossRef]
- Barreto, A.; Santos, J.; Amorim, M.J.B.; Maria, V.L. How Can Nanoplastics Affect the Survival, Reproduction, and Behaviour of the Soil Model Enchytraeus crypticus? Appl. Sci. 2020, 10, 7674. [Google Scholar] [CrossRef]
- Heinlaan, M.; Viljalo, K.; Richter, J.; Ingwersen, A.; Vija, H.; Mitrano, D.M. Multi-generation exposure to polystyrene nanoplastics showed no major adverse effects in Daphnia magna. Environ. Pollut. 2023, 323, 121213. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, D.J.; da Silva, A.C.D.O.; da Silva, M.L.N.; Vicentini, D.S.; Matias, W.G. Individual and combined multigenerational effects induced by polystyrene nanoplastic and glyphosate in Daphnia magna (Strauss, 1820). Sci. Total Environ. 2022, 811, 151360. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, L.; Ru, S.; Eom, J.; Wang, D.; Wang, J. Nanoplastics induce more severe multigenerational life-history trait changes and metabolic responses in marine rotifer Brachionus plicatilis: Comparison with microplastics. J. Hazard. Mater. 2023, 449, 131070. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-W.; Luk, T.C.; Liao, V.H.-C. Long-term nanoplastics exposure results in multi and trans-generational reproduction decline associated with germline toxicity and epigenetic regulation in Caenorhabditis elegans. J. Hazard. Mater. 2021, 412, 125173. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liao, K.; Wang, D. Comparison of transgenerational reproductive toxicity induced by pristine and amino modified nanoplastics in Caenorhabditis elegans. Sci. Total Environ. 2021, 768, 144362. [Google Scholar] [CrossRef] [PubMed]
- Fountain, M.T.; Hopkin, S.P. Folsomia candida (collembola): A “Standard” Soil Arthropod. Annu. Rev. Entomol. 2004, 50, 201–222. [Google Scholar] [CrossRef]
- Wu, P.; Tang, Y.; Cao, G.; Li, J.; Wang, S.; Chang, X.; Dang, M.; Jin, H.; Zheng, C.; Cai, Z. Determination of Environmental Micro(Nano)Plastics by Matrix-Assisted Laser Desorption/Ionization–Time-of-Flight Mass Spectrometry. Anal. Chem. 2020, 92, 14346–14356. [Google Scholar] [CrossRef]
- Test No. 232: Collembolan Reproduction Test in Soil; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2016; ISBN 9789264264601.
- Maria, V.L.; Ribeiro, M.J.; Amorim, M.J.B. Oxidative stress biomarkers and metallothionein in Folsomia candida—Responses to Cu and Cd. Environ. Res. 2014, 133, 164–169. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Clairborne, A. Catalase activity. In Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Carlberg, I.; Mannervik, B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975, 250, 5475–5480. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases, The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.P.; Draper, H.H. [35] Comparative studies on different methods of malonaldehyde determination. Methods in Enzymology. 1984, 105, 299–305. [Google Scholar]
- Wilhelm Filho, D.; Tribess, T.; Gáspari, C.; Claudio, F.; Torres, M.; Magalhães, A.R. Seasonal changes in antioxidant defenses of the digestive gland of the brown mussel (Perna perna). Aquaculture 2001, 203, 149–158. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Guilhermino, L.; Lopes, M.C.; Carvalho, A.P.; Soares, A.M.V.M. Acetylcholinesterase activity in juveniles of Daphnia magna Straus. Bull. Environ. Contam. Toxicol. 1996, 57, 979–985. [Google Scholar] [CrossRef]
- Kawecki, D.; Nowack, B. Polymer-Specific Modeling of the Environmental Emissions of Seven Commodity Plastics as Macro- and Microplastics. Environ. Sci. Technol. 2019, 53, 9664–9676. [Google Scholar] [CrossRef] [PubMed]
- Rist, S.; Baun, A.; Hartmann, N.B. Ingestion of micro- and nanoplastics in Daphnia magna—Quantification of body burdens and assessment of feeding rates and reproduction. Environ. Pollut. 2017, 228, 398–407. [Google Scholar] [CrossRef]
- Rist, S.; Baun, A.; Almeda, R.; Hartmann, N.B. Ingestion and effects of micro- and nanoplastics in blue mussel (Mytilus edulis) larvae. Mar. Pollut. Bull. 2019, 140, 423–430. [Google Scholar] [CrossRef]
- Pathan, S.I.; Arfaioli, P.; Bardelli, T.; Ceccherini, M.T.; Nannipieri, P.; Pietramellara, G. Soil Pollution from Micro- and Nanoplastic Debris: A Hidden and Unknown Biohazard. Sustainability 2020, 12, 7255. [Google Scholar] [CrossRef]
- Mowla, M.; Shakiba, S.; Louie, S.M. Selective quantification of nanoplastics in environmental matrices by asymmetric flow field-flow fractionation with total organic carbon detection. Chem. Commun. 2021, 57, 12940–12943. [Google Scholar] [CrossRef]
- Wahl, A.; Le Juge, C.; Davranche, M.; El Hadri, H.; Grassl, B.; Reynaud, S.; Gigault, J. Nanoplastic occurrence in a soil amended with plastic debris. Chemosphere 2021, 262, 127784. [Google Scholar] [CrossRef]
- Materić, D.; Kasper-Giebl, A.; Kau, D.; Anten, M.; Greilinger, M.; Ludewig, E.; van Sebille, E.; Röckmann, T.; Holzinger, R. Micro- and Nanoplastics in Alpine Snow: A New Method for Chemical Identification and (Semi)Quantification in the Nanogram Range. Environ. Sci. Technol. 2020, 54, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Materić, D.; Ludewig, E.; Brunner, D.; Röckmann, T.; Holzinger, R. Nanoplastics transport to the remote, high-altitude Alps. Environ. Pollut. 2021, 288, 117697. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-X.; He, S.; Gao, Y.; Li, Z.-C.; Chi, H.-Y.; Li, C.-J.; Wang, D.-J.; Yan, B. Protein Corona-Mediated Extraction for Quantitative Analysis of Nanoplastics in Environmental Waters by Pyrolysis Gas Chromatography/Mass Spectrometry. Anal. Chem. 2021, 93, 6698–6705. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Wang, X.; Luo, X.; Liu, G.; Zheng, H. Effects of polystyrene microplastics on the fitness of earthworms in an agricultural soil. IOP Conf. Ser. Earth Environ. Sci. 2017, 61, 12148. [Google Scholar] [CrossRef]
- Andrés, C.M.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of Hydrogen Peroxide Formation and Elimination in Mammalian Cells, and Its Role in Various Pathologies. Stresses 2022, 2, 256–274. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar]
- Singhal, S.S.; Singh, S.P.; Singhal, P.; Horne, D.; Singhal, J.; Awasthi, S. Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol. Appl. Pharmacol. 2015, 289, 361–370. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [PubMed]
- Barreto, A.; Silva, A.R.R.; Capitão, A.; Sousa, É.M.L.; Calisto, V.; Maria, V.L. Nanoplastics increase the toxicity of a pharmaceutical, at environmentally relevant concentrations—A mixture design with Daphnia magna. Environ. Toxicol. Pharmacol. 2023, 103, 104258. [Google Scholar] [PubMed]
- Hu, M.; Palić, D. Micro- and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 2020, 37, 101620. [Google Scholar]
- Wu, D.; Liu, Z.; Cai, M.; Jiao, Y.; Li, Y.; Chen, Q.; Zhao, Y. Molecular characterisation of cytochrome P450 enzymes in waterflea (Daphnia pulex) and their expression regulation by polystyrene nanoplastics. Aquat. Toxicol. 2019, 217, 105350. [Google Scholar]
- Xu, X.; Miao, Z.; Sun, M.; Wan, B. Epigenetic Mechanisms of Paternal Stress in Offspring Development and Diseases. Int. J. Genomics 2021, 2021, 6632719. [Google Scholar]
- McHardy, S.F.; Wang, H.-Y.L.; McCowen, S.V.; Valdez, M.C. Recent advances in acetylcholinesterase Inhibitors and Reactivators: An update on the patent literature (2012–2015). Expert Opin. Ther. Pat. 2017, 27, 455–476. [Google Scholar]
- Trang, A.; Khandhar, P.B. Physiology, Acetylcholinesterase; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Chen, Q.; Gundlach, M.; Yang, S.; Jiang, J.; Velki, M.; Yin, D.; Hollert, H. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ. 2017, 584–585, 1022–1031. [Google Scholar]
- Varó, I.; Perini, A.; Torreblanca, A.; Garcia, Y.; Bergami, E.; Vannuccini, M.L.; Corsi, I. Time-dependent effects of polystyrene nanoparticles in brine shrimp Artemia franciscana at physiological, biochemical and molecular levels. Sci. Total Environ. 2019, 675, 570–580. [Google Scholar]
- Guimarães, A.T.B.; de Lima Rodrigues, A.S.; Pereira, P.S.; Silva, F.G.; Malafaia, G. Toxicity of polystyrene nanoplastics in dragonfly larvae: An insight on how these pollutants can affect bentonic macroinvertebrates. Sci. Total Environ. 2021, 752, 141936. [Google Scholar]
- Shan, S.; Zhang, Y.; Zhao, H.; Zeng, T.; Zhao, X. Polystyrene nanoplastics penetrate across the blood-brain barrier and induce activation of microglia in the brain of mice. Chemosphere 2022, 298, 134261. [Google Scholar] [CrossRef] [PubMed]
- Prüst, M.; Meijer, J.; Westerink, R.H.S. The plastic brain: Neurotoxicity of micro- and nanoplastics. Part. Fibre Toxicol. 2020, 17, 24. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreto, A.; Santos, J.; Andrade, G.; Santos, M.; Maria, V.L. New Insights into Nanoplastics Ecotoxicology: Effects of Long-Term Polystyrene Nanoparticles Exposure on Folsomia candida. Toxics 2023, 11, 876. https://doi.org/10.3390/toxics11100876
Barreto A, Santos J, Andrade G, Santos M, Maria VL. New Insights into Nanoplastics Ecotoxicology: Effects of Long-Term Polystyrene Nanoparticles Exposure on Folsomia candida. Toxics. 2023; 11(10):876. https://doi.org/10.3390/toxics11100876
Chicago/Turabian StyleBarreto, Angela, Joana Santos, Gonçalo Andrade, Matilde Santos, and Vera L. Maria. 2023. "New Insights into Nanoplastics Ecotoxicology: Effects of Long-Term Polystyrene Nanoparticles Exposure on Folsomia candida" Toxics 11, no. 10: 876. https://doi.org/10.3390/toxics11100876
APA StyleBarreto, A., Santos, J., Andrade, G., Santos, M., & Maria, V. L. (2023). New Insights into Nanoplastics Ecotoxicology: Effects of Long-Term Polystyrene Nanoparticles Exposure on Folsomia candida. Toxics, 11(10), 876. https://doi.org/10.3390/toxics11100876








