Chemical Gas Telemetry System Based on Multispectral Infrared Imaging
Abstract
1. Introduction
2. Experimental Equipment and Methods
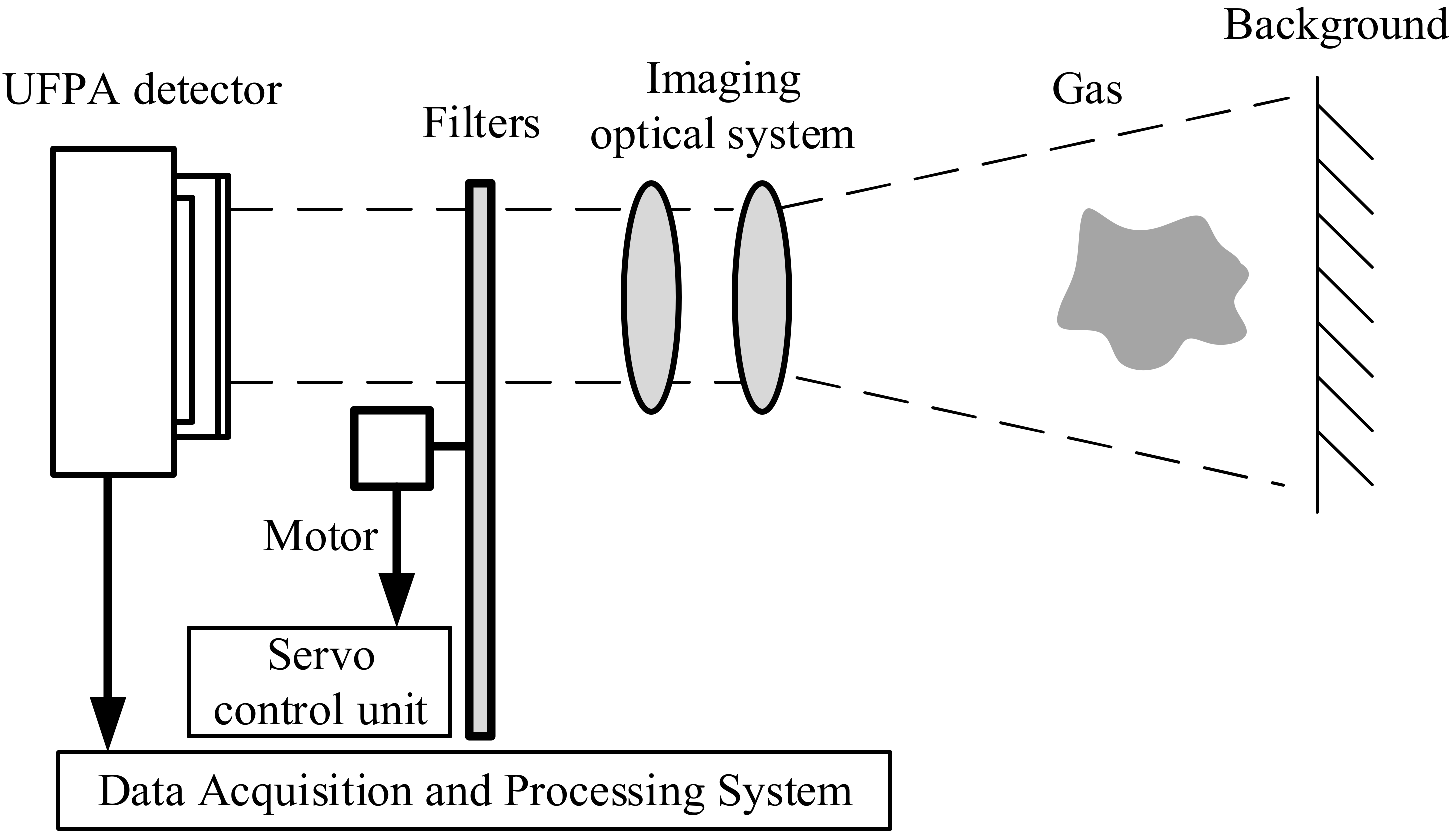
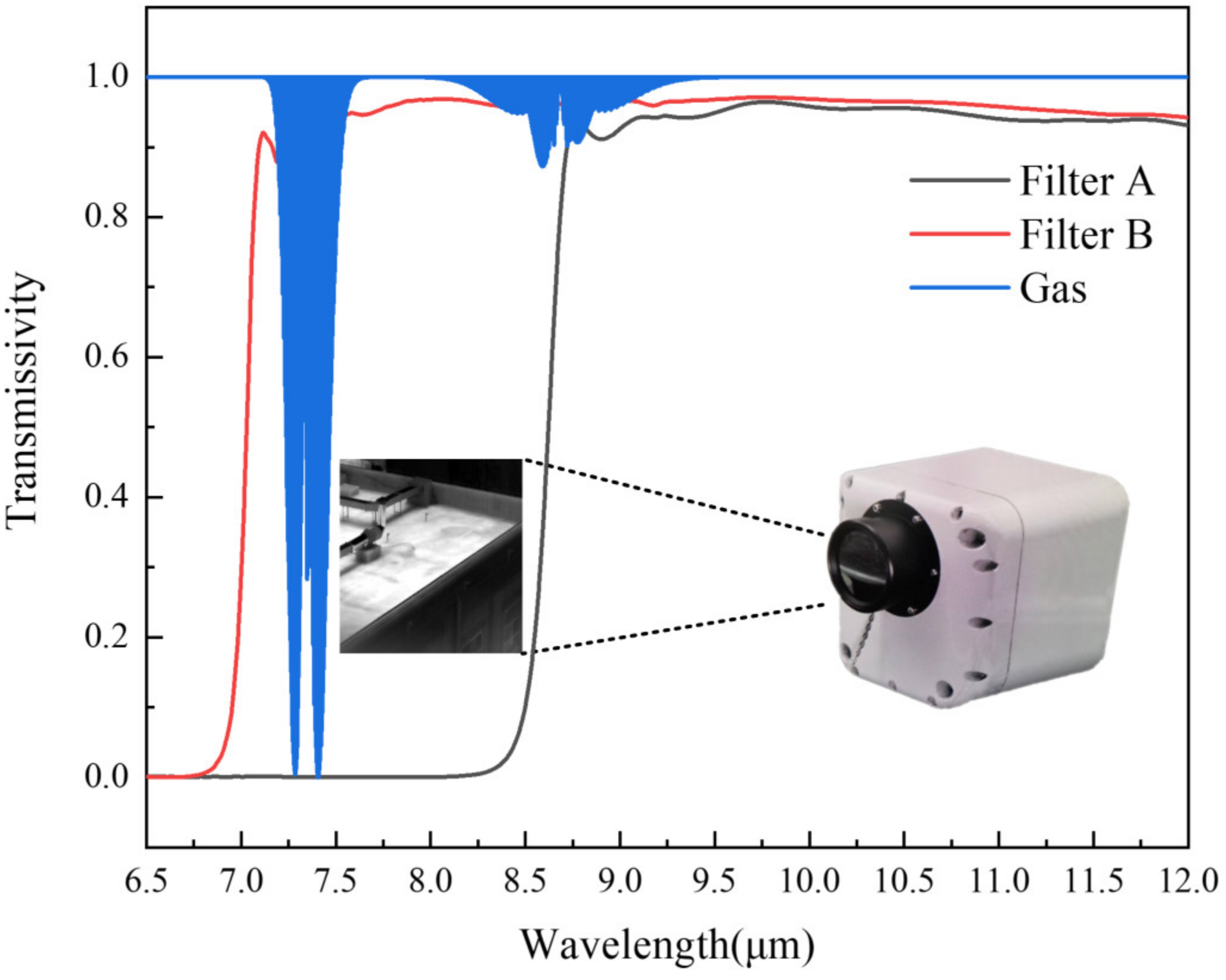
2.1. Camera and Detection Principle
2.2. Gas Detection Setups
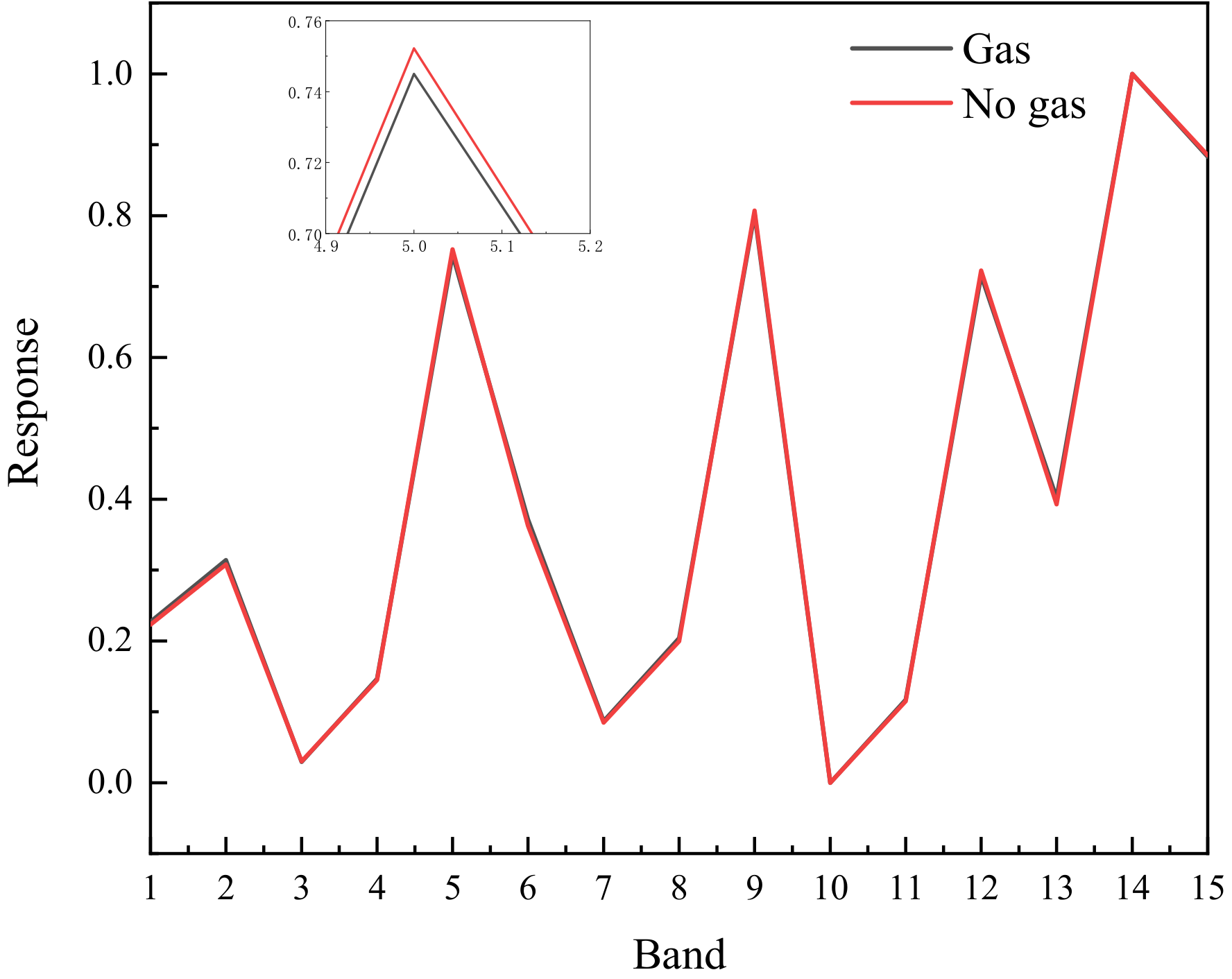
2.3. Data Processing
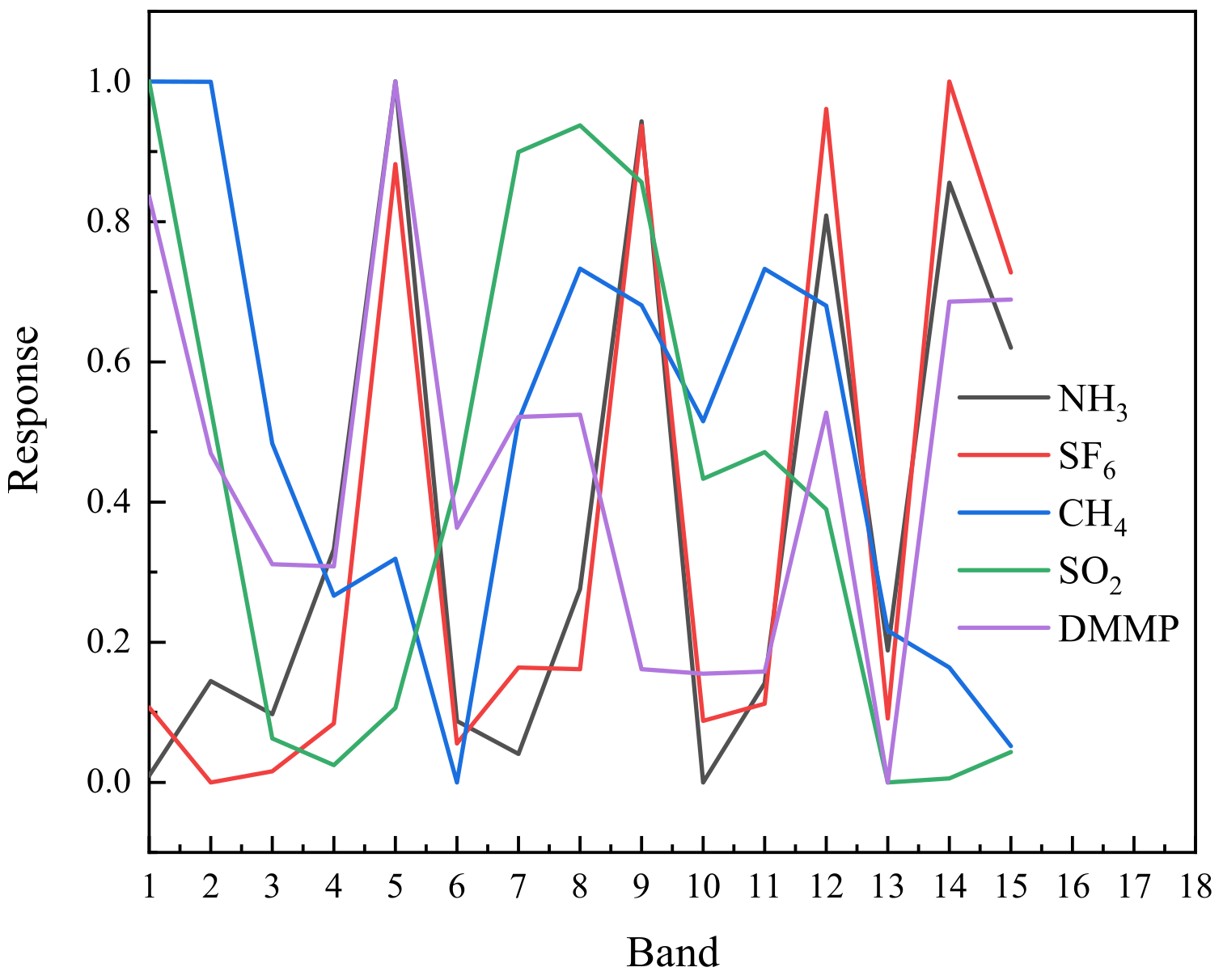
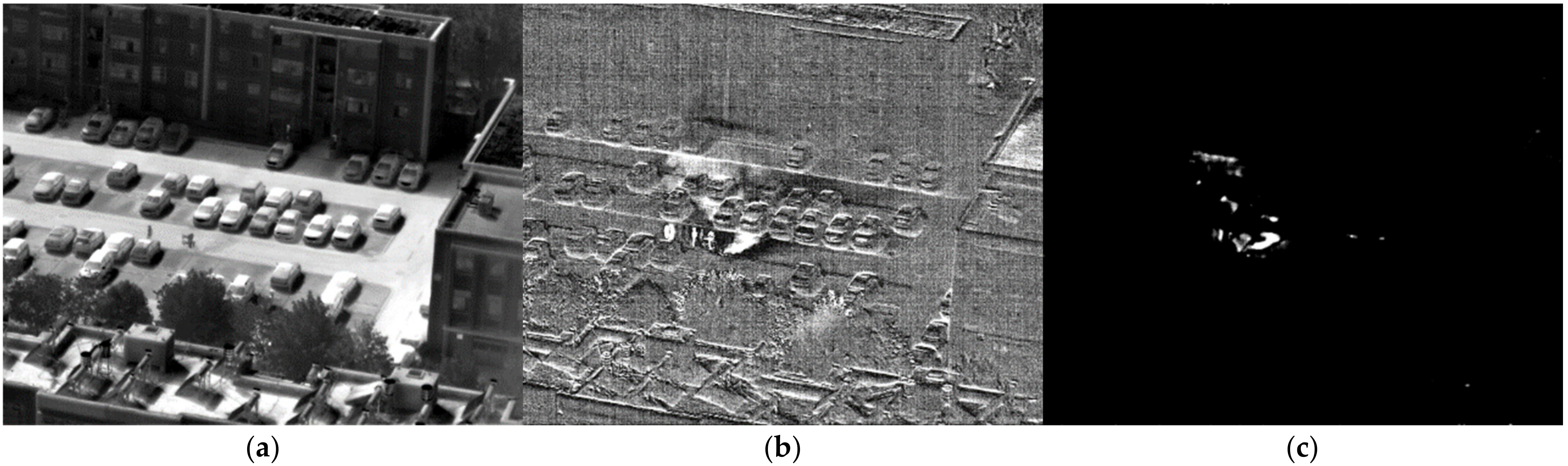
3. Results and Discussion
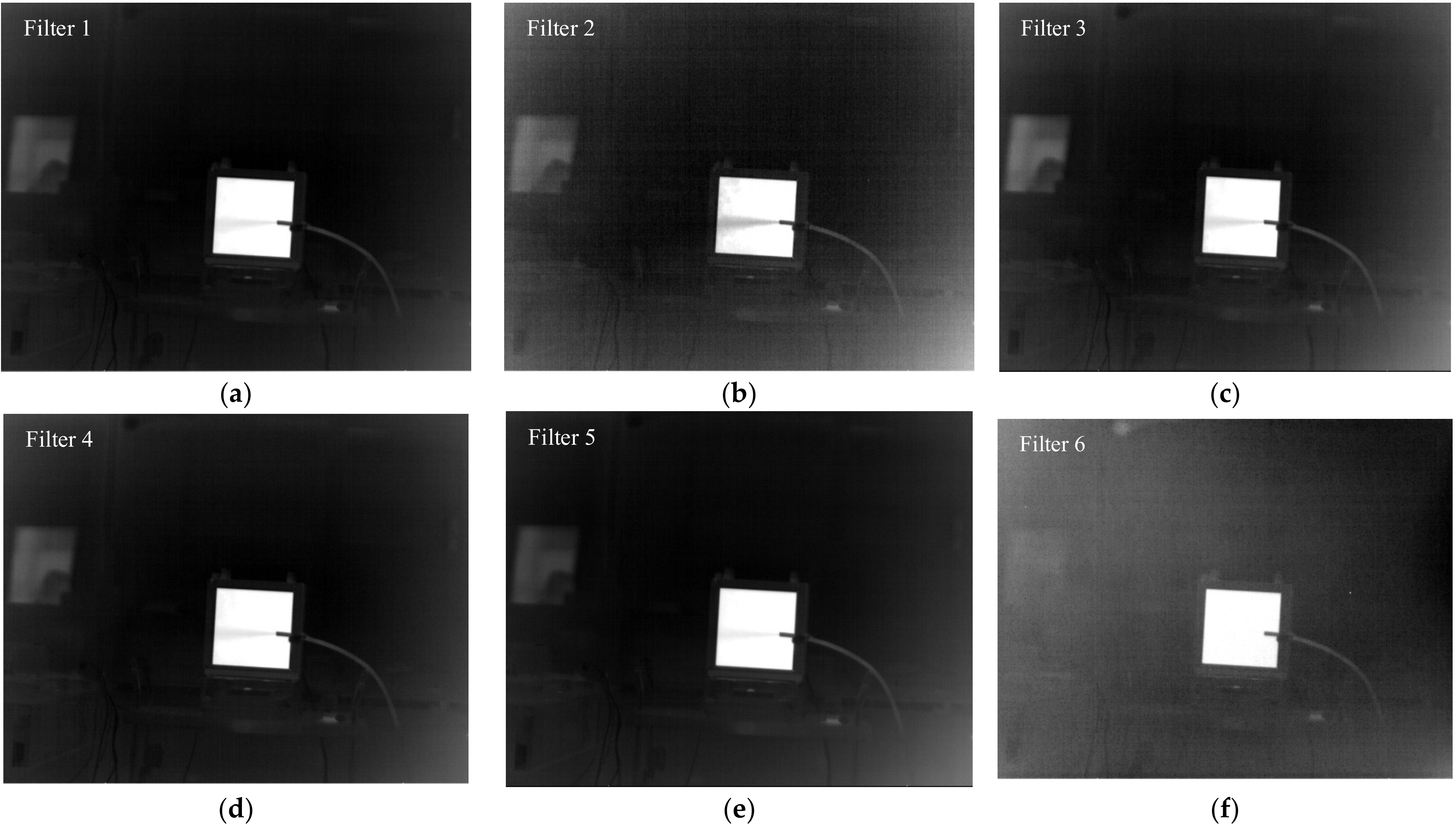
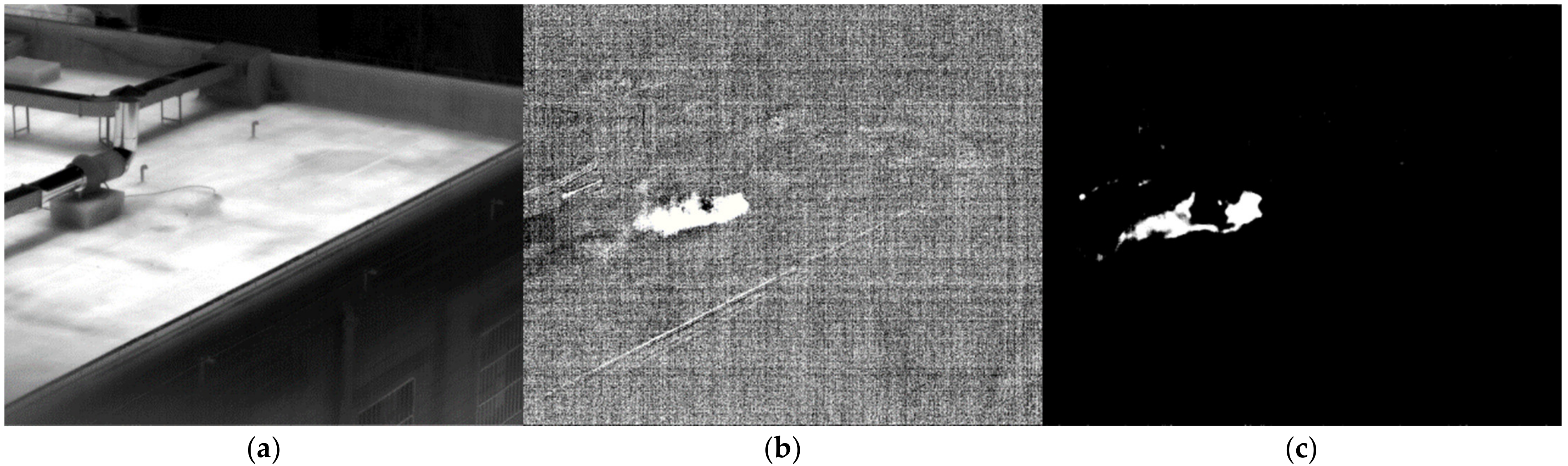
3.1. Validation of the Method in Laboratory Conditions
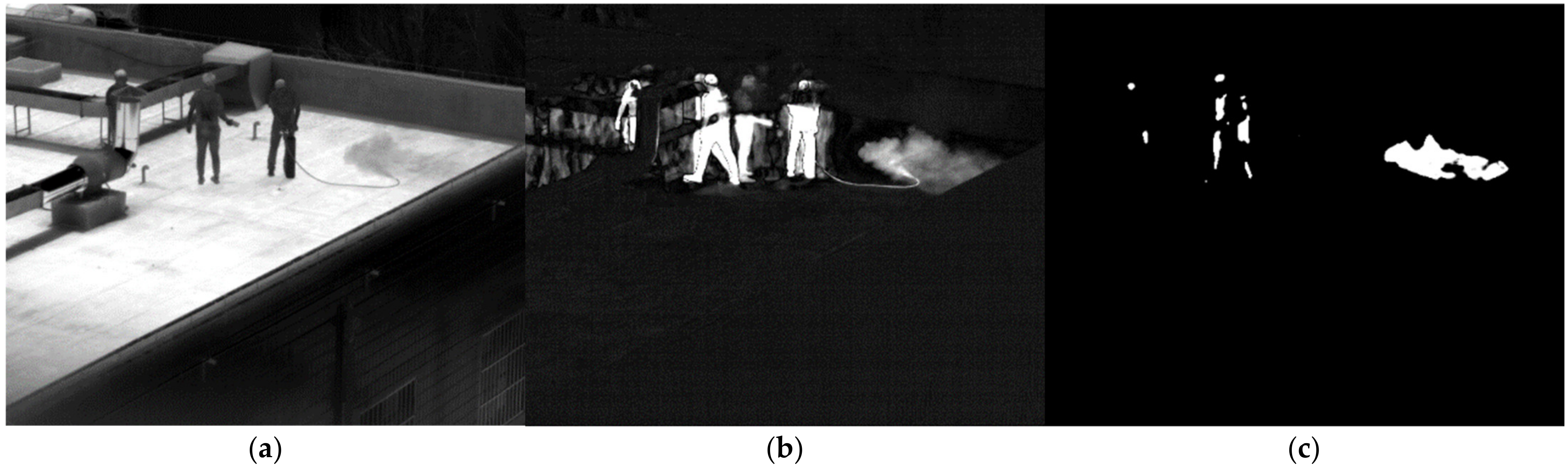
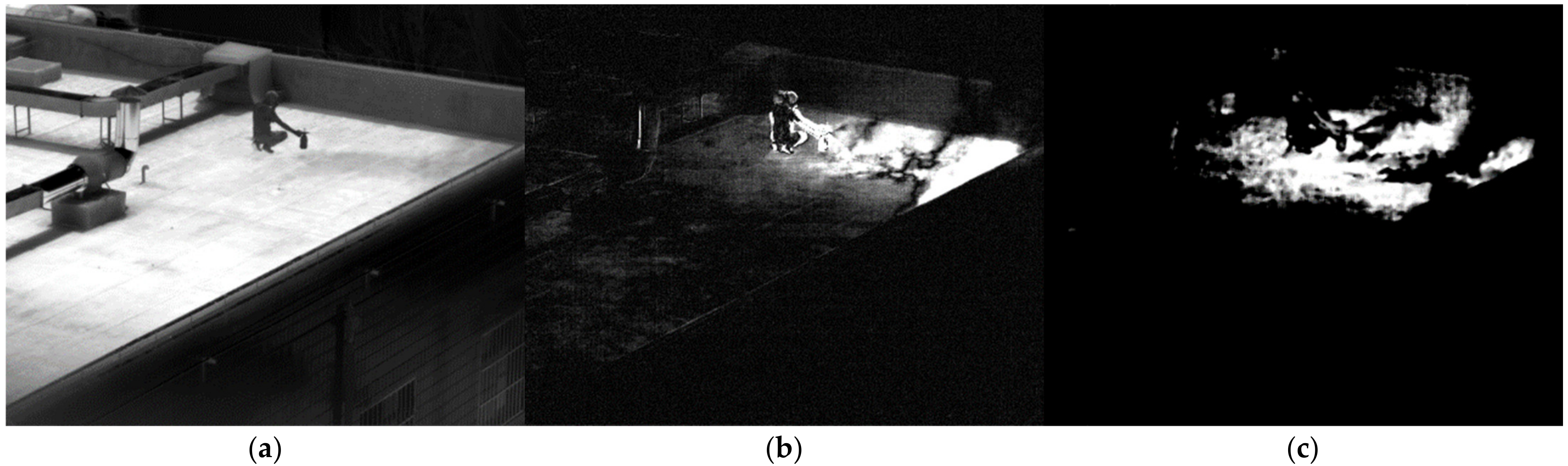
3.2. Validation in Real-World Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sandsten, J.; Weibring, P.; Edner, H.; Svanberg, S. Real-time gas-correlation imaging employing thermal background radiation. Opt. Express 2000, 6, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Hagen, N.; Kudenov, M.W. Review of snapshot spectral imaging technologies. Opt. Eng. 2013, 52, 090901. [Google Scholar] [CrossRef]
- Sandsten, J.; Edner, H.; Svanberg, S. Gas visualization of industrial hydrocarbon emissions. Opt. Express 2004, 12, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, B.; Yuan, M.; Yang, Z.; Yu, C.; Zheng, W. CO detection system based on TDLAS using a 4.625 μm interband cascaded laser. Int. J. Environ. Res. Public Health 2022, 19, 12828. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, D.; Pierre-Yves, F.; Stéphanie, D.; Xavier, W.; Sophie, J.; Emmanuel, V.; Hadrien, P. Test of SIMAGAZ: A LWIR cryogenic multispectral infrared camera for methane gas leak detection and quantification. In Proceedings of the SPIE 11727, Online Only, 12 April 2021. [Google Scholar]
- Emily, E.B.; David, W.M. Pollutant monitoring of aircraft exhaust with multispectral imaging. In Proceedings of the SPIE 10008, Edinburgh, UK, 26 October 2016. [Google Scholar]
- Wang, M.; Hong, H.; Huang, L. Infrared video based gas leak detection method using modified FAST features. In Proceedings of the SPIE 10611, Xiangyang, China, 8 March 2018. [Google Scholar]
- Christophe, C.; Sylvie, B.; Marcel, C.; Roland, D.; Yann, F.; Rémi, G.; Didier, H.; Marc, J.; Alain, K.; Philippe, P.; et al. SIELETERS, an airborne infrared dual-band spectro-imaging system for measurement of scene spectral signatures. Opt. Express 2015, 23, 16164–16176. [Google Scholar]
- Sylvain, F.; Aymeric, A.; Manon, V.; Romain, V.; Sébastien, B. Detecting unknown chemical clouds at distance with multispectral imagery. In Proceedings of the SPIE 10183, Anaheim, CA, USA, 3 May 2017. [Google Scholar]
- Fanny, L.; Elodie, C.; Philippe, H.; Thomas, F.; Karen, R.; Stéphanie, G. Design and performances of micro cameras and photometers instruments on TARANIS satellite for an advanced characterization of Transient Luminous Event in the upper atmosphere. In Proceedings of the SPIE 9640, Toulouse, France, 16 October 2015. [Google Scholar]
- Jacob, L.H.; Brent, A.R.; David, L.B.; Jay, P.G.; Kevin, C.G. Imaging fourier-transform spectrometer measurements of a turbulent nonpremixed jet flame. Opt. Lett. 2014, 39, 2350–2353. [Google Scholar]
- Tadashi, I.; Jumpei, M.; Akihiko, K.; Hiroshi, S.; Ryota, S. Ground-based demonstration of imaging SWIR-FTS for space-based detection of air pollution and greenhouse gases. In Proceedings of the Imaging and Applied Optics, Arlington, VA, USA, 23–27 June 2013. [Google Scholar]
- Zheng, W.J.; Yang, Z.X.; Huang, S.J.; Wang, C.Y.; Liao, N.F.; Fang, Y.H.; Su, J.H. Two Dimensional Spatial and Temporal Hybrid Modulation LWIR Hyperspectral Imaging for Remote Gas Detection. Spectrosc. Spect. Anal. 2016, 36, 67–68. [Google Scholar]
- Su, L.; Yan, Y.; Xiangli, B.; Huang, F.; Zhou, S. Spectrum reconstruction method for airborne temporally–spatially modulated fourier transform imaging spectrometers. IEEE Trans. Geosci. Remote Sens. 2014, 52, 3720–3728. [Google Scholar] [CrossRef]
- Zhao, B.; Lv, J.; Ren, J.; Qin, Y.; Wang, W. Data processing and performance evaluation of a tempo-spatially mixed modulation imaging fourier transform spectrometer based on stepped micro-mirror. Opt. Express 2020, 28, 6320–6335. [Google Scholar] [CrossRef] [PubMed]
- Philippe, F.B.; Audrey, E.; Franck, F.; Jean-Baptiste, H. Stand-off CWA imaging system: Second sight MS. In Proceedings of the SPIE 8358, Baltimore, MD, USA, 4 May 2012. [Google Scholar]
- Gagnon, M.A.; Jahjah, K.A.; Marcotte, F.; Tremblay, P.; Farley, V.; Chamberland, M. Time-resolved thermal infrared multispectral imaging of gases and minerals. In Proceedings of the SPIE 9263, Beijing, China, 18 November 2014. [Google Scholar]
- Jin., W.; Li, J.; Dun, X.; Jin., M.; Wang, X. Wide-band gas leak imaging detection system using UFPA. In Proceedings of the SPIE 9301, Beijing, China, 24 November 2014. [Google Scholar]
- Naranjo, E.; Baliga, S.; Bernascolle, P. IR gas imaging in an industrial setting. In Proceedings of the SPIE 7661, Orlando, FL, USA, 3 May 2010. [Google Scholar]
- Zeng, X.; Huang, L. Gas leak detection in infrared video with background modeling. In Proceedings of the SPIE 10611, Xiangyang, China, 8 March 2018. [Google Scholar]







| Number | Operating Wavelength |
|---|---|
| 1 | 6.5 μm~12.5 μm |
| 2 | 10 μm~15 μm |
| 3 | 8.5 μm~15 μm |
| 4 | 7 μm~15 μm |
| 5 | 6.5 μm~15 μm |
| 6 | 6.5 μm~9 μm |
| Number | Sequence of Image Subtraction | Detection Range |
|---|---|---|
| 1 | 1–2 | 6.5 μm~10 μm; 12.5 μm~15 μm |
| 2 | 1–3 | 6.5 μm~8.5 μm; 12.5 μm~15 μm |
| 3 | 1–4 | 6.5 μm~7 μm; 12.5 μm~15 μm |
| 4 | 1–5 | 12.5 μm~15 μm |
| 5 | 1–6 | 9 μm~12.5 μm |
| 6 | 2–3 | 8.5 μm~10 μm |
| 7 | 2–4 | 7 μm~10 μm |
| 8 | 2–5 | 6.5 μm~10 μm |
| 9 | 2–6 | 6.5 μm~9 μm; 10 μm~15 μm |
| 10 | 3–4 | 7 μm~8.5 μm |
| 11 | 3–5 | 6.5 μm~8.5 μm |
| 12 | 3–6 | 6.5 μm~8.5 μm; 9 μm~15 μm |
| 13 | 4–5 | 6.5 μm~7 μm |
| 14 | 4–6 | 6.5 μm~7 μm; 9 μm~15 μm |
| 15 | 5–6 | 9 μm~15 μm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Duan, S.; Pang, L.; Li, W.; Yang, Z.; Hu, Y.; Yu, C. Chemical Gas Telemetry System Based on Multispectral Infrared Imaging. Toxics 2023, 11, 83. https://doi.org/10.3390/toxics11010083
Li K, Duan S, Pang L, Li W, Yang Z, Hu Y, Yu C. Chemical Gas Telemetry System Based on Multispectral Infrared Imaging. Toxics. 2023; 11(1):83. https://doi.org/10.3390/toxics11010083
Chicago/Turabian StyleLi, Kun, Shaoli Duan, Lingling Pang, Weilai Li, Zhixiong Yang, Yaohang Hu, and Chunchao Yu. 2023. "Chemical Gas Telemetry System Based on Multispectral Infrared Imaging" Toxics 11, no. 1: 83. https://doi.org/10.3390/toxics11010083
APA StyleLi, K., Duan, S., Pang, L., Li, W., Yang, Z., Hu, Y., & Yu, C. (2023). Chemical Gas Telemetry System Based on Multispectral Infrared Imaging. Toxics, 11(1), 83. https://doi.org/10.3390/toxics11010083






