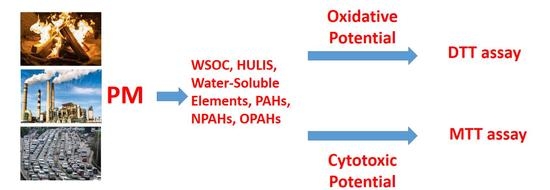
Size-Resolved Redox Activity and Cytotoxicity of Water-Soluble Urban Atmospheric Particulate Matter: Assessing Contributions from Chemical Components
Abstract
1. Introduction
- Τo gain a better understanding of the size distribution of the OP of urban PM.
- Τo investigate its linkage to redox-active PM components.
- To assess the relationship to cytotoxicity responses measured by cellular assays.
2. Materials and Methods
2.1. Site and Sampling Description
2.2. Chemical Analyses
2.2.1. Determination of WSOC, HULIS and Water-Soluble Elements
2.2.2. DTT Assay
2.2.3. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl Tetrazolium Bromide (MTT) Test
2.3. Quality Assurance/Control
2.4. Statistical Analysis
3. Results
3.1. Size Distribution of PM
3.2. Size Distribution of Water-Soluble Organic Carbon (WSOC)
3.3. Size Distribution of Humic-like Substances (HULIS)
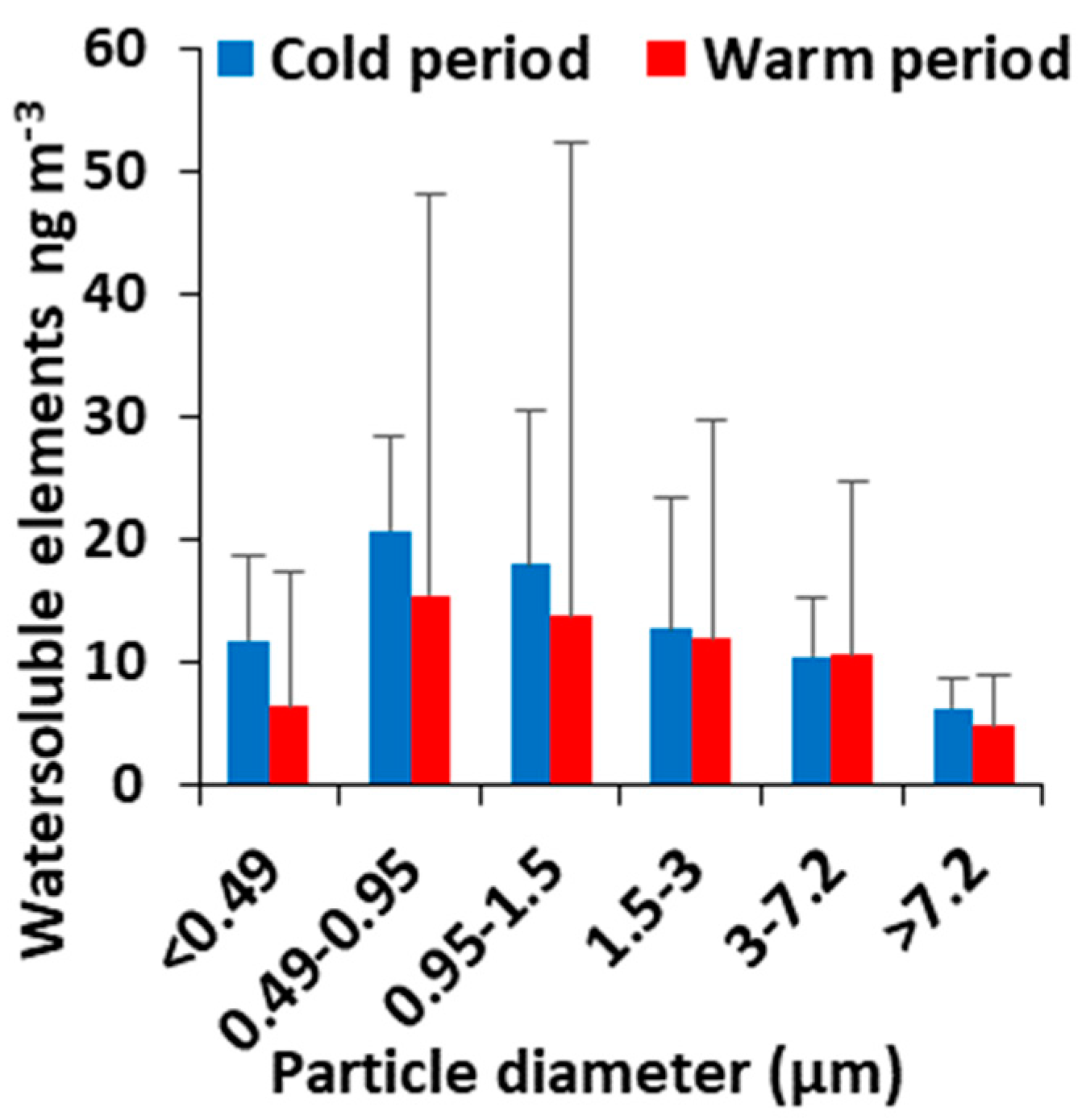
3.4. Size Distribution of Water-Soluble Elements
3.5. Size Distribution of PAHs, OPAHs and NPAHs
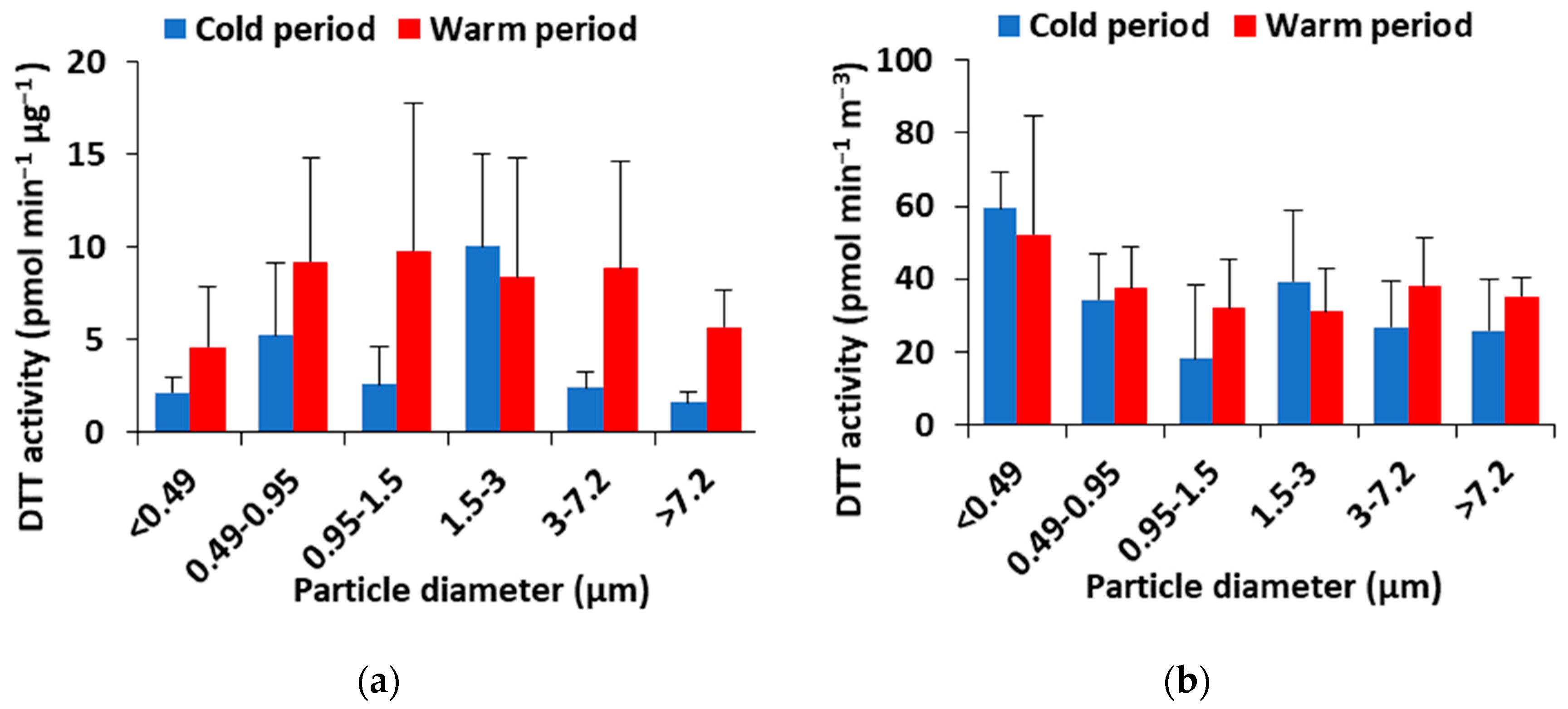
3.6. DTT Redox Activity of Size-Resolved PM
3.7. MTT Cytotoxicity of Size-Resolved PM
3.8. Relationships between OPDTT and Chemical Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lakey, P.S.J.; Berkemeier, T.; Tong, H.; Arangio, A.M.; Lucas, K.; Pöschl, U.; Shiraiwa, M. Chemical Exposure-Response Relationship between Air Pollutants and Reactive Oxygen Species in the Human Respiratory Tract. Sci. Rep. 2016, 6, 32916. [Google Scholar] [CrossRef]
- Nel, A. Air Pollution-Related Illness: Effects of Particles. Science 2005, 308, 804–806. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary Oxidative Stress, Inflammation and Cancer: Respirable Particulate Matter, Fibrous Dusts and Ozone as Major Causes of Lung Carcinogenesis through Reactive Oxygen Species Mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef]
- Janssen, N.A.H.; Strak, M.; Yang, A.; Hellack, B.; Kelly, F.J.; Kuhlbusch, T.A.J.; Harrison, R.M.; Brunekreef, B.; Cassee, F.R.; Steenhof, M.; et al. Associations between Three Specific A-Cellular Measures of the Oxidative Potential of Particulate Matter and Markers of Acute Airway and Nasal Inflammation in Healthy Volunteers. Occup. Environ. Med. 2015, 72, 49–56. [Google Scholar] [CrossRef]
- Cho, A.K.; Sioutas, C.; Miguel, A.H.; Kumagai, Y.; Schmitz, D.A.; Singh, M.; Eiguren-Fernandez, A.; Froines, J.R. Redox Activity of Airborne Particulate Matter at Different Sites in the Los Angeles Basin. Environ. Res. 2005, 99, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Verma, V.; Bates, J.T.; Abrams, J.; Klein, M.; Strickland, M.J.; Sarnat, S.E.; Chang, H.H.; Mulholland, J.A.; Tolbert, P.E.; et al. Oxidative Potential of Ambient Water-Soluble PM2.5 in the Southeastern United States: Contrasts in Sources and Health Associations between Ascorbic Acid (AA) and Dithiothreitol (DTT) Assays. Atmos. Chem. Phys. 2016, 16, 3865–3879. [Google Scholar] [CrossRef]
- Shafer, M.M.; Hemming, J.D.C.; Antkiewicz, D.S.; Schauer, J.J. Oxidative Potential of Size-Fractionated Atmospheric Aerosol in Urban and Rural Sites across Europe. Faraday Discuss. 2016, 189, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Samara, C. On the Redox Activity of Urban Aerosol Particles: Implications for Size Distribution and Relationships with Organic Aerosol Components. Atmosphere 2017, 8, 205. [Google Scholar] [CrossRef]
- Puthussery, J.V.; Zhang, C.; Verma, V. Development and Field Testing of an Online Instrument for Measuring the Real-Time Oxidative Potential of Ambient Particulate Matter Based on Dithiothreitol Assay. Atmos. Meas. Tech. 2018, 11, 5767–5780. [Google Scholar] [CrossRef]
- Pietrogrande, M.C.; Russo, M.; Zagatti, E. Review of PM Oxidative Potential Measured with Acellular Assays in Urban and Rural Sites across Italy. Atmosphere 2019, 10, 626. [Google Scholar] [CrossRef]
- Velali, E.; Papachristou, E.; Pantazaki, A.; Choli-Papadopoulou, T.; Planou, S.; Kouras, A.; Manoli, E.; Besis, A.; Voutsa, D.; Samara, C. Redox Activity and in Vitro Bioactivity of the Water-Soluble Fraction of Urban Particulate Matter in Relation to Particle Size and Chemical Composition. Environ. Pollut. 2016, 208, 774–786. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Guascito, M.R.; Giordano, M.E.; Caricato, R.; De Bartolomeo, A.R.; Romano, M.P.; Conte, M.; Dinoi, A.; Contini, D. Oxidative Potential, Cytotoxicity, and Intracellular Oxidative Stress Generating Capacity of PM10: A Case Study in South of Italy. Atmosphere 2021, 12, 464. [Google Scholar] [CrossRef]
- Jiang, H.; Ahmed, C.M.S.; Canchola, A.; Chen, J.Y.; Lin, Y.-H. Use of Dithiothreitol Assay to Evaluate the Oxidative Potential of Atmospheric Aerosols. Atmosphere 2019, 10, 571. [Google Scholar] [CrossRef]
- Kumagai, Y.; Koide, S.; Taguchi, K.; Endo, A.; Nakai, Y.; Yoshikawa, T.; Shimojo, N. Oxidation of Proximal Protein Sulfhydryls by Phenanthraquinone, a Component of Diesel Exhaust Particles. Chem. Res. Toxicol. 2002, 15, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Charrier, J.G.; Anastasio, C. On Dithiothreitol (DTT) as a Measure of Oxidative Potential for Ambient Particles: Evidence for the Importance of Soluble Transition Metals. Atmos. Chem. Phys. 2012, 12, 11317–11350. [Google Scholar] [CrossRef]
- Verma, V.; Rico-Martinez, R.; Kotra, N.; King, L.; Liu, J.; Snell, T.W.; Weber, R.J. Contribution of Water-Soluble and Insoluble Components and Their Hydrophobic/Hydrophilic Subfractions to the Reactive Oxygen Species-Generating Potential of Fine Ambient Aerosols. Environ. Sci. Technol. 2012, 46, 11384–11392. [Google Scholar] [CrossRef]
- Lin, P.; Yu, J.Z. Generation of Reactive Oxygen Species Mediated by Humic-like Substances in Atmospheric Aerosols. Environ. Sci. Technol. 2011, 45, 10362–10368. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Guo, H.; Cheng, T.; Li, X. Particle Size Distributions of Oxidative Potential of Lung-Deposited Particles: Assessing Contributions from Quinones and Water-Soluble Metals. Environ. Sci. Technol. 2018, 52, 6592–6600. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lu, X.; Li, X.; Liu, Y.; Wang, X.; Chen, H.; Chen, J.; Yang, X.; Fu, T.-M.; Zhao, Q.; et al. ROS-Generation Potential of Humic-like Substances (HULIS) in Ambient PM2.5 in Urban Shanghai: Association with HULIS Concentration and Light Absorbance. Chemosphere 2020, 256, 127050. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Carraway, M.S.; Madden, M.C. Composition of Air Pollution Particles and Oxidative Stress in Cells, Tissues, and Living Systems. J. Toxicol. Environ. Health B Crit. Rev. 2012, 15, 1–21. [Google Scholar] [CrossRef]
- Yu, Y.-X.; Chen, L.; Yang, D.; Pang, Y.-P.; Zhang, S.-H.; Zhang, X.-Y.; Yu, Z.-Q.; Wu, M.-H.; Fu, J.-M. Polycyclic Aromatic Hydrocarbons in Animal-Based Foods from Shanghai: Bioaccessibility and Dietary Exposure. Food Addit. Contam. Part A 2012, 29, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Besis, A.; Gallou, D.; Avgenikou, A.; Serafeim, E.; Samara, C. Size-Dependent in Vitro Inhalation Bioaccessibility of PAHs and O/N PAHs—Implications to Inhalation Risk Assessment. Environ. Pollut. 2022, 301, 119045. [Google Scholar] [CrossRef] [PubMed]
- Latronico, S.; Giordano, M.E.; Urso, E.; Lionetto, M.G.; Schettino, T. Effect of the Flame Retardant Tris (1,3-Dichloro-2-Propyl) Phosphate (TDCPP) on Na(+)-K(+)-ATPase and Cl(-) Transport in HeLa Cells. Toxicol. Mech. Methods 2018, 28, 599–606. [Google Scholar] [CrossRef]
- Voliotis, A.; Prokeš, R.; Lammel, G.; Samara, C. New Insights on Humic-like Substances Associated with Wintertime Urban Aerosols from Central and Southern Europe: Size-Resolved Chemical Characterization and Optical Properties. Atmos. Environ. 2017, 166, 286–299. [Google Scholar] [CrossRef]
- Viana, M.; Maenhaut, W.; Ten Brink, H.M.; Chi, X.; Weijers, E.; Querol, X.; Alastuey, A.; Mikuška, P.; Večeřa, Z. Comparative Analysis of Organic and Elemental Carbon Concentrations in Carbonaceous Aerosols in Three European Cities. Atmos. Environ. 2007, 41, 5972–5983. [Google Scholar] [CrossRef]
- Duarte, R.M.B.O.; Mieiro, C.L.; Penetra, A.; Pio, C.A.; Duarte, A.C. Carbonaceous Materials in Size-Segregated Atmospheric Aerosols from Urban and Coastal-Rural Areas at the Western European Coast. Atmos. Res. 2008, 90, 253–263. [Google Scholar] [CrossRef]
- Fan, X.; Song, J.; Peng, P. Temporal Variations of the Abundance and Optical Properties of Water Soluble Humic-Like Substances (HULIS) in PM2.5 at Guangzhou, China. Atmos. Res. 2016, 172–173, 8–15. [Google Scholar] [CrossRef]
- Voisin, D.; Jaffrezo, J.-L.; Houdier, S.; Barret, M.; Cozic, J.; King, M.D.; France, J.L.; Reay, H.J.; Grannas, A.; Kos, G.; et al. Carbonaceous Species and Humic like Substances (HULIS) in Arctic Snowpack during OASIS Field Campaign in Barrow. J. Geophys. Res. Atmos. 2012, 117, D00R19. [Google Scholar] [CrossRef]
- Dinar, E.; Taraniuk, I.; Graber, E.R.; Katsman, S.; Moise, T.; Anttila, T.; Mentel, T.F.; Rudich, Y. Cloud Condensation Nuclei Properties of Model and Atmospheric HULIS. Atmos. Chem. Phys. 2006, 6, 2465–2482. [Google Scholar] [CrossRef]
- Hoffer, A.; Gelencsér, A.; Guyon, P.; Kiss, G.; Schmid, O.; Frank, G.P.; Artaxo, P.; Andreae, M.O. Optical Properties of Humic-like Substances (HULIS) in Biomass-Burning Aerosols. Atmos. Chem. Phys. 2006, 6, 3563–3570. [Google Scholar] [CrossRef]
- Zheng, G.; He, K.; Duan, F.; Cheng, Y.; Ma, Y. Measurement of Humic-like Substances in Aerosols: A Review. Environ. Pollut. 2013, 181, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Kiss, G.; Varga, B.; Galambos, I.; Ganszky, I. Characterization of Water-Soluble Organic Matter Isolated from Atmospheric Fine Aerosol. J. Geophys. Res. Atmos. 2002, 107, ICC 1-1–ICC 1-8. [Google Scholar] [CrossRef]
- Krivácsy, Z.; Kiss, G.; Ceburnis, D.; Jennings, G.; Maenhaut, W.; Salma, I.; Shooter, D. Study of Water-Soluble Atmospheric Humic Matter in Urban and Marine Environments. Atmos. Res. 2008, 87, 1–12. [Google Scholar] [CrossRef]
- Samburova, V.; Szidat, S.; Hueglin, C.; Fisseha, R.; Baltensperger, U.; Zenobi, R.; Kalberer, M. Seasonal Variation of High-Molecular-Weight Compounds in the Water-Soluble Fraction of Organic Urban Aerosols. J. Geophys. Res. Atmos. 2005, 110, D23210. [Google Scholar] [CrossRef]
- Graber, E.R.; Rudich, Y. Atmospheric HULIS: How Humic-like Are They? A Comprehensive and Critical Review. Atmos. Chem. Phys. 2006, 6, 729–753. [Google Scholar] [CrossRef]
- Lin, P.; Huang, X.-F.; He, L.-Y.; Zhen Yu, J. Abundance and Size Distribution of HULIS in Ambient Aerosols at a Rural Site in South China. J. Aerosol. Sci. 2010, 41, 74–87. [Google Scholar] [CrossRef]
- Salma, I.; Mészáros, T.; Maenhaut, W. Mass Size Distribution of Carbon in Atmospheric Humic-like Substances and Water Soluble Organic Carbon for an Urban Environment. J. Aerosol. Sci. 2013, 56, 53–60. [Google Scholar] [CrossRef]
- Shirmohammadi, F.; Wang, D.; Hasheminassab, S.; Verma, V.; Schauer, J.J.; Shafer, M.M.; Sioutas, C. Oxidative Potential of On-Road Fine Particulate Matter (PM2.5) Measured on Major Freeways of Los Angeles, CA, and a 10-Year Comparison with Earlier Roadside Studies. Atmos. Environ. 2017, 148, 102–114. [Google Scholar] [CrossRef]
- Liu, W.; Xu, Y.; Liu, W.; Liu, Q.; Yu, S.; Liu, Y.; Wang, X.; Tao, S. Oxidative Potential of Ambient PM2.5 in the Coastal Cities of the Bohai Sea, Northern China: Seasonal Variation and Source Apportionment. Environ. Pollut. 2018, 236, 514–528. [Google Scholar] [CrossRef]
- Verma, V.; Pakbin, P.; Cheung, K.L.; Cho, A.K.; Schauer, J.J.; Shafer, M.M.; Kleinman, M.T.; Sioutas, C. Physicochemical and Oxidative Characteristics of Semi-Volatile Components of Quasi-Ultrafine Particles in an Urban Atmosphere. Atmos. Environ. 2011, 45, 1025–1033. [Google Scholar] [CrossRef]
- Saffari, A.; Daher, N.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Seasonal and Spatial Variation in Dithiothreitol (DTT) Activity of Quasi-Ultrafine Particles in the Los Angeles Basin and Its Association with Chemical Species. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2014, 49, 441–451. [Google Scholar] [CrossRef]
- Perrone, M.G.; Zhou, J.; Malandrino, M.; Sangiorgi, G.; Rizzi, C.; Ferrero, L.; Dommen, J.; Bolzacchini, E. PM Chemical Composition and Oxidative Potential of the Soluble Fraction of Particles at Two Sites in the Urban Area of Milan, Northern Italy. Atmos. Environ. 2016, 128, 104–113. [Google Scholar] [CrossRef]
- Charrier, J.G.; Richards-Henderson, N.K.; Bein, K.J.; McFall, A.S.; Wexler, A.S.; Anastasio, C. Oxidant Production from Source-Oriented Particulate Matter—Part 1: Oxidative Potential Using the Dithiothreitol (DTT) Assay. Atmos. Chem. Phys. 2015, 15, 2327–2340. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Bachmann, K.A.; Bailer, A.J.; Bolger, P.M.; Borak, J.; Cai, L.; Cedergreen, N.; Cherian, M.G.; Chiueh, C.C.; Clarkson, T.W.; et al. Biological Stress Response Terminology: Integrating the Concepts of Adaptive Response and Preconditioning Stress within a Hormetic Dose–Response Framework. Toxicol. Appl. Pharmacol. 2007, 222, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Sthijns, M.M.J.P.E.; Thongkam, W.; Albrecht, C.; Hellack, B.; Bast, A.; Haenen, G.R.M.M.; Schins, R.P.F. Silver Nanoparticles Induce Hormesis in A549 Human Epithelial Cells. Toxicol. In Vitro 2017, 40, 223–233. [Google Scholar] [CrossRef]
- Argyropoulos, G.; Besis, A.; Voutsa, D.; Samara, C.; Sowlat, M.H.; Hasheminassab, S.; Sioutas, C. Source Apportionment of the Redox Activity of Urban Quasi-Ultrafine Particles (PM0.49) in Thessaloniki Following the Increased Biomass Burning Due to the Economic Crisis in Greece. Sci. Total Environ. 2016, 568, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.T.; Weber, R.J.; Abrams, J.; Verma, V.; Fang, T.; Klein, M.; Strickland, M.J.; Sarnat, S.E.; Chang, H.H.; Mulholland, J.A.; et al. Reactive Oxygen Species Generation Linked to Sources of Atmospheric Particulate Matter and Cardiorespiratory Effects. Environ. Sci. Technol. 2015, 49, 13605–13612. [Google Scholar] [CrossRef]
- Vinson, A.; Sidwell, A.; Black, O.; Roper, C. Seasonal Variation in the Chemical Composition and Oxidative Potential of PM2.5. Atmosphere 2020, 11, 1086. [Google Scholar] [CrossRef]
- Visentin, M.; Pagnoni, A.; Sarti, E.; Pietrogrande, M.C. Urban PM(2.5) Oxidative Potential: Importance of Chemical Species and Comparison of Two Spectrophotometric Cell-Free Assays. Environ. Pollut. 2016, 219, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Misra, C.; Sempf, J.; Wang, M.; Oberley, T.; Froines, J.; Nel, A. Ultrafine Particulate Pollutants Induce Oxidative Stress and Mitochondrial Damage. Environ. Health Perspect. 2003, 111, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, M.; Bramble, L.A.; Schmitz, D.A.; Schauer, J.J.; Sioutas, C.; Harkema, J.R.; Nel, A.E. The Adjuvant Effect of Ambient Particulate Matter Is Closely Reflected by the Particulate Oxidant Potential. Environ. Health Perspect. 2009, 117, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Øvrevik, J. Oxidative Potential Versus Biological Effects: A Review on the Relevance of Cell-Free/Abiotic Assays as Predictors of Toxicity from Airborne Particulate Matter. Int. J. Mol. Sci. 2019, 20, 4772. [Google Scholar] [CrossRef] [PubMed]
- Steenhof, M.; Gosens, I.; Strak, M.; Godri, K.J.; Hoek, G.; Cassee, F.R.; Mudway, I.S.; Kelly, F.J.; Harrison, R.M.; Lebret, E.; et al. In Vitro Toxicity of Particulate Matter (PM) Collected at Different Sites in the Netherlands Is Associated with PM Composition, Size Fraction and Oxidative Potential—The RAPTES Project. Part. Fibre Toxicol. 2011, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Calas, A.; Uzu, G.; Kelly, F.J.; Houdier, S.; Martins, J.M.F.; Thomas, F.; Molton, F.; Charron, A.; Dunster, C.; Oliete, A.; et al. Comparison between Five Acellular Oxidative Potential Measurement Assays Performed with Detailed Chemistry on PM10 Samples from the City of Chamonix (France). Atmos. Chem. Phys. 2018, 18, 7863–7875. [Google Scholar] [CrossRef]

| Particle Diameter (μm) | PM (μg m−3) | WSOC (μg m−3) | HULIS-C (μg m−3) | HULIS (μg m−3) | ||||
|---|---|---|---|---|---|---|---|---|
| Cold Period | Warm Period | Cold Period | Warm Period | Cold Period | Warm Period | Cold Period | Warm Period | |
| <0.49 | 31.8 ± 15 | 11.8 ± 3 | 3.51 ± 0.41 | 1.48 ± 0.42 | 0.73 ± 0.23 | 0.24 ± 0.07 | 1.39 ± 0.43 | 0.47 ± 0.13 |
| 0.49–0.95 | 8.7 ± 4.3 | 5.1 ± 3.2 | 0.81 ± 0.14 | 0.5 ± 0.16 | 0.13 ± 0.02 | 0.09 ± 0.04 | 0.25 ± 0.03 | 0.17 ± 0.08 |
| 0.95–1.5 | 5.9 ± 3.4 | 5 ± 3.4 | 0.34 ± 0.07 | 0.34 ± 0.13 | 0.06 ± 0.02 | 0.05 ± 0.02 | 0.11 ± 0.03 | 0.1 ± 0.04 |
| 1.5–3 | 4.6 ± 2.4 | 4.9 ± 2.4 | 0.28 ± 0.09 | 0.31 ± 0.07 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.1 ± 0.03 | 0.1 ± 0.02 |
| 3–7.2 | 11.1 ± 4.2 | 6.1 ± 3.9 | 0.46 ± 0.15 | 0.32 ± 0.09 | 0.11 ± 0.03 | 0.08 ± 0.02 | 0.21 ± 0.05 | 0.16 ± 0.04 |
| >7.2 | 15.6 ± 3.8 | 6.8 ± 2.6 | 0.8 ± 0.3 | 0.29 ± 0.1 | 0.23 ± 0.06 | 0.08 ± 0.02 | 0.43 ± 0.12 | 0.15 ± 0.05 |
| Total | 77.7 ± 33.5 | 39.5 ± 18.5 | 6.2 ± 0.52 | 3.23 ± 0.76 | 1.31 ± 0.25 | 0.61 ± 0.15 | 2.5 ± 0.48 | 1.15 ± 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besis, A.; Romano, M.P.; Serafeim, E.; Avgenikou, A.; Kouras, A.; Lionetto, M.G.; Guascito, M.R.; De Bartolomeo, A.R.; Giordano, M.E.; Mangone, A.; et al. Size-Resolved Redox Activity and Cytotoxicity of Water-Soluble Urban Atmospheric Particulate Matter: Assessing Contributions from Chemical Components. Toxics 2023, 11, 59. https://doi.org/10.3390/toxics11010059
Besis A, Romano MP, Serafeim E, Avgenikou A, Kouras A, Lionetto MG, Guascito MR, De Bartolomeo AR, Giordano ME, Mangone A, et al. Size-Resolved Redox Activity and Cytotoxicity of Water-Soluble Urban Atmospheric Particulate Matter: Assessing Contributions from Chemical Components. Toxics. 2023; 11(1):59. https://doi.org/10.3390/toxics11010059
Chicago/Turabian StyleBesis, Athanasios, Maria Pia Romano, Eleni Serafeim, Anna Avgenikou, Athanasios Kouras, Maria Giulia Lionetto, Maria Rachele Guascito, Anna Rita De Bartolomeo, Maria Elena Giordano, Annarosa Mangone, and et al. 2023. "Size-Resolved Redox Activity and Cytotoxicity of Water-Soluble Urban Atmospheric Particulate Matter: Assessing Contributions from Chemical Components" Toxics 11, no. 1: 59. https://doi.org/10.3390/toxics11010059
APA StyleBesis, A., Romano, M. P., Serafeim, E., Avgenikou, A., Kouras, A., Lionetto, M. G., Guascito, M. R., De Bartolomeo, A. R., Giordano, M. E., Mangone, A., Contini, D., & Samara, C. (2023). Size-Resolved Redox Activity and Cytotoxicity of Water-Soluble Urban Atmospheric Particulate Matter: Assessing Contributions from Chemical Components. Toxics, 11(1), 59. https://doi.org/10.3390/toxics11010059