Airborne Pesticides from Agricultural Practices: A Critical Review of Pathways, Influencing Factors, and Human Health Implications
Abstract
1. Introduction
2. Methods
3. Existence and Dispersion of Pesticides in the Atmosphere
3.1. Existence of Pesticides in Outdoor Air
3.2. Pesticide Properties and Dispersion Behavior
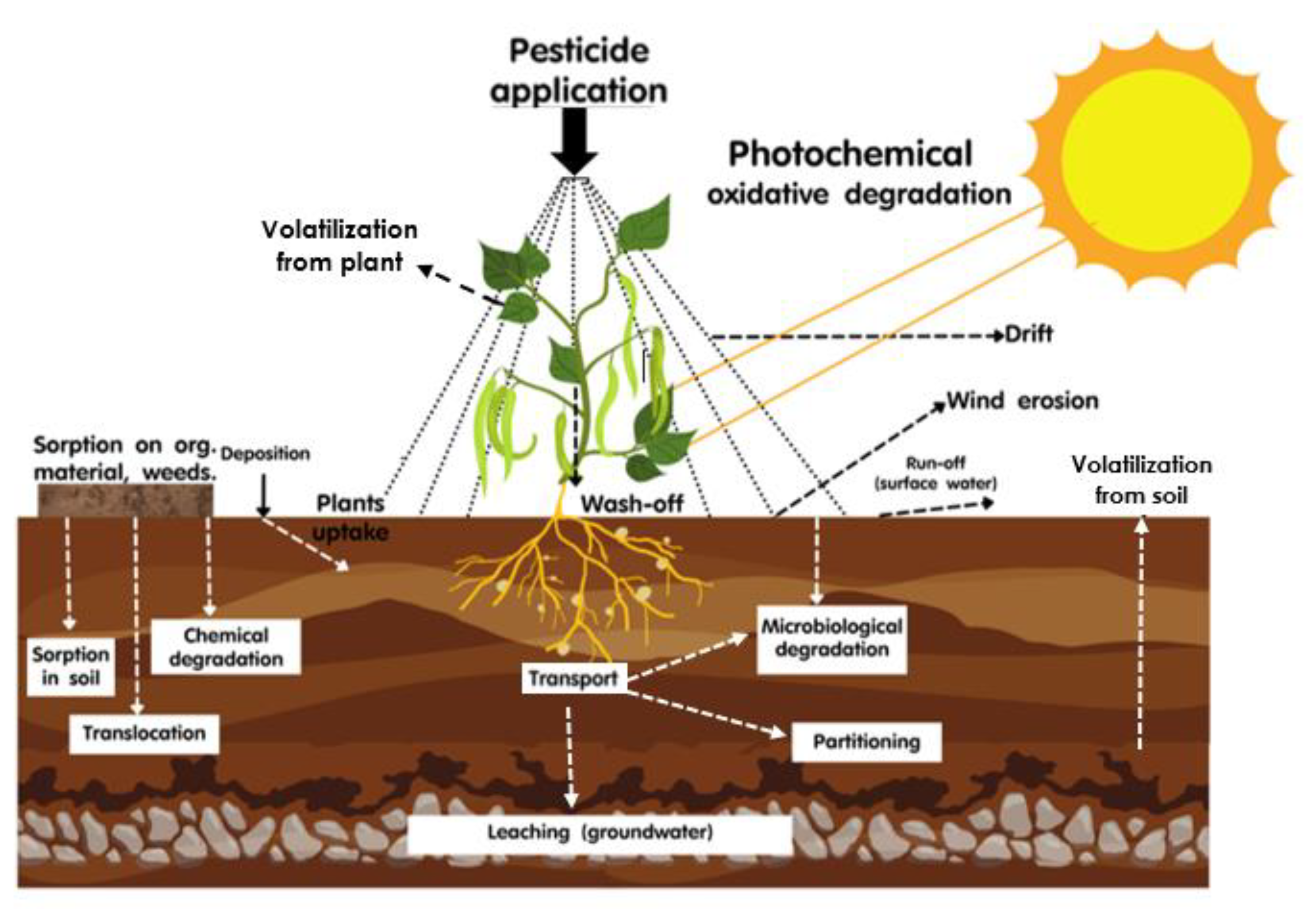
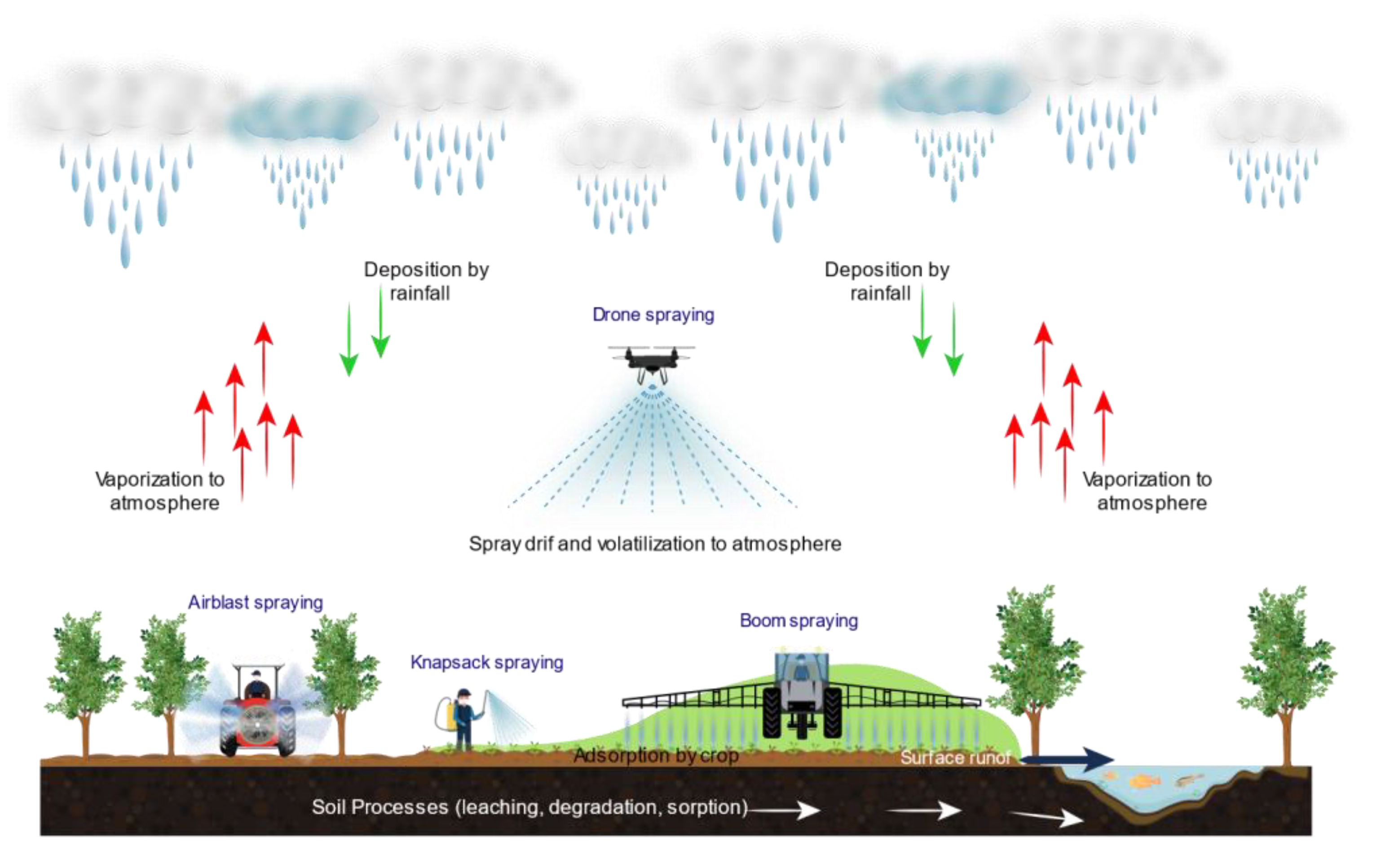
3.3. Pesticide Applications, Spray Drift, and Volatilization Process
3.4. Dispersion and Volatilization of Pesticide to Atmosphere
3.5. Influence of Environmental Factors on Pesticide Dispersion
4. Environmental and Human Health Consequences from Pesticides as Airborne Pollutants
4.1. Environmental Pollution from Airborne Pesticides
4.2. Human Health Risk from Airborne Pesticide
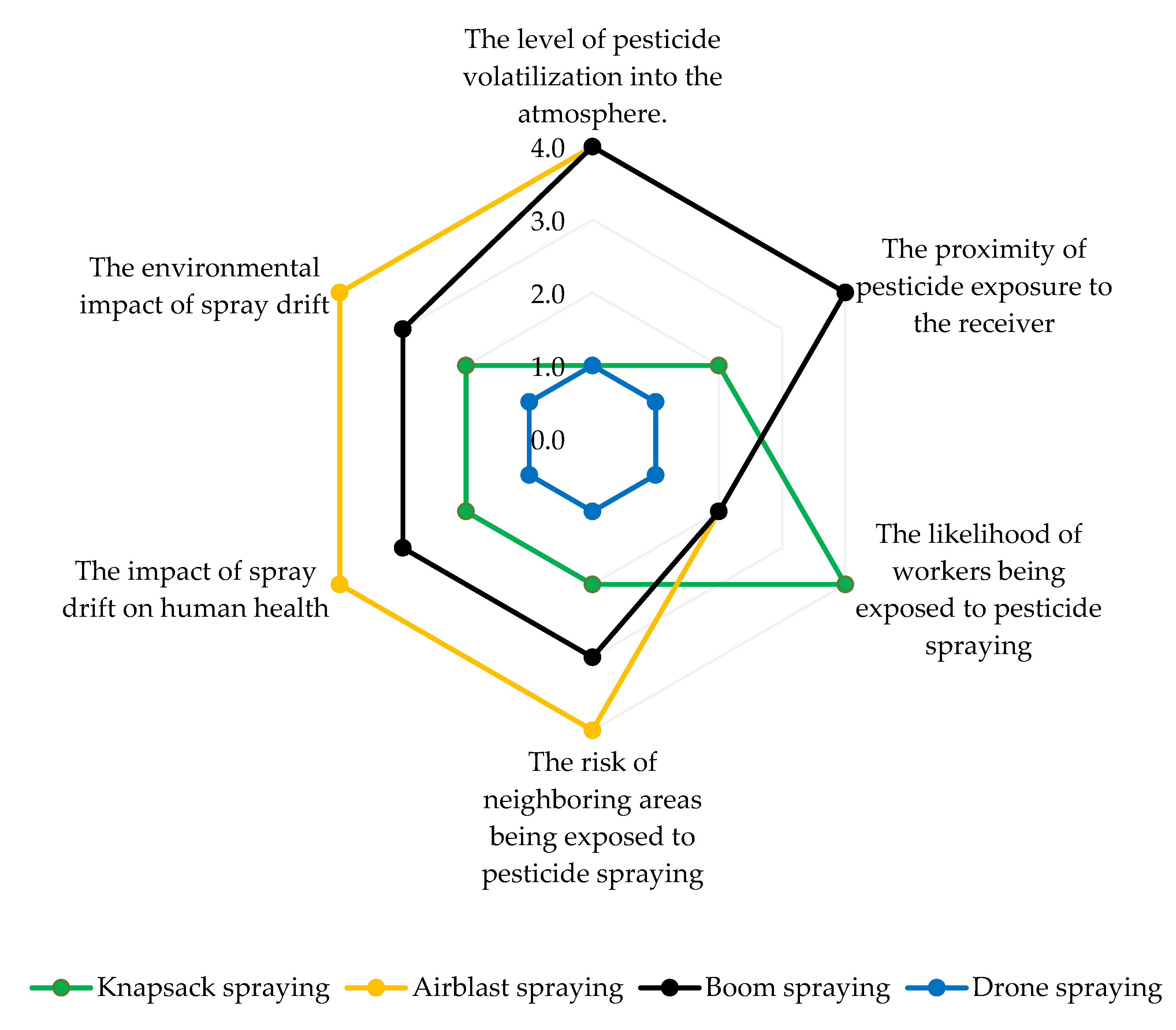
5. Mitigating the Risks to the Environment and Human Health Associated with Pesticide Spraying Methods
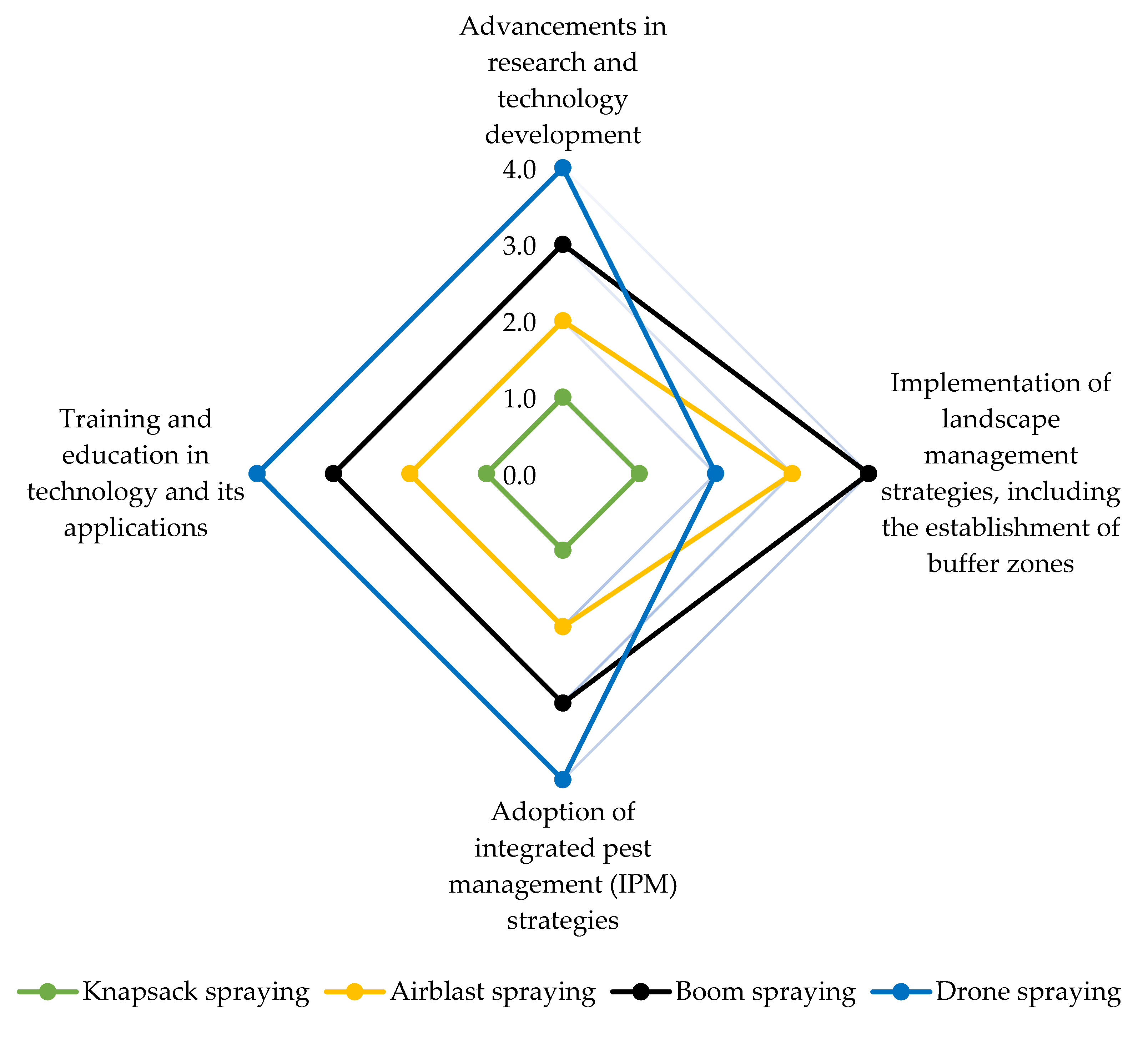
6. Effective Strategies for Minimizing Pesticide Exposure
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and health: A progress update. Lancet Planet. Health 2022, 6, e535–e547. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). FAOSTAT Database: Pesticides Use. 2020. Available online: http://www.fao.org/faostat/en/#data/RP (accessed on 15 March 2023).
- Degrendele, C.; Okonski, K.; Melymuk, L.; Landlová, L.; Kukučka, P.; Audy, O.; Kohoutek, J.; Čupr, P.; Klánová, J. Pesticides in the atmosphere: A comparison of gas-particle partitioning and particle size distribution of legacy and current-use pesticides. Atmos. Chem. Phys. 2016, 16, 1531–1544. [Google Scholar] [CrossRef]
- Gil, Y.; Sinfort, C. Emission of pesticides to the air during sprayer application: A bibliographic review. Atmos. Environ. 2005, 39, 5183–5193. [Google Scholar] [CrossRef]
- Nascimento, M.M.; da Rocha, G.O.; de Andrade, J.B. Pesticides in fine airborne particles: From a green analysis method to atmospheric characterization and risk assessment. Sci. Rep. 2017, 7, 2267. [Google Scholar] [CrossRef]
- Coscollà, C.; Yusà, V.; Beser, M.I.; Pastor, A. Multi-residue analysis of 30 currently used pesticides in fine airborne particulate matter (PM 2.5) by micorwave-assisted extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 8817–8827. [Google Scholar] [CrossRef] [PubMed]
- Polyrakis, I.T. Environmental pollution from pesticides. In Predictive Modeling and Risk Assessment. Integrating safety and Environmental Knowledge into Food Studies towards European Sustainable Development; Costa, R., Kristbergsson, K., Eds.; Springer: Boston, MA, USA, 2009; Volume 4, pp. 201–224. [Google Scholar] [CrossRef]
- Wong, H.L.; Garthwaite, D.G.; Ramwell, C.T.; Brown, C.D. How does exposure to pesticides vary in space and time for residents living near to treated orchards? Environ. Sci. Pollut. Res. Int. 2017, 24, 26444–26461. [Google Scholar] [CrossRef]
- Socorro, J.; Durand, A.; Temime-Roussel, B.; Gligorovski, S.; Wortham, H.; Quivet, E. The persistence of pesticides in atmospheric particulate phase: An emerging air quality issue. Sci. Rep. 2016, 6, 33456. [Google Scholar] [CrossRef] [PubMed]
- Lichota, G.B.; McAdie, M.; Ross, P.S. Endangered Vancouver Island marmots (Marmota vancouverensis): Sentinels of atmospheric delivered contaminants to British Columbia, Canada. Environ. Toxicol. Chem. 2004, 23, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Bedos, C.; Cellier, P.; Calvet, R.; Barriuso, E. Occurrence of pesticides in the atmosphere in France. Agronomie 2002, 22, 35–49. [Google Scholar] [CrossRef]
- Van den Berg, F.; Kubiak, R.; Benjey, W.G.; Majewsjki MSYates, S.R.; Reeves, G.L.; Smelt, J.H.; Van der Linden, A.M.A. Emission of pesticides in the air. Water Air Soil Pollut. 1999, 115, 195–218. [Google Scholar] [CrossRef]
- Hapeman, C.J.; McConnell, L.L.; Rice, C.P. Current United States Department of Agriculture—Agricultural research service research on understanding agrochemical fate and transport to prevent and mitigate adverse environmental impacts. Pest Manag. Sci. 2003, 59, 681–690. [Google Scholar] [CrossRef]
- Coscollà, C.; Muñoz, A.; Borrás, E.; Vera, T.; Ródenas, M.; Yusà, V. Particle size distributions of currently used pesticides in ambient air of an agricultural Mediterranean area. Atmos. Environ. 2014, 95, 29–35. [Google Scholar] [CrossRef]
- Sarigiannis, D.A.; Kontoroupis, P.; Solomou, E.S.; Nikolaki, S.; Karabelas, A. Inventory of pesticide emissions into the air in Europe. Atmos. Environ. 2013, 75, 6–14. [Google Scholar] [CrossRef]
- Fang, Y.; Nie, Z.; Die, Q.; Tian, Y.; Liu, F.; He, J.; Huang, Q. Organochlorine pesticides in soil, air and vegetation at and around a contaminated site in southwestern of China: Concentration, transmission and risk evaluation. Chemosphere 2017, 178, 340–349. [Google Scholar] [CrossRef]
- Veludo, A.F.; Figueiredo, D.M.; Degrendele, C.; Masinyana, L.; Curchod, L.; Kohoutek, J.; Kukučka, P.; Martiník, J.; Přibylová, P.; Klánová, J.; et al. Seasonal variations in air concentrations of 27 organochlorine pesticides (OCPs) and 25 current-use pesticides (CUPs) across three agricultural areas of South Africa. Chemosphere 2022, 289, 133162. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, R.; Yu, K.; Han, M.; Wang, Y.; Huang, X.; Wang, R.; Liu, F. First report on organochlorine pesticides (OCPs) in coral tissues and the surrounding air-seawater system from the South China Sea: Distribution, source, and environmental fate. Chemosphere 2022, 286, 131711. [Google Scholar] [CrossRef]
- Coscollà, C.; Colin, P.; Yahyaoui, A.; Petrique, O.; Yusà, V.; Mellouki, A.; Pastor, A. Occurrence of currently used pesticides in ambient air of Centre Region (France). Atmos. Environ. 2010, 44, 3915–3925. [Google Scholar] [CrossRef]
- Syed, J.H.; Malik, R.N.; Liu, D.; Xu, Y.; Wang, Y.; Li, J.; Zhang, G.; Jones, K.C. Organochlorine pesticides in air and soil and estimated air-soil exchange in Punjab, Pakistan. Sci. Total Environ. 2013, 444, 491–497. [Google Scholar] [CrossRef]
- Li, J.; Zhu, T.; Wang, F.; Qiu, X.H.; Lin, W.L. Observation of organochlorine pesticides in the air of the Mt. Everest region. Ecotoxicol. Environ. Saf. 2006, 63, 33–41. [Google Scholar] [CrossRef]
- Zaller, J.G.; Kruse-Plaß, M.; Schlechtriemen, U.; Gruber, E.; Peer, M.; Nadeem, I.; Formayer, H.; Hutter, H.P.; Landler, L. Pesticides in ambient air, influenced by surrounding land use and weather, pose a potential threat to biodiversity and humans. Sci. Total Environ. 2022, 838, 156012. [Google Scholar] [CrossRef] [PubMed]
- Casey, A.; Bush, B.; David, O.; Carpenter, D.O. PCBs in indoor air and human blood in Pittsfield Massachusetts. Chemosphere 2022, 293, 133551. [Google Scholar] [CrossRef] [PubMed]
- Kruse-Plaß, M.; Hofmann, F.; Wosniok, W.; Schlechtriemen, U.; Kohlschuetter, N. Pesticides and pesticide-related products in ambient air in Germany. Environ. Sci. Eur. 2021, 33, 18–32. [Google Scholar] [CrossRef]
- Han, D.H. Airborne concentrations of organophosphorus pesticides in Korean pesticide manufacturing/formulation workplaces. Ind. Health 2011, 49, 703–713. [Google Scholar] [CrossRef]
- Degrendele, C.; Klánová, J.; Prokeš, R.; Příbylová, P.; Šenk, P.; Šudoma, M.; Röösli, M.; Dalvie, M.A.; Fuhrimann, S. Current use pesticides in soil and air from two agricultural sites in South Africa: Implications for environmental fate and human exposure. Sci. Total Environ. 2022, 807, 150455. [Google Scholar] [CrossRef]
- Gamboa, D.L.; Diaz, S.K.; Ruepert, C.; van Wendel de Joode, B. Passive monitoring techniques to evaluate environmental pesticide exposure: Results from the Infant’s Environmental Health study (ISA). Environmetal Res. 2020, 184, 109243. [Google Scholar] [CrossRef]
- Degrendele, C.; Kanduč, T.; Kocman, D.; Lammel, G.; Cambelová, A.; Dos Santos, S.G.; Horvat, M.; Kukučka, P.; Šmejkalvá, A.H.; Mikeš, O.; et al. NPAHs and OPAHs in the atmosphere of two central European cities: Seasonality, urban-to-background gradients, cancer risks and gas-to-particle partitioning. Sci. Total Environ. 2021, 793, 148528. [Google Scholar] [CrossRef]
- Fuentes, E.; López, A.; Ibáñez, M.; Yusà, V.; Muñoz, A.; Vera, T.; Borrás, E.; Calvete-Sogo, H.; Coscollà, C. Pesticide inhalation exposure of applicators and bystanders using conventional and innovative cropping systems in the Valencian Region, Spain. Atmosphere 2021, 12, 631. [Google Scholar] [CrossRef]
- Morshed, M.; Omar, R.D.; Mohamad, S.; Abd, S.W. Determination of glyphosate through passive and active sampling methods in a treated field atmosphere. Afr. J. Agric. Res. 2011, 6, 4010–4018. [Google Scholar]
- Chang, F.-C.; Simcik, M.F.; Capel, P.D. Occurrence and fate of the herbicide glyphosate and its degradate aminomethylphosphonic acid in the atmosphere. Environ. Toxicol. Chem. 2010, 30, 548–555. [Google Scholar] [CrossRef]
- Ravier, S.; Désert, M.; Gille, G.; Armengaud, A.; Wortham, H. Monitoring of Glyphosate, Glufosinate-ammonium, and (Aminomethyl) phosphonic acid in ambient air of Provence-Alpes-Côte-d’ Azur Region, France. Atmos. Environ. 2019, 204, 102–109. [Google Scholar] [CrossRef]
- Feltracco, M.; Barbaro, E.; Maule, F.; Bortolini, M.; Gabrieli, J.; De Blasi, F.; Cairns, W.R.L.; Dallo, F.; Zangrando, R.; Barbante, C.; et al. Airborne polar pesticides in rural and mountain sites of North-Eastern Italy: An emerging air quality issue. Environ. Pollut. 2022, 308, 119657. [Google Scholar] [CrossRef] [PubMed]
- Leistra, M.; Van der Linden AM, A.; Boesten JJ, T.I.; Tiktak, A.; Van den Berg, F. PEARL Model for Pesticide Behaviour and Emissions in Soil-Plant Systems; Description of the Processes in FOCUS PEARL v 1.1.1; Alterra-rapport 013/RIVM Rep. 711401009; Alterra: Wageningen, The Netherlands, 2001; 115p. [Google Scholar]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Bidleman, T.F. Atmospheric processes: Wet and dry deposition of organic compound are controlled by their vapour-particle partitioning. Environ. Sci. Technol. 1998, 22, 361–367. [Google Scholar] [CrossRef]
- Glotfelty, D.E.; Taylor, A.W.; Turner, B.C.; Zoller, W.H. Volatilization of surface-applied pesticides from fallow soil. J. Agric. Food Chem. 1984, 32, 638–643. [Google Scholar] [CrossRef]
- Van Wesenbeeck, I.; Driver, J.; Ross, J. Relationship between the evaporation rate and vapor pressure of moderately and high volatile chemicals. Bull. Environ. Contam. Toxicol. 2008, 80, 315–318. [Google Scholar] [CrossRef]
- Woodrow, J.E.; Seiber, J.N.; Dary, C. Predicting pesticide emissions and downwind concentrations using correlations with estimated vapor pressures. J. Agric. Food Chem. 2001, 49, 3841–3846. [Google Scholar] [CrossRef]
- Guth, J.A.; Reischmann, F.J.; Allen, R.; Arnold, D.; Hassink, J.; Leake, C.R. Volatilisation of crop protection chemicals from crop and soil surfaces under controlled conditions—Prediction of volatile losses from physico-chemical properties. Chemosphere 2004, 57, 871–887. [Google Scholar] [CrossRef]
- Lichiheb, N.; Personne, E.; Bedos, C.; Van den Berg, F.; Barriuso, E. Implementation of the effects of physicochemical properties on the foliar penetration of pesticides and its potential for estimating pesticide volatilization from plants. Sci. Total Environ. 2016, 550, 1022–1031. [Google Scholar] [CrossRef]
- Kubiak, R.; Burkle, L.; Cousins, I.; Hourdakis, A.; Jarvis, T.; Jene, B.; Koch, W.; Kreuger, J.; Maier, W.-M.; Van den Berg, F.; et al. Pesticides in Air: Considerations for Exposure Assessment. FOCUS Working Group. European Commission SANCO/10553/2006. 2008. Available online: https://esdac.jrc.ec.europa.eu/public_path/projects_data/focus/air/docs/FOCUS_AIR_GROUP_REPORT-FINAL.pdf (accessed on 12 November 2021).
- Cetin, B.; Ozer, S.; Sofuoglu, A.; Odabasi, M. Determination of Henry’s law constants of organochlorine pesticides in deionized and saline water as a function of temperature. Atmos. Environ. 2006, 40, 4538–4546. [Google Scholar] [CrossRef][Green Version]
- Odabasi, M.; Cetin, B.; Sofuoglu, A. Henry’s law constant, octanol–air partition coefficient and supercooled liquid vapor pressure of carbazole as a function of temperature: Application to gas/particle partitioning in the atmosphere. Chemosphere 2006, 62, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Suntio, L.R.; Shiu, W.Y.; Mackay, D.; Seiber, J.N.; Glotfelty, D. Critical review of Henry’s law constant of pesticides. In Reviews of Environmental Contamination and Toxicology. Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer: New York, NY, USA, 1988; Volume 103. [Google Scholar] [CrossRef]
- Goodarzi, M.; Ortiz, E.V.; Coelho, L.S.; Duchowicz, P.R. Linear and non-linear relationships mapping the Henry’s law parameters of organic pesticides. Atmos. Environ. 2010, 44, 3179–3186. [Google Scholar] [CrossRef]
- Herman-Fetcho, J.A.; McConnell, L.L.; Rice, C.P.; Baker, J.E. Wet deposition and air−water gas exchange of currently used pesticides to a subestuary of the Chesapeake Bay. Environ. Sci. Technol. 2000, 34, 1462–1468. [Google Scholar] [CrossRef]
- Spencer, W.F.; Cliath, M.M.; Jury, W.A.; Zhang, L.Z. Volatilization of organic chemicals from soil as related to their Henry’s law constant. J. Environ. Qual. 1988, 17, 505–509. [Google Scholar] [CrossRef]
- Feigenbrugel, V.; Le Calvé, S. Temperature dependence of Henry’s law constants of fenpropidin and pyrimethanil: Impact on their atmospheric partitionings and lifetimes. J. Environ. Sci. Public Health 2021, 5, 180–199. [Google Scholar] [CrossRef]
- Mackay, D.; Shiu, W.; Ma, K.C.; Lee, S.C. Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Davie-Martin, C.L.; Hageman, K.J.; Chin, Y.-P.; Rouge, V.; Fujita, Y. Influence of temperature, relative humidity, and soil properties on the soil−air partitioning of semivolatile pesticides: Laboratory measurements and predictive models. Environ. Sci. Technol. 2015, 49, 10431–10439. [Google Scholar] [CrossRef]
- Wu, J. Soil-Air partition coefficients of persistent organic pollutants decline from climate warming: A case study in Yantai County, Shandong Province, China. Water Air Soil Pollut. 2020, 231, 371. [Google Scholar] [CrossRef]
- EPA. Acetamide Herbicides Registration Review; US Environmental Protection Agency: Washington, DC, USA, 2010. [Google Scholar]
- WHO. Acetamide Herbicides: Diazinon, Chlormequat, Chlorpropham, and Methabenzthiazuron; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Laskowski, D.A.; Hopkin, S.P. Understanding Biomarkers: An Introduction to Molecular Biotechnology; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Kohli, R.K.; Singh, R. Biodegradation of alachlor by Aspergillus niger isolated from agricultural soil. J. Environ. Biol. 2016, 37, 187–194. [Google Scholar]
- ATSDR. Toxicological Profile for Chlordane (Update); Public Health Service, US Department of Health and Human Services: Washington, DC, USA, 1994. [Google Scholar]
- EPA-738-R-03-012; Reregistration Eligibility Decision (RED): Atrazine. US Environmental Protection Agency: Washington, DC, USA, 2003.
- EPA 738-R-06-033; Reregistration Eligibility Decision (RED): Simazine. US Environmental Protection Agency: Washington, DC, USA, 2006.
- NPIC. Atrazine Technical Fact Sheet; National Pesticide Information Center: Corvallis, OR, USA, 2021. [Google Scholar]
- Tomlin, C.D.S. (Ed.) The Pesticide Manual, 15th ed.; British Crop Production Council: Hampshire, UK, 2006. [Google Scholar]
- EPA. Captan: Re-Registration Eligibility Decision; US Environmental Protection Agency: Washington, DC, USA, 2007; Available online: https://www.epa.gov/ingredients-used-pesticide-products/captan-re-registration-eligibility-decision-red-facts (accessed on 11 March 2023).
- EPA 738-R-06-030; Reregistration Eligibility Decision (RED): Carbamate Pesticides. US Environmental Protection Agency: Washington, DC, USA, 2006.
- WHO. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2003–2004; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- EPA 738-R-06-031; Reregistration Eligibility Decision (RED): Carbaryl. US Environmental Protection Agency: Washington, DC, USA, 2006.
- EPA. Carbendazim: Reregistration Eligibility Decision for Fungicide Uses; US Environmental Protection Agency: Washington, DC, USA, 2009; Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_PC-054601_1-Jun-09.pdf (accessed on 11 March 2023).
- EPA. Chlorothalonil: Revised Human Health Risk Assessment for the Reregistration Eligibility Decision; US Environmental Protection Agency: Washington, DC, USA, 2008; Available online: https://www.epa.gov/sites/default/files/2016-09/documents/chlorothalonil_red.pdf (accessed on 11 March 2023).
- NPIC. Pesticide Information Profiles—Chlorpyrifos and Diazinon; National Pesticide Information Center: Corvallis, OR, USA, 2022. [Google Scholar]
- WHO. DDT and Its Derivatives-Environmental Aspects. Environmental Health Criteria 83; World Health Organization: Geneva, Switzerland, 1989; pp. 235–236. [Google Scholar]
- EPA. Pesticide Fact Sheet: DDT and DDE; US Environmental Protection Agency: Washington, DC, USA, 2016. [Google Scholar]
- EPA. Integrated Risk Information System (IRIS)—Substance Table; US Environmental Protection Agency: Washington, DC, USA, 2019; Available online: https://www.epa.gov/iris/substance-tables-iris (accessed on 11 March 2023).
- Battaglin, W.A.; Sandstrom, M.W.; Kuivila, K.M.; Kolpin, D.W. Glyphosate, Other Herbicides, and Transformation Products in Midwestern Streams, 2002–2010; US Geological Survey: Reston, VA, USA, 2011. [Google Scholar]
- PPDB. Pesticide Properties Database. 2023. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/ (accessed on 30 March 2023).
- Battaglin, W.A.; Meyer, M.T.; Kuivila, K.M.; Dietze, J.E. Glyphosate and its degradation product AMPA occur frequently and widely in US soils, surface water, groundwater, and precipitation. J. Am. Water Resour. Assoc. 2014, 50, 275–290. [Google Scholar] [CrossRef]
- EPA 738-R-06-032; Reregistration Eligibility Decision (RED): Metazachlor. US Environmental Protection Agency: Washington, DC, USA, 2006.
- CCOHS. Metazachlor; Canadian Centre for Occupational Health and Safety: Hamilton, ON, Canda, 2021. [Google Scholar]
- EPA 738-R-06-008; Reregistration Eligibility Decision (RED): Metribuzin. US Environmental Protection Agency: Washington, DC, USA, 2006.
- ECHA Propiconazole. European Chemicals Agency. 2010. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.000.328 (accessed on 11 March 2023).
- EPA. Reregistration Eligibility Decision (RED) for Tebuconazole; US Environmental Protection Agency: Washington, DC, USA, 2008; Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_PC-065002_1-May-08.pdf (accessed on 11 March 2023).
- Zhou, X.; Wang, P.; Luo, Y.; Chen, S.; Huang, Q. Simultaneous adsorption of atrazine and terbuthylazine on montmorillonite: Kinetics, thermodynamics and competitive adsorption. J. Environ. Manag. 2016, 168, 145–153. [Google Scholar]
- NPIC. Trifluralin Technical Fact Sheet; National Pesticide Information Center: Corvallis, OR, USA, 2016; Available online: https://npic.orst.edu/factsheets/triftech.pdf (accessed on 12 March 2023).
- Ochoa, V.; Maestroni, B. Chapter 9—Pesticides in Water, Soil, and Sediments. In Integrated Analytical Approaches for Pesticide Management; Academic Press: Cambridge, MA, USA, 2018; pp. 133–147. ISBN 9780128161555. [Google Scholar] [CrossRef]
- Hong, S.W.; Zhao, L.; Zhu, H. SAAS, a computer program for estimating pesticide spray efficiency and drift of air-assisted pesticide applications. Comput. Electron. Agric. 2018, 155, 58–68. [Google Scholar] [CrossRef]
- Ramaprasad, J.; Tsai, M.Y.; Elgethun, K.; Hebert, V.R.; Felsot, A.; Yost, M.G.; Fenske, R.A. The Washington aerial spray drift study: Assessment of off-target organophosphorus insecticide atmospheric movement by plant surface volatilization. Atmos. Environ. 2004, 38, 5703–5713. [Google Scholar] [CrossRef]
- Kira, O.; Linker, R.; Dubowski, Y. Estimating drift of airborne pesticides during orchard spraying using active Open Path FTIR. Atmos. Environ. 2016, 142, 264–270. [Google Scholar] [CrossRef]
- Bourodimos, G.; Koutsiaras, M.; Psiroukis, V.; Balafoutis, A.; Fountas, S. Development and Field Evaluation of a Spray Drift Risk Assessment Tool for Vineyard Spraying Application. Agriculture 2019, 9, 181. [Google Scholar] [CrossRef]
- Gil, Y.; Sinfort, C.; Brunet, Y.; Polveche, V.; Bonicelli, B. Atmospheric loss of pesticides above an artificial vineyard during air-assisted spraying. Atmos. Environ. 2007, 41, 2945–2957. [Google Scholar] [CrossRef]
- Pezzi, F.; Rondelli, V. The performance of an air-assisted sprayer operating in Vines. J. Agric. Eng. Res. 2000, 76, 331–340. [Google Scholar] [CrossRef]
- Cross, J.V.; Walklate, P.J.; Murray, R.A.; Richardson, G.M. Spray deposits and losses in different sized apple trees from an axial fan orchard sprayer:1. Effects of spray liquid flow rate. Crop Prot. 2001, 20, 13–30. [Google Scholar] [CrossRef]
- Cross, J.V.; Walklate, P.J.; Murray, R.A.; Richardson, G.M. Spray deposits and losses in different sized apple trees from an axial fan orchard sprayer: 2. Effects of spray quality. Crop Prot. 2001, 20, 333–343. [Google Scholar] [CrossRef]
- Cross, J.V.; Walklate, P.J.; Murray, R.A.; Richardson, G.M. Spray deposits and losses in different sized apple trees from an axial fan orchard sprayer:3. Effects of air volumetric flow rate. Crop Prot. 2003, 22, 381–394. [Google Scholar] [CrossRef]
- Hewitt, A.J.; Miller, P.C.H.; Bagley, W.E. Interaction of Tank Mix and Nozzle Design on Spray Performance and Drift Potential; ASAE Paper: Washington, DC, USA, 2001; p. 01-011081. [Google Scholar]
- Hewitt, A.J. Spray optimization through application and liquid physical property variables-I. Environmentalist 2008, 28, 25–30. [Google Scholar] [CrossRef]
- de Jong, F.M.W.; de Snoo, G.R.; van de Zande, J.C. Estimated nationwide effects of pesticide spray drift on terrestrial habitats in the Netherlands. J. Environ. Manag. 2008, 86, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Voutsas, E.; Vavva, C.; Magoulas, K.; Tassios, D. Estimation of the volatilization of organic compounds from soil surfaces. Chemosphere 2005, 58, 751–758. [Google Scholar] [CrossRef]
- Woodrow, J.E.; Gibson, K.A.; Seiber, J.N. Pesticides and related toxicants in the atmosphere. Rev. Environ. Contam. Toxicol. 2019, 247, 147–196. [Google Scholar] [CrossRef] [PubMed]
- Muir, D.C.G.; Teixeira, C.; Wania, F. Empirical and modeling evidence of regional atmospheric transport of current-use pesticides. Environ. Toxicol. Chem. 2004, 23, 2421–2432. [Google Scholar] [CrossRef] [PubMed]
- Zivan, O.; Segal-Rosenheimer, M.; Dubowski, Y. Airborne organophosphate pesticides drift in Mediterranean climate: The importance of secondary drift. Atmos. Environ. 2016, 127, 155–162. [Google Scholar] [CrossRef]
- Bedos, C.; Cellier, P.; Calvet, R.; Barriuso, E.; Gabrielle, B. Mass transfer of pesticides into the atmosphere by volatilization from soils and plants: Overview. Agronomie 2002, 22, 21–33. [Google Scholar] [CrossRef]
- Wolters, A. Pesticide Volatilization from Soil and Plant Surfaces: Measurements at Different Scales versus Model Predictions. 2003. Available online: https://d-nb.info/968370667/34 (accessed on 15 November 2021).
- Dickerson, R.R.; Huffman, G.J.; Luke, W.T.; Nunnermacker, L.J.; Pickering, K.E.; Leslie, A.C.D.; Lindsey, C.G.; Slinn, W.G.N.; Kelly, T.J.; Daum, P.H.; et al. Thunderstorms—An important mechanism in the transport of air-pollutants. Science 1987, 235, 460–464. [Google Scholar] [CrossRef]
- Bansal, O.P. Fate of pesticides in the environment. J. Indian Chem. Soc. 2011, 88, 1525–1532. [Google Scholar]
- Quan, G.; Yin, C.; Chen, T.; Yan, J. Degradation of Herbicide Mesotrione in Three Soils with Differing Physicochemical Properties from China. J. Environ. Qual. 2015, 44, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K. Pesticides and Environment. Pestic. Chem. Toxicol. 2012, 1, 114–122. [Google Scholar]
- Maul, J.D.; Brennan, A.A.; Harwood, A.D.; Lydy, M.J. Effect of sediment-associated pyrethroids, fipronil, and metabolites on Chironomus tentans growth rate, body mass, condition index, immobilization, and survival. Environ. Toxicol. Chem. 2008, 27, 2582–2590. [Google Scholar] [CrossRef] [PubMed]
- Katagi, T. Direct photolysis mechanism of pesticide in water. J. Pestic. Sci. 2018, 43, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, J.P.; Williams, R.J.; Gooddy, D.C.; Cape, J.N.; Guha, P. Impacts of climate change on the fate and behaviour of pesticides in surface and groundwater—A UK perspective. Sci. Total Environ. 2006, 369, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Bedos, C.; Rousseau-Djabri, M.F.; Gabrielle, B.; Flura, D.; Durand, B.; Barriuso, E.; Cellier, P. Measurement of trifluralin volatilization in the field: Relation to soil residue and effect of soil incorporation. Environ. Pollut. 2006, 144, 958–966. [Google Scholar] [CrossRef]
- Schneider, M.; Endo, S.; Goss, K.U. Volatilization of pesticides from the bare soil surface: Evaluation of the humidity effect. J. Environ. Qual. 2013, 42, 844–851. [Google Scholar] [CrossRef]
- Chen, L.; Feng, Q.; He, Q.; Huang, Y.; Zhang, Y.; Jiang, G.; Zhao, W.; Gao, B.; Lin, K.; Xu, Z. Sources, atmospheric transport and deposition mechanism of organochlorine pesticides in soils of the Tibetan Plateau. Sci. Total Environ. 2017, 577, 405–412. [Google Scholar] [CrossRef]
- Chowdhury, I.F.; Rohan, M.; Stodart, B.J.; Chen, C.; Wu, H.; Doran, G.S. Persistence of atrazine and trifluralin in a clay loam soil undergoing different temperature and moisture conditions. Environ. Pollut. 2021, 276, 116687. [Google Scholar] [CrossRef]
- Reichman, R.; Yates, S.R.; Skaggs, T.H.; Rolston, D.E. Effects of soil moisture on the diurnal pattern of pesticide emission: Comparison of simulations with field measurements. Atmos. Environ. 2013, 66, 52–62. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Cao, A.; Xu, J.; Fang, W.; Yan, D.; Li, Y.; Wang, Q. Effects of soil type, moisture content and organic amendment rate on dimethyl disulfide distribution and persistency in soil. Environ. Pollut. 2021, 285, 117198. [Google Scholar] [CrossRef]
- Martinez, C.O.; de Souza Silva, C.M.M.; Fay, E.F.; Abakerli, R.B.; Maia, A.H.N.N.; Durrant, L.R. The effects of moisture and temperature on the degradation of sulfentrazone. Geoderma 2008, 147, 56–62. [Google Scholar] [CrossRef]
- Tariq, M.I.; Afzal, S.; Hussain, I. Degradation and persistence of cotton pesticides in sandy loam soils from Punjab, Pakistan. Environ. Res. 2006, 100, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Đurović, R.; Gajić-Umiljendić, J.; Đorđević, T. Effects of organic matter and clay content in soil on pesticide adsorption processes. Pestic. Phytomed. 2009, 24, 51–57. [Google Scholar] [CrossRef]
- Riaz, R.; Malik, R.N.; de Wit, C.A. Soil-air partitioning of semivolatile organic compounds in the Lesser Himalaya region: Influence of soil organic matter, atmospheric transport processes and secondary emissions. Environ. Pollut. 2021, 291, 118006. [Google Scholar] [CrossRef]
- Mamy, L.; Barriuso, E. Desorption and time-dependent sorption of herbicides in soils. Eur. J. Soil Sci. 2007, 58, 174–187. [Google Scholar] [CrossRef]
- Wołejko, E.; Jabłońska-Trypuć, A.; Wydro, U.; Butarewicz, A.; Łozowicka, B. Soil biological activity as an indicator of soil pollution with pesticides—A review. Appl. Soil Ecol. 2020, 147, 103356. [Google Scholar] [CrossRef]
- Spencer, W.F.; Cliath, M.M.; Yates, S.R. Soil-pesticide interactions and their impact on the volatilization process. In Environmental Impact of Soil Component Interactions, Volume I, Natural and Anthropogenic Organics; CRC Press: Boca Raton, FL, USA; Lewis Publishers Inc.: Chelsea, MI, USA, 1995; pp. 371–382. [Google Scholar]
- Galon, L.; Bragagnolo, L.; Korf, E.P. Mobility and environmental monitoring of pesticides in the atmosphere—A review. Environ. Sci. Pollut. Res. 2021, 28, 32236–32255. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, R.; Li, Y.; Wang, P.; Zhu, Y.; Yuan, G.; Zhang, Q.; Jiang, G. Accumulation and fate processes of organochlorine pesticides (OCPs) in soil profiles in Mt. Shergyla, Tibetan Plateau: A comparison on different forest types. Chemosphere 2019, 231, 571–578. [Google Scholar] [CrossRef]
- Wang, X.; Xue, Y.; Gong, P. Organochlorine pesticides and polychlorinated biphenyls in Tibetan forest soil: Profile distribution and processes. Environ. Sci. Pollut. Res. 2014, 21, 1897–1904. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, X.; Gong, P. Distribution and vertical migration of polycyclic aromatic hydrocarbons in forest soil pits of southern Tibet. Environ. Geochem. Health 2018, 40, 1941–1953. [Google Scholar] [CrossRef]
- Das, S.; Hageman, K.J. Influence of adjuvants on pesticide soil-air partition coefficients: Laboratory measurements and predicted effects on volatilization. Environ. Sci. Technol. 2020, 54, 7302–7308. [Google Scholar] [CrossRef]
- Herzfeld, D.; Sargent, K. Private pesticide applicator safety education manual. In Protecting the Environment, 19th ed.; University of Minnesota Extension: St Paul, MN, USA, 2011. [Google Scholar]
- Spencer, W.F.; Cliath, M.M. Movement of pesticides from soil to the atmosphere. In Long Range Transport of Pesticides; Lewis Publication: Lynden, WA, USA, 1990; pp. 1–16. [Google Scholar]
- Schweizer, A.; Turgut, C.; Hurle, K. Studies on the volatilization pf pesticides from plant and surfaces and their dependence on temperature and relative humidity. Z. Für Pflanzenkrankh. Und Pflanzenschutz 2000, 17, 791–798. [Google Scholar]
- Bailey, S.W. Climate Change and decreasing herbicide persistence. Pest Manag. Sci. 2004, 60, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Tepper, G. Weather Essentials for Pesticide Application, Grains Research and Development Corporation; GRDC Manager Integrated Publications: Kingston, Australia, 2012; pp. 10–15. [Google Scholar]
- Waymann, B.; Rudel, H. Influence of air velocity, application dose, and test area size on the volatilization of lindane. Int. J. Environ. Anal. Chem. 1995, 58, 371–378. [Google Scholar] [CrossRef]
- Cessna, A.J.; Larney, F.J.; Kerr, L.A.; Bullock, M.S. Transport of trifluralin on wind-eroded sediment. Can. J. Soil Sci. 2006, 86, 545–554. [Google Scholar] [CrossRef]
- Bronsert, A.; Niehoff, D.; Burger, G. Effects of climate and land-use change on storm runoff generation: Present knowledge and modelling capabilities. Hydrol. Process. 2002, 16, 509–529. [Google Scholar] [CrossRef]
- Lei, Y.D.; Wania, F. Is rain or snow a more efficient scavenger of organic chemicals? Atmos. Environ. 2004, 38, 3557–3571. [Google Scholar] [CrossRef]
- Thurman, E.M.; Cromwell, A.E. Atmospheric transport, deposition, and fate of triazine herbicides and their metabolites in Pristine Areas at Isle Royale National Park. Environmental. Sci. Technol. 2000, 34, 3079–3085. [Google Scholar] [CrossRef]
- Mast, M.A.; Foreman, W.T.; Skaates, S.V. Current-use pesticides and organochlorine compounds in precipitation and lake sediment from two high-elevation national parks in the Western United States. Arch. Environ. Contam. Toxicol. 2007, 52, 294–305. [Google Scholar] [CrossRef]
- Majewski, M.S.; Capel, P.D. Pesticides in the Atmosphere—Pesticides in the Hydrologic System; Ann Arbor Press: Chelsea, MI, USA, 1995. [Google Scholar]
- Daly, G.L.; Lei, Y.D.; Teixeira, C.; Muir, D.C.G.; Wania, F. Pesticides in Western Canadian mountain air and soil. Environ. Sci. Technol. 2007, 41, 6020–6025. [Google Scholar] [CrossRef]
- Ding, Y.; Hayward, S.J.; Westgate, J.N.; Brown, T.N.; Lei, Y.D.; Wania, F. Legacy and current-use pesticides in Western Canadian mountain air: Influence of pesticide sales, source proximity, and altitude. Atmos. Environ. 2023, 308, 119882. [Google Scholar] [CrossRef]
- Arias, L.A.; Garzón, A.; Ayarza, A.; Aux, S.; Bojacá, C.R. Environmental fate of pesticides in open field and greenhouse tomato production regions from Colombia. Environ. Adv. 2021, 3, 100031. [Google Scholar] [CrossRef]
- Robinson, D.E.; Mansingh, A.; Dasgupta, T.P. Fate and transport of ethoprophos in the Jamaican environment. Sci. Total Environ. 1999, 238, 373–378. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, Y.; Zhang, Z.; Xu, X.; Li, H.; Zuo, L. Halogenated organic pollutants in marine biota from the Xuande Atoll, South China Sea: Levels, biomagnification and dietary exposure. Mar. Pollut. Bull. 2017, 118, 413–419. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Ali, U.; Syed, J.H.; Malik, R.N.; Katsoyiannis, A.; Li, J.; Zhang, G. Organochlorine pesticides (OCPs) in South Asian region: A review. Sci. Total Environ. 2014, 476–477, 705–717. [Google Scholar] [CrossRef]
- Wong, F.; Alegria, H.A.; Jantunen, L.M.; Bidleman, T.F.; Salvador-Figueroa, M.; Gold-Bouchot, G.; Ceja-Moreno, V.; Waliszewski, S.M.; Infanzon, R. Organochlorine pesticides in soils and air of southern Mexico: Chemical profiles and potential for soil emissions. Atmos. Environ. 2008, 42, 7737–7745. [Google Scholar] [CrossRef]
- Chakraborty, P.; Zhang, G.; Li, J.; Sivakumar, A.; Jones, K.C. Occurrence and sources of selected organochlorine pesticides in the soil of seven major Indian cities: Assessment of air–soil exchange. Environ. Pollut. 2015, 204, 74–80. [Google Scholar] [CrossRef]
- Terry, F.B.; Leone, A.D.; Wong, F.; van Vliet, L.; Szeto, S.; Ripley, B.D. Emission of legacy chlorinated pesticides from agricultural and orchard soils in British Columbia, Canada. Environ. Toxicol. Chem. 2006, 25, 1448–1457. [Google Scholar] [CrossRef]
- Balmer, J.E.; Morris, A.D.; Hung, H.; Jantunen, L.; Vorkamp, K.; Rigét, F. Levels and trends of current-use pesticides (CUPs) in the arctic: An updated review, 2010–2018. Emerg. Contam. 2019, 5, 70–88. [Google Scholar] [CrossRef]
- Guida, Y.d.S.; Meire, R.O.; Torres, J.P.M.; Malm, O. Air contamination by legacy and current-use pesticides in Brazilian mountains: An overview of national regulations by monitoring pollutant presence in pristine areas. Environ. Pollut. 2018, 242, 19–30. [Google Scholar] [CrossRef]
- Estellano, V.H.; Pozo, K.; Harner, T.; Franken, M.; Zaballa, M. Altitudinal and seasonal variations of persistent organic pollutants in the Bolivian Andes mountains. Environ. Sci. Technol. 2008, 42, 2528–2534. [Google Scholar] [CrossRef] [PubMed]
- Brühl, C.A.; Bakanov, N.; Köthe, S.; Eichler, L.; Sorg, M.; Hörren, T. Direct pesticide exposure of insects in nature conservation areas in Germany. Sci. Rep. 2021, 11, 24144. [Google Scholar] [CrossRef]
- Sultana, J.; Syed, J.H.; Mahmood, A.; Ali, U.; Rehman, M.Y.A.; Malik, R.N.; Li, J.; Zhang, G. Investigation of organochlorine pesticides from the Indus Basin, Pakistan: Source, air-soil exchange fluxes and risk assessment. Sci. Total Environ. 2014, 497–498, 113–122. [Google Scholar] [CrossRef]
- Qu, C.; Albanese, S.; Lima, A.; Li, J.; Doherty, A.L.; Qi, S.; de Vivo, B. Residues of hexachlorobenzene and chlorinated cyclodiene pesticides in the soils of the Campanian Plain, southern Italy. Environ. Pollut. 2017, 231, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Zhang, G.; Li, J.; Xu, Y.; Liu, X.; Tanabe, S.; Jones, K.C. Selected organochlorine pesticides in the atmosphere of major Indian cities: Levels, regional versus local variations, and sources. Environ. Sci. Technol. 2010, 44, 8038–8043. [Google Scholar] [CrossRef] [PubMed]
- Khuman, S.N.; Chakraborty, P. Air-water exchange of pesticidal persistent organic pollutants in the lower stretch of the transboundary river Ganga, India. Chemosphere 2019, 233, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; He, M.; Kong, F. Risk attitude, risk perception, and farmers’ pesticide application behavior in China: A moderation and mediation model. J. Clean. Prod. 2020, 276, 124241. [Google Scholar] [CrossRef]
- Grella, M.; Marucco, P.; Balafoutis, A.; Balsari, P. Spray drift generated in vineyard during under-row weed control and suckering: Evaluation of direct and indirect drift-reducing techniques. Sustainability 2020, 12, 5068. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, L.; Wu, Y.; Musiu, E.M.; Cheng, Z.; Wang, P. Numerical simulation of airflow field from a six–rotor plant protection drone using lattice Boltzmann method. Biosyst. Eng. 2020, 197, 336–351. [Google Scholar] [CrossRef]
- Williams, W.M.; Giddings, J.M.; Purdy, J.; Solomon, K.R.; Giesy, J.P. Exposures of aquatic organisms to the organophosphorus insecticide, chlorpyrifos resulting from use in the United States. Rev. Environ. Contam. Toxicol. 2014, 231, 77–118. [Google Scholar] [CrossRef] [PubMed]
- Farhan, D.M.; Wajid, A.; Hussain, T.; Jabeen, F.; Ishaque, U.; Iftikhar, M.; Daim, M.A.; Noureen, A. Investigation of oxidative stress enzymes and histological alterations in tilapia exposed to chlorpyrifos. Environ. Sci. Pollut. Res. Int. 2020, 28, 13105–13111. [Google Scholar] [CrossRef] [PubMed]
- Giddings, J.M.; Williams, W.M.; Solomon, K.R.; Giesy, J.P. Risks to aquatic organisms from use of chlorpyrifos in the United States. In Ecological Risk Assessment for Chlorpyrifos in Terrestrial and Aquatic Systems in the United States. Reviews of Environmental Contamination and Toxicology (Continuation of Residue Reviews); Giesy, J., Solomon, K., Eds.; Springer: Cham, Switzerland, 2014; Volume 231, pp. 119–162. [Google Scholar] [CrossRef]
- Yang, C.; Lim, W.; Song, G. Mediation of oxidative stress toxicity induced by pyrethroid pesticides in fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 234, 108758. [Google Scholar] [CrossRef] [PubMed]
- Sumon, K.A.; Rashid, H.; Peeters, E.T.H.M.; Bosma, R.H.; Van den Brink, P.J. Environmental monitoring and risk assessment of organophosphate pesticides in aquatic ecosystems of north-west Bangladesh. Chemosphere 2018, 206, 92–100. [Google Scholar] [CrossRef] [PubMed]
- DiBartolomeis, M.; Kegley, S.; Mineau, P.; Radford, R.; Klein, K. An assessment of acute insecticide toxicity loading (AITL) of chemical pesticides used on agricultural land in the United States. PLoS ONE 2019, 14, e0220029. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D.; Thompson, J.; Croombs, A. Rapid rise in toxic load for bees revealed by analysis of pesticide use in Great Britain. PeerJ 2018, 6, e5255. [Google Scholar] [CrossRef] [PubMed]
- Kudsk, P.; Jørgensen, L.N.; Ørum, J.E. Pesticide load—A new Danish pesticide risk indicator with multiple applications. Land Use Policy 2018, 70, 384–393. [Google Scholar] [CrossRef]
- Schulz, R.; Bub, S.; Petschick, L.L.; Stehle, S.; Wolfram, J. Applied pesticide toxicity shifts toward plants and invertebrates, even in GM crops. Science 2021, 372, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Tassin de Montaigu, C.; Goulson, D. Identifying agricultural pesticides that may pose a risk for birds. PeerJ 2020, 8, e9526. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Antoniou, M. Mammalian toxicity of herbicides used in intensive GM crop farming. In Herbicides: Chemistry, Efficacy, Toxicology, and Environmental Impacts; Mesnage, R., Zaller, J.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 143–180. [Google Scholar]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Özkara, A.; Akyil, D.; Konuk, M. Pesticides, Environmental Pollution, and Health, Environmental Health Risk—Hazardous Factors to Living Species. InTech 2016, 16, 3–25. [Google Scholar] [CrossRef]
- Mannucci, P.M.; Franchini, M. Health effects of ambient air pollution in developing countries. Int. J. Environ. Res. Public Health 2017, 14, 1048. [Google Scholar] [CrossRef] [PubMed]
- Hassaan, M.; El Nemr, A. Pesticides pollution: Classifications, human health impact, extraction and treatment techniques. Egypt. J. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Kaur, N.; Starling, A.P.; Calafat, A.M.; Sjodin, A.; Clouet-Foraison, N.; Dolan, L.M.; Imperatore, G.; Jensen, E.T.; Lawrence, J.M.; Ospina, M.; et al. Longitudinal association of biomarkers of pesticide exposure with cardiovascular disease risk factors in youth with diabetes. Environ. Res. 2020, 181, 108916. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; Tang, F.H.M.; Black, A.J.; Marks, G.B.; McBratney, A. The pesticide health risk index—An application to the world's countries. Sci. Total Environ. 2021, 801, 149731. [Google Scholar] [CrossRef]
- Matich, E.K.; Laryea, J.A.; Seely, K.A.; Stahr, S.; Su, L.J.; Hsu, P.C. Association between pesticide exposure and colorectal cancer risk and incidence: A systematic review. Ecotoxicol. Environ. Saf. 2021, 219, 112327. [Google Scholar] [CrossRef] [PubMed]
- Parween, T.; Jan, S. Chapter 7—Ecological effect of pesticide on microbial communities and human health. In Ecophysiology of Pesticides; Parween, T., Jan, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 223–263. [Google Scholar] [CrossRef]
- Tudi, M.; Li, H.; Li, H.; Wang, L.; Lyu, J.; Yang, L.; Tong, S.; Yu, Q.J.; Ruan, H.D.; Atabila, A.; et al. Exposure Routes and Health Risks Associated with Pesticide Application. Toxics 2022, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Gallart-Mateu, D.; Armenta, S.; de la Guardia, M. Indoor and outdoor determination of pesticides in air by ion mobility spectrometry. Talanta 2016, 161, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Banks, A.P.W.; He, C.; Drage, D.S.; Gallen, C.L.; Li, Y.; Li, Q.; Thai, P.K.; Mueller, J.F. Polycyclic aromatic hydrocarbons, polychlorinated biphenyls and legacy and current pesticides in indoor environment in Australia—Occurrence, sources and exposure risks. Sci. Total Environ. 2019, 693, 133588. [Google Scholar] [CrossRef] [PubMed]
- Abass, K.; Turpeinen, M.; Pelkonen, O. An Evaluation of The Cytochrome P450 Inhibition Potential of Selected Pesticides in Human Hepatic Microsomes. J. Environ. Sci. Health Part B 2009, 44, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.A.; Jeong, Y.; Jeon, H.P.; Kim, S. Comparing Passive Dosing and Solvent Spiking Methods to Determine the Acute Toxic Effect of Pentachlorophenol on Daphnia Magna. Ecotoxicology 2020, 29, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Uckun, M.; Yologlu, E.; Uckun, A.A.; Oz, O.B. Acute Toxicity of Insecticide Thiamethoxam to Crayfish (Astacus Leptodactylus): Alterations in Oxidative Stress Markers, Atpases and Cholinesterase. Acta Chim. Slov. 2021, 68, 521–531. [Google Scholar] [CrossRef]
- Choudhary, A.; Ali, A.S.; Ali, S.A. Adverse health effects of organophosphate pesticides among occupationally exposed farm sprayers: A case study of Bhopal Madhya Pradesh, India. Asian J. Biomed. Pharm. Sci. 2014, 4, 30–35. [Google Scholar] [CrossRef]
- Fareed, M.; Pathak, M.K.; Bihari, V.; Kamal, R.; Srivastava, A.K.; Kesavachandran, C.N. Adverse respiratory health and hematological alterations among agricultural workers occupationally exposed to organophosphate pesticides: A cross-sectional study in North India. PLoS ONE 2013, 8, e69755. [Google Scholar] [CrossRef]
- Langenbach, T.; Caldas, L.Q. Strategies for reducing airborne pesticides under tropical conditions. Ambio 2018, 47, 574–584. [Google Scholar] [CrossRef]
- Lu, C.; Fenske, R.A.; Simcox, N.J.; Kalman, D. Pesticide exposure of children in an agricultural community: Evidence of household proximity to farmland and take home exposure pathways. Environ. Res. 2000, 84, 290–302. [Google Scholar] [CrossRef] [PubMed]
- van Wendel de Joode, B.; Barraza, D.; Ruepert, C.; Mora, A.M.; Córdoba, L.; Öberg, M. Indigenous children living nearby plantations with chlorpyrifos-treated bags have elevated 3,5,6-trichloro-2-pyridinol (TCPy) urinary concentrations. Environ. Res. 2012, 117, 17–26. [Google Scholar] [CrossRef]
- Linhart, C.; Panzacchi, S.; Belpoggi, F.; Clausing, P.; Zaller, J.G.; Hertoge, K. Year-round pesticide contamination of public sites near intensively managed agricultural areas in South Tyrol. Environ. Sci. Eur. 2021, 33, 1–12. [Google Scholar] [CrossRef]
- Coscollà, C.; López, A.; Yahyaoui, A.; Colin, P.; Robin, C.; Poinsignon, Q.; Yusà, V. Human exposure and risk assessment to airborne pesticides in a rural French community. Sci. Total Environ. 2017, 584–585, 856–868. [Google Scholar] [CrossRef]
- Gupta, P.K. Pesticide exposure—Indian scene. Toxicology 2004, 198, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Rudzi, S.K.; Ho, Y.B.; Tan, E.S.S.; Jalaludin, J.; Ismail, P. Exposure to airborne pesticides and its residue in blood serum of paddy farmers in Malaysia. Int. J. Environ. Res. Public Health 2022, 19, 6806. [Google Scholar] [CrossRef]
- Mu, H.; Zhang, J.; Yang, X.; Wang, K.; Xu, W.; Zhang, H.; Liu, X.; Ritsema, C.J.; Geissen, V. Pesticide screening and health risk assessment of residential dust in a rural region of the North China Plain. Chemosphere 2022, 303, 135115. [Google Scholar] [CrossRef]
- Chetty-Mhlanga, S.; Fuhrimann, S.; Basera, W.; Eeftens, M.; Röösli, M.; Dalvie, M.A. Association of activities related to pesticide exposure on headache severity and neurodevelopment of school-children in the rural agricultural farmlands of the Western Cape of South Africa. Environ. Int. 2021, 146, 106237. [Google Scholar] [CrossRef]
- Tsai, M.S.; Chen, M.H.; Lin, C.C.; Liu, C.Y.; Chen, P.C. Children’s environmental health based on birth cohort studies of Asia (2)—Air pollution, pesticides, and heavy metals. Environ. Res. 2019, 179, 108754. [Google Scholar] [CrossRef]
- Guida, Y.; de Carvalho, G.O.; Capella, R.; Pozo, K.; Lino, A.S.; Azeredo, A.; Carvalho, D.F.P.; Braga, A.L.F.; Torres, J.P.M.; Meire, R.O. Atmospheric Occurrence of Organochlorine Pesticides and Inhalation Cancer Risk in Urban Areas at Southeast Brazil. Environ. Pollut. 2021, 271, 116359. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, M.J.; Wu, F.Y.; Kang, Y.; Wang, H.S.; Cheung, K.C.; Wong, M.H. Risk assessment of bioaccessible organochlorine pesticides exposure via indoor and outdoor dust. Atmos. Environ. 2013, 77, 525–533. [Google Scholar] [CrossRef]
- Rauh, V.A.; Perera, F.P.; Horton, M.K.; Whyatt, R.M.; Bansal, R.; Hao, X. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proc. Natl. Acad. Sci. USA 2012, 109, 7871–7876. [Google Scholar] [CrossRef] [PubMed]
- Perez-Fernandez, C.; Morales-Navas, M.; Guardia-Escote, L.; Colomina, M.T.; Giménez, E.; Sánchez-Santed, F. Postnatal exposure to low doses of chlorpyrifos induces longterm effects on 5C-SRTT learning and performance, cholinergic and GABAergic systems and BDNF expression. Exp. Neurol. 2020, 330, 113356. [Google Scholar] [CrossRef]
- Doǧanlar, Z.B.; Doǧanlar, O.; Tozkir, H.; Gökalp, F.D.; Doǧan, A.; Yamaç, F.; Askın, O.O.; Aktas, Ü.E. Nonoccupational exposure of agricultural area residents to pesticides: Pesticide accumulation and evaluation of genotoxicity. Arch. Environ. Contam. Toxicol. 2018, 75, 530–544. [Google Scholar] [CrossRef]
- Tang, M.; Zhao, M.; Zhou, S.; Chen, K.; Zhang, C.; Liu, W. Assessing the underlying breast cancer risk of Chinese females contributed by dietary intake of residual DDT from agricultural soils. Environ. Int. 2014, 73, 208–215. [Google Scholar] [CrossRef]
- Niu, L.; Xu, C.; Zhu, S.; Zhang, H.; Liu, W. Factors influencing the ecological and human health risks of DDTs in soils and air at the isomeric and enantiomeric levels. J. Hazard. Mater. 2018, 359, 316–324. [Google Scholar] [CrossRef]
- Gascon, M.; Sunyer, J.; Martinez, D.; Guerra, S.; Lavi, I.; Torrent, M.; Vrijheid, M. Persistent organic pollutants and children’s respiratory health: The role of cytokines and inflammatory biomarkers. Environ. Int. 2014, 69, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Karmaus, W.; Brooks, K.R.; Nebe, T.; Witten, J.; Obi-Osius, N.; Kruse, H. Immune function biomarkers in children exposed to lead and organochlorine compounds: A cross-sectional study. Environ. Heath 2005, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Balte, P.P.; Kühr, J.; Kruse, H.; Karmaus, W. Body burden of dichlorodiphenyl dichloroethane (DDE) and childhood pulmonary function. Int. J. Environ. Res. Public Health 2017, 14, 1376. [Google Scholar] [CrossRef]
- Raherison, C.; Baldi, I.; Pouquet, M.; Berteaud, E.; Moesch, C.; Bouvier, G.; Canal-Raffin, M. Pesticides exposure by air in vineyard rural area and respiratory health in children: A pilot study. Environ. Res. 2019, 169, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Lopez, J.; Amchich, F.; Murillo, J.; Denenberg, J. Blood pressure after a heightened pesticide spray period among children living in agricultural communities in Ecuador. Environ. Res. 2019, 175, 335–342. [Google Scholar] [CrossRef]
- Parrón, T.; Requena, M.; Hernández, A.F.; Alarcón, R. Environmental exposure to pesticides and cancer risk in multiple human organ systems. Toxicol. Lett. 2013, 230, 157–165. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Liu, R.; Gan, J.; Liu, J.; Liu, W. Endocrine-disrupting effects of pesticides through interference with human glucocorticoid receptor. Environ. Sci. Technol. 2016, 50, 435–443. [Google Scholar] [CrossRef]
- Kamel, F. Paths from pesticides to Parkinson’s. Science 2013, 341, 722–723. [Google Scholar] [CrossRef]
- Yan, D.; Zhang, Y.; Liu, L.; Yan, H. Pesticide exposure and risk of Alzheimer’s disease: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 32222. [Google Scholar] [CrossRef]
- Jallow, M.F.; Awadh, D.G.; Albaho, M.S.; Devi, V.Y.; Thomas, B.M. Pesticide Knowledge and Safety Practices among Farm Workers in Kuwait: Results of a Survey. Int. J. Environ. Res. Public Health 2017, 14, 340. [Google Scholar] [CrossRef]

| Pesticide | Name | Concentration Range (ng/m3) | Country | References |
|---|---|---|---|---|
| Organochlorine Insecticide | Aldrin | 0.36–1.18 | China | [17] |
| cis-Chlordane | 0.01 | South Africa | [18] | |
| trans-Chlordane | 0.02 | South Africa | [18] | |
| Chlordane | nd 1–1.369 | China | [19] | |
| 2,4′-DDD | 0.01 | South Africa | [18] | |
| 4,4′-DDD | 0.37–0.55 | France | [20] | |
| 0.011–0.087 | Pakistan | [21] | ||
| 4.57–154 | China | [17] | ||
| 0.02 | South Africa | [18] | ||
| 2,4′-DDE | 0.02 | South Africa | [18] | |
| 4,4′-DDE | 0.27–0.36 | France | [20] | |
| 0.002–0.007 | China | [22] | ||
| 2.05–25.60 | China | [17] | ||
| 0.012–0.240 | Pakistan | [21] | ||
| 1.20 | South Africa | [18] | ||
| 11.60–109.50 | Austria | [23] | ||
| nd–0.20 | USA | [24] | ||
| 2,4′-DDT | 0.06 | South Africa | [18] | |
| 4,4′-DDT | 0.1 | South Africa | [18] | |
| 0.009–0.068 | China | [19] | ||
| 12.50–15.50 | Austria | [23] | ||
| Dieldrin | 0.08 | South Africa | [18] | |
| Drins | nd–0.01 | China | [19] | |
| α-Endosulfan | 0.37–81.31 | France | [20] | |
| 0.011–0.028 | China | [22] | ||
| 0.52–1.09 | China | [17] | ||
| 0.00009–0.042 | China | [19] | ||
| 0.003–0.009 | Pakistan | [21] | ||
| 0.06–0.10 | South Africa | [18] | ||
| HCB | 0.05–0.43 | USA | [24] | |
| nd–0.026 | China | [19] | ||
| α-HCH | 0.0058 | South Africa | [18] | |
| β-HCH | 0.0027 | South Africa | [18] | |
| δ-HCH | 0.0005 | South Africa | [18] | |
| γ-HCH | 0.06 | South Africa | [18] | |
| HCHs | 0.00012–0.0184 | China | [19] | |
| 0–267.4 | Germany | [25] | ||
| Heptachlor | 0.0015 | South Africa | [18] | |
| Heptachlor epoxide | 0.00065 | South Africa | [18] | |
| 4,4′-methoxychlor | 0.0004–0.0088 | China | [19] | |
| Mirex | 0.00013 | South Africa | [18] | |
| nd–9.94 | USA | [24] | ||
| Oxychlordane | 0.00048 | South Africa | [18] | |
| Organophosphate Insecticide | Azinfos-methyl | 0.03 | South Africa | [18] |
| Chlorpyriphos | 10–12900 | Republic of Korea | [26] | |
| 0.11–0.15 | Spain | [15] | ||
| 0.10–1.00 | South Africa | [27] | ||
| 6.10–36.10 | Costa Rica | [28] | ||
| 15.50–287.0 | Austria | [23] | ||
| 16.20 | South Africa | [18] | ||
| Diazinon | 0.28–1.49 | France | [20] | |
| 1.40 | South Africa | [18] | ||
| Dimethoate | 0.19 | South Africa | [18] | |
| 0.10–1.00 | South Africa | [27] | ||
| Ethoprophos | 0.21–0.48 | France | [20] | |
| EPN | 0–4470 | Republic of Korea | [26] | |
| Malathion | 0.10–1.00 | South Africa | [29] | |
| 0.20 | South Africa | [18] | ||
| Parathion | 1000–61900 | Republic of Korea | [26] | |
| Phorate | 2100–33700 | Republic of Korea | [26] | |
| Carbamates | Carbaryl | 1.30 | South Africa | [18] |
| Oxamyl | 9.64 | Spain | [30] | |
| Acetamides | Metazachlor | 0.009 | South Africa | [18] |
| 0.17–3.13 | France | [16] | ||
| S-metolachlor | 0.29 | South Africa | [18] | |
| Triazinones/ Triazines/ Triazoles | Atrazine | 0.04 | South Africa | [18] |
| Metribuzin | 0.03 | South Africa | [18] | |
| Simazine | 0.88 | South Africa | [18] | |
| Terbuthylazine | 0.79 | South Africa | [18] | |
| Propiconazole | 0.08 | South Africa | [18] | |
| Herbicide | Alachlor | 0.12–6.03 | France | [20] |
| Alconifen | 0.23–4.15 | France | [16] | |
| Diuron | 0.12 | South Africa | [18] | |
| Dimethenamid | 0–1556.6 | Germany | [25] | |
| Glyphosate | 503.0–517.0 | Malaysia | [31] | |
| 0.24–0.48 | USA | [32] | ||
| 0.18–1.04 | France | [33] | ||
| 20.3–3176.8 | Germany | [25] | ||
| 0.10–0.30 | Italy | [34] | ||
| Metolachlor | 0–1273.3 | Germany | [25] | |
| 12.3–382.6 | Austria | [23] | ||
| Pendimethalin | 0–3916.8 | Germany | [25] | |
| 44.9–3932.4 | Austria | [23] | ||
| Prosulfocarb | 13.7–4357.8 | Austria | [23] | |
| Terbuthylazine | 0–905.9 | Germany | [25] | |
| Trifluralin | 0.12–40.74 | France | [16] | |
| Fungicide | Captan | 1.19–67.62 | France | [20] |
| 4.54–22.82 | France | [16] | ||
| Chlorothalonil | 0.11–107.93 | France | [20] | |
| 0–1866.2 | Germany | [25] | ||
| 30.6–554.4 | Austria | [23] | ||
| Carbendazim | 0.010–0.046 | Spain | [15] | |
| Difenoconazole | 77.43 | Spain | [30] | |
| Epoxiconazole | 0.12–3.99 | France | [20] | |
| 0–81.3 | Germany | [25] | ||
| Folpet | 7.90–82.2 | France | [16] | |
| 0–7613.8 | Germany | [25] | ||
| 35.5–1665.2 | Austria | [23] | ||
| Hexachlorobenzene | 0–46.3 | Germany | [25] | |
| Tebuconazole | 22.2 | South Africa | [29] | |
| 10.4–67.7 | Austria | [23] | ||
| Tetraconazole | 11.1–16.3 | Austria | [23] |
| Pesticide | Molecular Weight (g/mol) | Boiling Point (°C) | Density (g/cm3) | Log Kow 1 | Log Koc 2 | Water Solubility (mg/L) | Vapor Pressure (Pa) | Henry’s Law Constant (Pa.m3/mol) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Acetamides | 59.07–292 | N/A 3 | 0.85–2.9 | −1.52 | 1.15–3.3 | 10–99,500 | N/A 3 | N/A 3 | [54,55] |
| Alachlor | 269.8 | 108–109 | 1.14 | 3.36 | 2.91 | 191 | 0.17 | 2.24 | [56] |
| Alconifen | 328.9 | 400 | - | 6.2 | - | 0.03 | 1.8 × 10−9 | 2.46 | [57] |
| Aldrin (as Cl) | 364.9 | 175–177 | 1.57 | 5.2 | 5.3 | Insoluble | 0.027 | 0.697 | [58] |
| Atrazine | 215.7 | N/A 3 | 1.19–1.5 | 2.83 | 1.5–2.77 | 33–200,000 | 0.0004–0.4 | 3–110 | [59,60,61] |
| Azinfos-methyl | 214.6 | 96–98 | 1.3 | 2.3 | 2.36 | 1200 | 0.02 | 0.016 | [62] |
| Captan | 300.35 | Decomposes | 1.77 | 2.98 | 3.1 | 4.4 | 2.6 × 10−8 | N/A 3 | [63] |
| Carbamates | 86–389 | N/A 3 | 0.97–1.6 | −6.9 | 0.6–3.9 | 0.1–63,000 | N/A 3 | N/A 3 | [64,65] |
| Carbaryl | 201.2 | 142–143 | 1.23 | 1.88 | 1.94 | 42 | 0.0001 | 0.0005 | [66] |
| Carbendazim | 191.2 | Decomposes | 1.47 | 2.26 | 1.8 | 48.3 | 2.1 × 10−7 | N/A | [67] |
| α-Chlordane | 409.8 | 433–435 | 1.6 | 5.37 | 5.5 | 2.2 | 0.15 | 1.44 | [58] |
| γ-Chlordane | 409.8 | 433–435 | 1.6 | 5.37 | 5.5 | 2.2 | 0.15 | 1.44 | [58] |
| Chlordane | 409.8 | 433–435 | 1.6 | 5.37 | 5.5 | 2.2 | 0.15 | 1.44 | [58] |
| Chlorothalonil | 265.7 | Decomposes | 2.3 | 3.33 | 3.49 | 0.35 | 6.5 × 10−8 | N/A 3 | [68] |
| Chlorpyriphos | 350.6 | 156–157 | 1.49 | 4.8 | 4.7 | 0.6 | 0.00011 | 0.00044 | [69] |
| DDX | 320.9 | 150–155 | 1.66 | 5.25 | 5.28 | 0.06 | 0.0013 | 1.32 | [70] |
| 2,4′-DDD | 320.9 | 210–211 | 1.57 | 4.82 | 5.03 | Insoluble | 0.00011 | 0.0015 | [71] |
| 4,4′-DDD | 320.9 | 210–211 | 1.57 | 4.82 | 5.03 | Insoluble | 0.00011 | 0.0015 | [71] |
| 2,4′-DDE | 318.9 | 185–186 | 1.65 | 5.07 | 5.2 | 0.0017 | 0.00013 | 0.08 | [71] |
| 4,4′-DDE | 318.9 | 185–186 | 1.65 | 5.07 | 5.2 | 0.0017 | 0.00013 | 0.08 | [71] |
| 2,4′-DDT | 321 | 260–261 | 1.6 | 6.1 | 5.75 | Insoluble | 0.00005 | 0.0015 | [72] |
| 4,4′-DDT | 321 | 260–261 | 1.6 | 6.1 | 5.75 | Insoluble | 0.00005 | 0.0015 | [72] |
| Diazinon | 304.3 | 83–84 | 1.17 | 3.7 | 3.45 | 5.5 | 0.013 | 0.011 | [69] |
| Dichlorvos | 220.5 | 96–98 | 1.44 | 1.82 | 2.08 | 700 | 0.01 | 0.014 | [62] |
| Dieldrin | 380.9 | 385–386 | 1.7 | 4.64 | 4.85 | 0.05 | 0.0029 | 0.0057 | [72] |
| Dimethoate | 229.7 | 86–88 | 1.33 | 1.79 | 1.93 | 2000 | 0.13 | 0.038 | [62] |
| Diuron | 233.22 | Decomposes | 1.31 | 2.47 | 2.66 | 14.8 | 3.8 × 10−10 | 2.7 × 10−8 | [73] |
| Endosulfan | 406.9 | 408–409 | 1.86 | 4.8 | 4.97 | 0.03 | 0.00015 | 0.00038 | [72] |
| Epoxiconazole | 430.8 | 135–143 | 1.25 | 4.26 | 3.99 | 0.23 | 0.0018 | 3.3 × 10−6 | [74] |
| Ethoprophos | 240.3 | 88–90 | 1.34 | 2.63 | 2.68 | 16.2 | 0.04 | 0.023 | [62] |
| Folpet | 240.3 | 155–157 | 1.98 | 0.47 | N/A 3 | 2.2 | N/A 3 | N/A 3 | [74] |
| Glyphosate | 169.07 | Decomposes | 1.7 | −4.5 | −4.1 | 1.7 | 2.1 × 10−9 | N/A 3 | [75] |
| HCB | 284.8 | 288.5 | 1.3 | 4.06 | 4.09 | 0.16 | 0.0048 | 0.0089 | [72] |
| β-HCH | 290.8 | 288–289 | 1.3 | 4.29 | 4.34 | Insoluble | 0.00039 | 0.00068 | [72] |
| γ-HCH | 290.8 | 288–289 | 1.3 | 4.29 | 4.34 | Insoluble | 0.00039 | 0.00068 | [72] |
| α-HCH | 290.8 | 288–289 | 1.3 | 4.29 | 4.34 | Insoluble | 0.00039 | 0.00068 | [72] |
| Heptachlor | 373.4 | 205–208 | 1.5 | 5.21 | 5.28 | Insoluble | 0.0026 | 0.058 | [72] |
| Heptachlor epoxide | 389.8 | 370–380 | 1.8 | 4.87 | 4.94 | Insoluble | 0.00016 | 0.00027 | [72] |
| 4,4′-methoxychlor | 345.9 | 105–110 | 1.4 | 4.18 | 4.32 | Insoluble | 0.0027 | 0.0021 | [72] |
| Malathion | 330.3 | 156–157 | 1.19 | 2.91 | 3.01 | 17.5 | 0.0005 | 0.0013 | [62] |
| Metazachlor | 283.8 | N/A 3 | 1.34–1.36 | 4.03 | N/A 3 | 26–3800 | 0.005–0.2 | 1.5–22 | [76,77] |
| Metribuzin | 214.7 | 298.5 | 1.43–1.46 | 1.79 | 1.47 | 14–15,000 | 0.0069 | 0.52–2.1 | [78] |
| Mirex | 545.5 | 600–610 | 3.1 | 5.5 | 5.89 | Insoluble | 0.00025 | 0.00045 | [72] |
| Oxychlordane | 409.8 | 250 | 1.5 | 5.16 | 5.22 | Insoluble | 0.0002 | 0.00038 | [72] |
| Parathion-methyl | 263.8 | 150–152 | 1.23 | 3.84 | 3.54 | 8.7 | 0.019 | 0.065 | [62] |
| Propiconazole | 342.2 | 195–196 | 1.28 | 4.4 | - | 0.88 | 0.000013 | 1.24 | [79] |
| S-metolachlor | 345.9 | N/A 3 | 1.4 | 4.25 | N/A 3 | 5.5–11,000 | 0.013–0.05 | 0.7–37 | [77] |
| Simazine | 201.7 | 225.6 | 1.32–1.44 | 2.68 | 1.4–2.8 | 35–102,000 | 0.0003–0.06 | 2–53 | [59,60] |
| Tebuconazole | 307.8 | 130–133 | 1.25 | 3.94 | 3.67 | 68.8 | 0.00012 | 1.31 | [80] |
| Terbuthylazine | 285.3 | 135 | 1.28 | 2.68 | 2.32 | 60 | 0.00026 | 1.67 | [81] |
| Trifluralin | 335.84 | 120–123 | 1.4 | 6.24 | 4.9 | 0.2 | 5.5 × 10−7 | N/A 3 | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonupara, T.; Udomkun, P.; Khan, E.; Kajitvichyanukul, P. Airborne Pesticides from Agricultural Practices: A Critical Review of Pathways, Influencing Factors, and Human Health Implications. Toxics 2023, 11, 858. https://doi.org/10.3390/toxics11100858
Boonupara T, Udomkun P, Khan E, Kajitvichyanukul P. Airborne Pesticides from Agricultural Practices: A Critical Review of Pathways, Influencing Factors, and Human Health Implications. Toxics. 2023; 11(10):858. https://doi.org/10.3390/toxics11100858
Chicago/Turabian StyleBoonupara, Thirasant, Patchimaporn Udomkun, Eakalak Khan, and Puangrat Kajitvichyanukul. 2023. "Airborne Pesticides from Agricultural Practices: A Critical Review of Pathways, Influencing Factors, and Human Health Implications" Toxics 11, no. 10: 858. https://doi.org/10.3390/toxics11100858
APA StyleBoonupara, T., Udomkun, P., Khan, E., & Kajitvichyanukul, P. (2023). Airborne Pesticides from Agricultural Practices: A Critical Review of Pathways, Influencing Factors, and Human Health Implications. Toxics, 11(10), 858. https://doi.org/10.3390/toxics11100858









