Abstract
Endocrine disrupting chemicals (EDCs) are exogenous substances that alter the endocrine function of an organism, to result in adverse effects on growth and development, metabolism, and reproductive function. The kidney is one of the most important organs in the urinary system and an accumulation point. Studies have shown that EDCs can cause proteinuria, affect glomeruli and renal tubules, and even lead to diabetes and renal fibrosis in animal and human studies. In this review, we discuss renal accumulation of select EDCs such as dioxins, per- and polyfluoroalkyl substances (PFAS), bisphenol A (BPA), and phthalates, and delineate how exposures to such EDCs cause renal lesions and diseases, including cancer. The regulation of typical EDCs with specific target genes and the activation of related pathways are summarized.
1. Introduction
Endocrine disrupting chemicals (EDCs) are a class of hormone-like chemicals that exist in the environment and interfere with the production, transport, metabolism, regulation, degradation, and/or action of hormones. The ability of EDCs to interfere with endogenous hormones can lead to adverse effects on development, reproduction, immune, endocrine, and nervous systems of the organism. The cascade of events can also result in endocrine and metabolic imbalances in offspring as well [1,2]. EDCs are present in wastewater, textiles, cosmetics, waste residues produced by industrial and agricultural processes and in several domestic and household goods [3,4,5], and ultimately end up in landfills. Common contaminants include per- and polyfluoroalkyl substances (PFAS), polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), dioxins, bisphenol A (BPA), and phthalates, as well as heavy metals such as Cd, Pb, Hg [6]. Upon entry into the human system, EDCs often indirectly interact to cause endocrine imbalance in the organism by affecting the formation, secretion, transport, and metabolism of hormones in the organism, and thus, affecting growth, development, and reproduction.
Due to the environmental persistence of EDCs and their mobility in water bodies, these chemicals can migrate for a long distance along with water bodies [7,8,9]. At present, EDCs such as PFAS, BPA, PCBs and PAHs have been found in main water bodies worldwide [10,11]. Due to the limitations in existing wastewater treatment technologies, EDCs from livestock and poultry as well as domestic and industrial wastewater enter natural water bodies with wastewater discharge and continue to migrate and transform. EDCs in the environment can also be enriched through the food chain and accumulate in organisms at all trophic levels. The higher the trophic level, the higher the accumulation, eventually leading to toxicity [12,13,14]. There are several standard methods for extracting and estimating the concentration of EDCs in biological and environmental samples, including solid-phase extraction (SPE) [15], liquid chromatography-mass spectrometry (LC-MS) [16], gas chromatography-mass spectrometry (GC-MS) [17,18] and high-performance liquid chromatography (HPLC) [15,19]. It is important to use appropriate sample extraction and estimation methods for accurate and reliable detection of the levels of EDCs in different types of samples. With extensive advances in the chemical industry and the ubiquitous presence of plastics, EDCs entering the environment are on the rise both in terms of quantity and variety (new materials), and consequently, their hazards to humans and animals have attracted increased attention by societal and regulatory agencies (Figure 1).
Figure 1.
Common sources of EDCs.
The kidney is one of the main target organs of EDCs for accumulation. Patients with kidney disease usually show decreased renal function and proteinuria, which affects recovery from the disease [20]. Common renal diseases include chronic nephritis, renal calculi, renal failure, and renal cysts. These diseases are usually associated with the glomerular filtration rate (GFR) of the kidney, pathological damage, and abnormal blood or urine composition [21,22].
EDCs have attracted considerable public, regulatory, and scientific attention given their impact on kidney diseases and kidney cancer. Despite past efforts, less is known on mechanisms of action of the different EDCs on kidney function. In this review, we focus on the toxicity of non-metallic and non-agricultural EDCs that are used as additives (plasticizers phthalates and bisphenol A) and industrial chemicals (polychlorinated biphenyls, dioxins, PFAS, flame retardants) in triggering major renal diseases including cancer associated with exposures (Table 1).

Table 1.
Typical EDCs and kidney diseases.
2. Dioxin
Dioxins were identified as toxic compounds in the 1960s. Dioxins are a group of structurally related chemicals composed of two coplanar benzene rings (Figure 2a). These compounds induce a similar spectrum of toxic phenotypes, with a wide range of potency. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is one of the most toxic compounds in this group of chemicals [23]. Incineration is the largest source of dioxin emissions in the environment [24,25]. Dioxins enter the ambient air through chimneys, spread continuously, and accumulate in the surrounding area of the incineration plant. Routes of exposure to dioxins include inhalation, dust ingestion, and skin contact. Dioxins accumulate in the tissues of various animals [26,27,28,29,30,31] and cause chloracne, embryotoxicity [32,33] and nephrotoxicity [34].
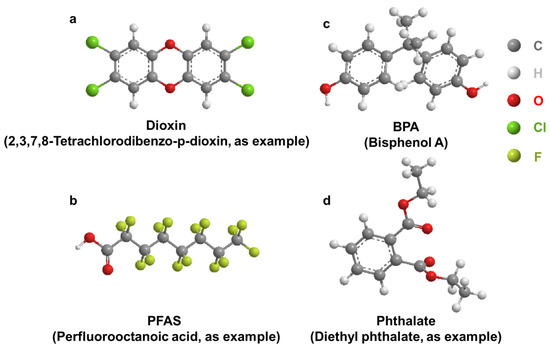
Figure 2.
General structure of select EDCs. (a): Dioxin; (b): BPA; (c): PFAS; (d): Phthalate.
2.1. Accumulation of Dioxins in the Kidney
Animal studies: Several studies, but not all, suggest that TCDD may accumulate in the kidney to impart toxicity. Examination of aquatic organisms by analyzing ten-year-old fish in heavily polluted lakes in China showed that a large amount of dioxin accumulated in the kidneys [35]. Numerous studies have indicated that the accumulation of TCDD can cause the ballooning degeneration or even necrosis of the renal tubules of zebrafish [36]. Polybrominated diphenyl ethers (PBDEs), which have similar structures to PCB, have also been associated with renal histopathological changes [37] and significantly reduced catalase activity [38]. Exposure of infant mice to PCB caused hyperuricemia in adults, leading to secondary nephrotoxicity such as renal hypertrophy and fibrosis [39]. After 12 days of exposure, the combined exposure to TCDD and PCB was more likely to induce nephrotoxicity through high expression of CYP1A1 (Cytochrome P450 Family 1 Subfamily A Member 1), compared with the control group (equivalent volume of olive oil). TCDD and PCB exposure also significantly increased serum creatinine and blood urea nitrogen levels, renal oxidative stress and histopathological changes compared to control in rats [40]. Further studies will shed light on TCDD and PCDD mediated carcinogenesis [41].
Human studies: Shalat et al. [42] reported that three young male utility workers developed kidney cancer after chronic exposure to PCB-containing transformers. Residents from an e-waste dismantling area showed increased accumulations of PCB, which could have contributed to abnormal changes in markers of kidney injury [43]. The screening of environmental chemicals in the soil of an e-waste recycling area and human cancer risk assessment calculations showed that dioxins have the highest potential cancer risk to residents, followed by PCBs [44]. Through a multiple linear regression model analysis of 150 pregnant women, it was found that exposure to environmental pollutants may have negative effects, while exposure to greenspace may have positive effects on fetal renal function during pregnancy [45]. Epidemiological evidence indicated that the development of diabetes and chronic kidney disease was also associated with long-term exposure and accumulation of dioxins [46,47,48]. Jain [48] analyzed data from US adults from 1999 to 2004 to investigate concentration changes of four dioxin homologs and four separate furan homologs at various stages of renal function decline and found that renal dysfunction was associated with high dioxin/furan concentrations.
2.2. Effect of Dioxin on AHR Regulation/Activity and RCC
The AHR is a ligand activated transcription factor that mediates the toxic effects of TCDD. The AHR is mostly expressed in the nucleus of advanced clear cell renal cell carcinoma (RCC) and tumor infiltrating lymphocytes, and its expression is related to the stage and histological grade of pathological tumors [49]. Numerous studies have shown a complex association between the AHR and cancer characteristics, including increased malignant cell invasion, migration, metastasis, and survival [50,51,52]. The primary structure of the AHR is considered to be critical to determining the sensitivity and specificity of animal responses to dioxins.
Animal studies: In a constructed adenine diet model of chronic kidney disease, female AHR knockout mice showed inflammatory and pro-fibrotic gene expression and acute tubular injury [53]. After exposure to TCDD, 9 renal function was significantly reduced in wild-type male mice, indicating that the AHR plays a major role in mouse kidney development [54]. Due to poor renal excretion in patients with chronic kidney disease, the accumulation of toxic substances was found to increase CYP1A1 expression. Because of the higher inducibility of polymorphic genotypes, the pathway may become more deleterious in individuals with homozygous mutant alleles [55].
TCDD-induced fetal hydronephrosis (TiFH) is a type of obstructive hydronephrosis characterized by the presence of dilated ureter or ureteral effusion. The relationship between TiFH and AHR was investigated in both rats and mice. In mice, Cox-2 (cyclooxygenase-2) plays a key role in TiFH [56,57]. The induction effect of TCDD in the mouse kidney does not require translocation of AHR to the nucleus. TCDD induction of Cox-2 in mouse kidney is primarily mediated by a non-genomic pathway that activates AHR. In rats, TiFH is also induced and may be an endogenous ligand for AHR and/or a protein interacting with AHR. In contrast to rats, mice lacking AHR did not develop hydronephrosis or hydronephrosis in the absence of TCDD [58]. To better explore the role of AHR in normal development and chemical response, AHR knockout (AHR-KO) models were created in rats and mice, respectively. However, in AHR-KO rats, hydronephrosis and hydroureter were observed and AHR was found to play significantly different roles in tissue development and virulence in rodent species [59].
Human studies: In a study of more than 300 chronic kidney disease (CKD) patients and healthy controls, overexpression of polymorphic variants of CYP1A1 were associated with free radical production related enzymes after exposure to environmental pollutants, and with induction of renal dysfunction. Because of the different effects of AHR in rats and mice, it was not possible to directly use animal models to verify the effect of TCDD in the human kidney. To better detect the effects of dioxins on human health and reduce the differences between species, the mouse AHR was replaced with human AHR cDNA by knock-in strategy. Human AHR can be expressed in mice to mediate the development of TCDD-induced hydronephrosis [60]. Transcriptional analysis of human AHR was performed and compared in the liver and kidney, but dioxin exposure in the kidney altered only 17 genes, including many AHR target genes [61].
Overall, the relationship between CYP1A1, TCDD, and the AHR is complex and involves the metabolism of TCDD by CYP1A1 and the activation of the AHR by TCDD. The activation of the AHR by TCDD may lead to the expression of various genes that could contribute to nephrotoxicity, including genes involved in inflammation and oxidative stress. CYP1A1 is involved in the metabolism of TCDD, and the activation of the AHR by TCDD may also lead to the expression of CYP1A1. More research is needed to fully understand the mechanisms by which TCDD causes adverse health effects.
3. Per- and Polyfluoroalkyl Substances
Per- and polyfluoroalkyl substances are a class of chemicals (Figure 2b) used in many industrial and consumer products with the main function of resisting heat, stains, water, and grease [62,63]. Classic examples include Teflon, coating on fast-food wrappers, non-stick pans, floor polish, carpets, furniture fabrics, firefighting foams, clothing treatments, and many others [64,65]. It has been estimated that over 98% of Americans have these chemicals in their body [66,67,68,69]. PFAS can be classified into long chain and short chain chemicals based on the number of carbon atoms (usually between 4 and 12) and they have a long half-life and are difficult to metabolize (because of the strong covalent C–F bond). In general, shorter chain chemicals (n ≤ 6) are easier to excrete than longer chain chemicals (n ≥ 6). Previous studies have found that urine was the main mode of excretion of PFAS in mice and monkeys [70,71]. High concentrations of PFAS were detected in human urine, and renal excretion in humans and accounts for approximately one-fifth of the total excretion based on their serum half-lives [72,73]. Analysis of blood and urine samples from adults showed that a variety of PFAS were detected, including perfluorooctane sulfonates (PFOS), perfluorooctanoic acid (PFOA), perfluorononanoate (PFNA), perfluorohexanesulfonic acid (PFHxS), and perfluorinated decanoic acid (PFDA), and that the level of PFAS in urine was positively correlated with that in blood [74].
3.1. Accumulation of PFAS in the Kidney
Renal excretion is an important excretory pathway of PFAS in humans and animals, and the kidney plays an essential role in the metabolism and transport of PFAS. After exposure to PFAS in rainbow trout, the degree of accumulation was blood > kidney > liver > gallbladder [75,76]. The distribution of 24 types of PFAS in eight different tissues of Orcinus orca was reported, and the highest concentration of PFAS was found in the liver, followed by the blood and kidney [77]. PFAS were measured in samples from 31 harbor porpoises (Phocoena relicta) stranded on the Black Sea coast and PFOS was found to account for 90% of all PFAS, which were highest in the liver (327 ± 351 ng/g wet weight) and kidney (147 ± 262 ng/g wet weight) tissues [78]. Both long-chain and short-chain PFAS were also detected in the organs and tissues of the sea leopard (Phoca vitulina) in the Netherlands [79,80]. In another study, PFAS accumulation in the tissues and organs of the Baltic guillemots (Uria algge) were noted. PFOS remained the most abundant, with a median concentration of 127 ng/g weight in the kidney [81]. The distribution of 16 PFAS in the liver, blood, kidney, adipose tissue, and muscle of 18 Arctic foxes aged 1–3 years was also noted, with the concentration of PFAS being highest in the liver, followed by the blood and kidney [82]. These data suggest that the tissue distribution and accumulation patterns of PFAS in organisms vary considerably. However, the kidney has always been an important organ for PFAS accumulation in animal species. Compared with long-chain PFAS, short-chain PFAS (e.g., perfluorobutyric acid, PFBA) have a relatively short half-life in blood and do not accumulate at high levels in human kidney tissue based on the analysis of seven lung and nine kidney samples from cancer patients [83]. Since studies on kidney toxicology are sparse, this makes a strong argument for investigations on chronic exposure in relation to accumulation and kidney diseases.
3.2. Relationship between PFAS and CKD
PFAS are ubiquitous and difficult to metabolize, and thus, their possible toxic effects on animals and humans have attracted significant attention [84,85,86,87,88,89,90]. However, some studies suggest that there may be no causal relationship between chronic kidney disease (CKD) and PFAS. To investigate the longitudinal association between PFOA and PFOS exposure and the incidence of CKD in patients with diabetes, PFOA and PFOS levels in serum were measured in nearly 1000 patients with diabetes and it was found that PFOS levels were significantly associated with a lower risk of CKD incidence. The interaction between PFOA and PFOS exposure and CKD was not statistically significant [91]. In a study of 73 normal weight pregnant women who did not have gestational diabetes mellitus or preeclampsia, the concentration of PFNA, PFOA, PFOS, and PFHxS in the blood changed from early to late pregnancy. The levels of these PFAS decreased during pregnancy, but were not linked to changes in the estimated glomerular filtration rate (eGFR) or glomerular pore size. Collectively, these studies suggest that changes in renal function are not caused by PFAS [92].
On the contrary, other studies have shown that PFAS was closely related to a decline in renal function. EGFR was one of the diagnostic factors of CKD, and studies have shown that PFAS can affect the level of eGFR. PFAS levels in 61 CKD patients were found to be significantly higher than in the healthy control group [93]. The levels of hemoglobin, serum albumin, and eGFR were significantly lower while the levels of potassium and uric acid were higher in the CKD group. PFOS levels were found to be significantly higher in CKD patients than controls. By measuring serum concentrations of PFOA, PFOS, PFNA, and PFHxS in adolescents living near chemical plants and comparing their eGFR with another 10,000 adolescents, the association between eGFR and serum PFOA might serve as an indicator of decreased renal function [94]. Serum samples from 53,650 adults (5210 with diabetes) were evaluated for four different PFAS. All PFAS had a strong positive correlation with eGFR in patients with CKD or anemia, and these relationships were more significantly associated in patients with diabetes. PFAS are inversely associated with renal function in CKD and diabetes, suggesting that exposure to PFAS could be associated with decreased renal function [95,96,97,98]. After following 1237 non-diabetic women aged 45 to 56 years until 2017, a study by Park [99] suggested that PFAS may increase the risk of diabetes in middle-aged women.
3.3. Effect of PFAS on RCC and Renal Function
Several discrepancies exist in studies on the association between PFAS and kidney cancer. Reports from 18 epidemiological studies on the association between PFOS exposure and human cancer risk indicated that the relative risks of PFOA and PFOS do not indicate a dose–response relationship between exposure and kidney cancer. Positive associations with PFOA exposure were detected in community settings, but because occupational exposure to PFOS was one to two orders of magnitude higher than environmental exposure, epidemiologic evidence does not support the hypothesis of a causal dose–response relationship between PFOS exposure and human cancer [100].
In contrast, other studies have demonstrated that the kidney was one of the target organs of PFAS. Data from 324 patients showed that a doubling of serum PFOA concentration was associated with an approximately 70% increase in RCC risk when PFAS concentrations were serially simulated. Higher concentrations of certain PFAS and the highest incidence of RCC were also observed in African Americans compared to non-Hispanic whites [101,102]. This study was also supported by the study from Steenland et al. [103], in which the association between PFOA and RCC was examined in nearly 1000 renal cancer cases. The pooled analysis indicated that PFOA was associated with renal cancer in human studies, for every 1 ng/mL increase of serum PFOA, the logarithmic ratio of RCC increased by 0.1349. These results provide strong evidence that PFOA is a renal carcinogen.
Since the association between PFOA and RCC has yielded conflicting conclusions in different studies, PFOA exposure was converted to serum PFOA concentrations and meta-analysis was used to estimate cancer risk. In the meta-analysis, risk for RCC rose 16% for each 10 ng/mL increase in serum PFOA. Thus, the association of PFOA with RCC is most likely causal and makes a strong argument for further research on the mechanisms underlying this association [104].
Recently, studies are beginning to emerge on the effect of PFAS in select cancers (liver, prostate, kidney, etc.) [105], but mechanistic studies on the role of PFAS are sparse. Although epidemiological evidence points to kidney cancer as one of the cancers triggered by PFAS, very few studies have examined the role of PFAS in kidney disease or renal cell carcinoma.
A study by Park [106] showed that after being absorbed by the human body, PFAS are first combined with serum proteins and then deposited in various organs of the body such as the liver, kidney, and testis, and that PFAS cannot be excreted, resulting in a variety of toxic outcomes. The effect of PFAS on gap junctional intercellular communication (GJIC) in a dolphin renal epithelial cell line showed that PFOS, perfluorooctane sulfonamide (PFOSA) and perfluorohexane sulfonic acid (PFHA) could rapidly inhibit GJIC, while perfluorobutane sulfonic acid (PFBS) had no significant effect on GJIC. In humans, urinary excretion of short-chain PFAS was found to be higher, whereas other elimination pathways were found to be more dominant for long-chain chemicals [107,108]. These results suggest that the toxic effects of PFAS on organisms could possibly depend on its structure rather than the properties of its functional groups.
Only a few studies exist on the mechanism of PFAS exposure and kidney response. Examination of over 70 studies studies in the fields of epidemiology, pharmacokinetics, and toxicology showed that PFAS exposure causes renal tubular tissue and cellular changes. PFAS exposure altered several pathways associated with kidney disease, including oxidative stress, peroxisome proliferator-activated receptor (PPAR) pathway, and epithelial–mesenchymal transition (EMT) [109]. By evaluating the role of PFAS in Xenopus A6 renal epithelial cells, the inductive effects of these chemicals were attributed to the stimulation of DNA/RNA, secondary protein structures, lipids, and fatty acids, which ultimately lead to cell death [110]. PFOA exposed to male and female rats showed accumulation in liver, kidney, and small intestinal microsomes. Significant uridine diphosphate -glucuronosyltransferase (UDPGT) activity was observed in all tests, but no evidence of PFOA-glucuronide formation was observed either by highly sensitive radio-chromatography or by liquid chromatography-mass spectrometry (LC/MS) [111]. Recent studies by Wen et al. [112] showed that exposure to PFOS resulted in increased expression of renal injury markers Acta2 and Bcl2l1, decreased DNA methylation, and the upregulation of histone demethylases Kdm1a and Kdm4c. In addition, PFOS was shown to induce renal injury and upregulation of transcription factors Nef2l2, Hes1, Ppara, and Ppard. These results suggest a potential adverse effect of PFOS on renal fibrosis and carcinogenesis.
A number of studies have shown that the kidney may be at risk due to exposure to EDCs, including PFAS. However, there are still several important knowledge gaps in our understanding of kidney disease and kidney cancer due to the overlapping populations, limited modeling boundaries, and lack of mechanistic studies. Elucidating the effects of PFAS exposure on genetic, biological, environmental, occupational, and other risk factors is critical. Further studies on the direct and indirect mechanisms of nephrotoxicity, including experimental models, metabolic analysis, and translation to epidemiology is warranted. In addition, how environmental toxicants such as PFAS drive differences in kidney disease among different populations remains poorly understood.
Occupational workers are commonly exposed to high levels of PFAS with greater risk compared to the general population. A study (data from 1950–2009) analyzing cancers and cancer deaths among ~30,000 firefighters (21–29 years of employment) found that cancers of respiratory, digestive, and urinary systems were the highest [113]. In a study of 40 occupational workers and 52 population-based controls in China, a total of 14 biomarkers related to oxidative stress, fatty acid β-oxidation disorder and kidney injury were identified in occupational workers [114]. HEK-293 cells were exposed to three widely used aqueous film forming foams (AFFFs). All AFFFs induced cytotoxicity and markedly inhibited cell proliferation when exposed to only one-tenth of the working concentration of fire suppression [115]. Therefore, the health effects of occupational exposure to PFAS on workers should not be ignored [116]. Studies from meta-analysis focused on cancer risk among firefighters indicated that firefighters were at increased risk of developing multiple myeloma, non-Hodgkin lymphoma, prostate, kidney, lung, and testicular cancer. Eight additional cancers were also listed as having a “possible” association with exposures to fire training activities [117,118]. Risk for these cancers among firefighters may be related to direct exposures to complex toxicants during fire training activities through inhalation and contact with PPE contributing to the modulation of biochemical or physiological pathways that put firefighters at increased baseline risk of developing cancer [119]. Cancer is also one of the leading causes of death among firefighters according to the Centers for Disease Control and Prevention [113,117,118]. Research is needed to reveal toxicity due to occupational exposure in the study of PFAS.
Currently, many traditional PFAS are being phased out and are being replaced by commercial compounds such as GenX, F-53B, and short-chain variants. Even more challenging is the existence of hundreds of undiscovered PFAS compounds whose health effects are unknown. Because of the dramatic increase in the production of novel alternative PFAS compounds, there is an urgent need to understand the relationship between PFAS exposure and kidney disease to find key biomarkers triggered due to PFAS toxicity.
4. Bisphenol A
Bisphenol A is an environmental toxicant with a phenolic ring and structural similarity to phenols (Figure 2c). It is an endocrine disrupter involved in the synthesis of a variety of plastics and it has been used since the 1960s to make the inner coating of plastic bottles, food packaging, and medical devices [120].
4.1. Accumulation of BPA in the Kidney
BPA can be detected in virtually 100% of human urine samples [121,122,123]. Pharmacokinetic and biomonitoring data indicate that BPA is rapidly and efficiently metabolized after ingestion [124]. Low doses of BPA are effectively reduced in the gastrointestinal tract and liver of adults of all species, with final exposures typically below 1% of the total. However, it is worth noting that, compared with neonatal primates, newborn rodents are immature and do not have the ability to metabolize and excrete BPA, leading to BPA accumulation in serum or tissues, including fetal tissues [125,126].
A population survey in China from 2003 to 2006 showed that urinary excretion of BPA decreased with decreasing renal function. A correlation was shown between urinary excretion of BPA and albuminuria and the association of BPA with renal injury [127]. The decline of renal function was found to be not conducive to the elimination of BPA, leading to its accumulation, forming a vicious cycle [128,129,130].
4.2. Effect of BPA on Renal Function
BPA is cytotoxic and mutagenic in aquatic organisms, animals, and humans. BPA exposure is known to cause various adverse effects on the immune, endocrine, reproductive, developmental and nervous systems [131,132].
Animal studies: Histopathological evaluation of rats exposed to BPA for 30 days showed infiltrative and dilatative changes in renal tissues, leading to renal failure [133,134,135]. Significant increases in sodium, potassium and calcium concentrations were observed in response to BPA, indicating that BPA disrupts electrolyte balance, leading to renal dysfunction [136,137]. Fetal glomerular abnormalities and decreased glomerular formation were observed after being exposed to BPA during pregnancy [138]. Histological examination of the kidneys of BPA-exposed fish revealed glomerular atrophy and distortion, uriniferous tubule edema, necrosis and atrophy, severe hyperemia, and blood hemolysis [139].
Human studies: Substantial evidence shows that high levels of BPA in the blood are associated with kidney disease. Despite the inconsistent urinary BPA concentrations observed in patients with kidney disease, the statistical association with eGFR supports the association between BPA and glomerular filtration, suggesting BPA as a possible environmental factor that induces kidney injury [140]. Low doses of BPA can cause cytotoxicity in renal mouse podocytes, and studies in human kidney cells have also shown that BPA promotes kidney injury [141,142,143]. In a study of patients with CKD, an increase in serum BPA was found to be accompanied by a decrease in eGFR and renal function, potentially due to BPA accumulation [144,145,146]. A positive correlation between urinary BPA levels and serum uric acid levels in children [147] was noted. Interestingly, urinary BPA levels were lower in children with CKD than in healthy people. BPA exposure had no effect on renal function in children with CKD, possibly because pediatric patients with CKD may have healthier eating habits than the general population [148].
4.3. Mechanisms of BPA Kidney Disease Promotion
To better inform on the biomarkers of renal disease, it is important to understand the molecular mechanism of BPA-induced nephrotoxicity and carcinogenicity [149]. In vitro experiments showed that BPA reduced superoxide dismutase (SOD) activity and glutathione (GSH) levels, while promoting cellular apoptosis, reactive oxygen species (ROS) production, and DNA damage [150,151,152]. A study in rats showed that proteinuria and glomerular damage were associated with increased lipid peroxidation and decreased levels of the antioxidants, glutathione and superoxide dismutase. BPA is known to directly act on kidney mitochondria to cause oxidative stress and mitochondrial dysfunction, resulting in subsequent damage to the whole organ [153,154,155,156]. Longer exposure times involve transcriptional responses of immune-related genes, potentially due to BPA-related oxidative stress in inducing inflammatory responses in macrophages [157,158,159]. Evaluation of epigenetic toxicity has shown that BPA can lead to DNA methylation. Long interspersed transposable element-1 (Line1) and CCGG global methylation rates in fetal kidney were 77.9% and 77.0%, respectively [160]. Changes in the methylation of CpG promoter were detected in the Rassf1a and C-myc genes in cells [161].
BPA was found to activate autophagy and apoptosis-related signaling pathways. BPA exposure activated autophagy-related proteins (ATG5, ATG7 and MAP1LC3B/LC3B) and decreased the expression of NRF-2 and HO-1 proteins [162,163,164,165]. BPA also activated the AMPK/mTOR (The 5′-adenosine monophosphate-activated protein kinase and mammalian target of rapamycin) signaling pathway, which triggers P62/Lc3/Beclin1 signaling, leading to the formation of autophagosomes and autolysosomes, and ultimately, stimulating autophagy in renal cells [166,167]. BPA exposure down-regulated the expression of PI3K (phosphatidylinositide 3-kinases) and AKT (protein kinase B) and activated the Bcl/Bax-Caspase 9-Caspase 3 (B-cell lymphoma/BCL2-Associated X-Caspase 9-Caspase 3) signaling pathways, leading to apoptosis and necrosis of renal cells [168]. Recent studies have shown that BPA-induced renal dysfunction was associated with ferriosis, which depends on ferritin phagocytosis, through the activation of AMPK-mTOR-ULK1 (Unc-51-like kinase 1) axis [169].
Further studies are needed to assess the molecular mechanisms of BPA-induced nephrotoxicity. In addition, some studies interestingly suggest that when BPA is co-exposed with other environmental pollutants, compensatory mechanisms exist that can reverse the damage caused by each toxic substance on its own. Additional research is needed to better understand the underlying synergistic mechanisms [170].
5. Phthalates
Phthalates are planar aromatic hydrocarbons with side chains (Figure 2d). They are often used as universal plasticizers for polyvinyl chloride (PVC) [3]. Phthalates also are widely used in many consumer products and medical devices, resulting in a significant burden on human health [171,172,173,174,175,176]. They can accumulate in the kidney and cause renal dysfunction and decline [177,178,179,180].
5.1. Accumulation of Phthalates in the Kidney
Similar to BPA, phthalates are rapidly metabolized and absorbed in the gastrointestinal tract and eventually excreted in urine. When ingested orally, phthalates are mainly found in the liver and kidneys, but do not accumulate as much in other organs or tissues [181,182,183,184]. However, phthalates are almost always detected in animals due to their continuous exposure.
5.2. Effect of Phthalates on Renal Function
Animal studies: Several studies suggest that phthalates only affect kidney function in rodents, and that primate kidneys are not target organs. Previous research has shown that oral administration of a high dose of phthalates (500 mg/kg/day) affects the liver and kidney in rodents, but not in primates [185,186]. Phthalates can induce the epithelial–mesenchymal transition in rat renal cells and aggravate renal fibrosis [187]. Exposure to phthalates in the μg/liter range in drinking water induces significant perturbation of metabolic profiles and renal dysfunction in mice [188]. To assess the effects of phthalates in primates, young adult male cynomolgus monkeys were exposed to high doses (500 mg/kg/day) of phthalates via endogastric cannulators for 14 consecutive days. Histopathological examination revealed no significant treatment-related effects on the kidneys, even at the indicated doses in rodents [185].
Human studies: Recent studies have shown that phthalates can affect kidney function in children, pregnant women, and adults [3,148,189,190,191,192]. Phthalates have a strong relationship with the urinary albumin creatinine ratio (ACR) and eGFR in children with CKD. Urinary phthalate levels of CKD children were lower than healthy children, possibly because children with CKD may have healthier eating habits [3,148,193]. A study in more than 400 women in Korea showed a significant positive correlation between phthalates and ACR in both single-pollutant models and multi-pollutant models [191]. In the co-exposure model, the phthalates have an additive effect on ACR. In a hypertensive population, phthalates were found to be positively correlated with ACR, β2-microglobulin, cystatin C, and negatively correlated with eGFR. Hypertensive people are more sensitive to early kidney injury due to phthalate exposure [4,194]. Compared with normal albuminuria patients, patients with microalbuminuria and macroalbuminuria had higher levels of phthalates urinary metabolites, which is unrelated to eGFR [195,196]. Over time, however, tubular damage from phthalates and increased oxidative stress follow a pattern that can affect kidney function due to long-term exposure, revealing an unexplored relationship between phthalates and the determinants of renal status [131].
5.3. Mechanisms of Phthalate Toxicity in the Kidney
Studies indicate that phthalates cause kidney disease through multiple mechanisms [192,197,198]. In human kidney embryonic cells (HEK-293T), phthalates were found to directly regulate mRNA translation, which was manifested as the inhibition of both cap-dependent and -independent mRNA translation in vivo [199]. In rodents, phthalates significantly increase oxidative damage and phosphorylated extracellular regulated protein kinases 1/2 (ERK1/2) expression [195]. Phthalate exposure also increased ROS, malondialdehyde (MDA) and DNA-protein cross-linked (DPC) levels and decreased GSH levels also in a dose-dependent manner [200]. An increase in protein levels of NRF-2, HO-1, and GCLC (responsible for GSH synthesis) was also observed. In addition, upregulation of P53 and BAX proteins and the downregulation of BCL-2 by phthalate exposure suggest that phthalate-induced apoptosis was activated by the mitochondrial pathway [201,202].
Phthalate exposure has also been shown to cause toxicity by promoting autophagy [187]. Oral administration of phthalates in pregnant rats increased LC3II/I protein expression, increased hedgehog interacting protein (Hhip) gene and protein expression, and inhibited hedgehog signaling, leading to autophagy in renal tubule cells and resulting in renal fibrosis [203]. In addition, maternal exposure to phthalates can activate the Rhoa/Rock pathway in the kidney of the offspring, inducing the occurrence of EMT, which may eventually lead to renal fibrosis in the offspring [204]. Peroxisome proliferator-activated receptors (PPARγ) significantly inhibited phthalate-mediated EMT induction [205]. Phthalate exposure reduced the protein expression of PPARα and PPARγ both in vivo and in vitro. Phthalates induce large accumulation of AHR and AHR nuclear transporter (Arnt) in the nucleus [206], exacerbating chronic kidney diseases.
These results suggest that phthalates can induce oxidative stress and apoptosis in the kidney through dermal, oral, and other exposure routes, ultimately leading to histopathological changes in the kidney. However, most studies have focused on rodents, and internal doses, which makes it difficult to assess the potential risk aspects of phthalates in humans.
6. Conclusions
This review summarizes and discusses the effects of EDCs on kidneys (Figure 3). EDCs cause proteinuria, affect eGFR, perturb the intracellular REDOX balance, and activate apoptosis and AHR pathways, eventually resulting in kidney injury and renal fibrosis. Most previous studies are l focused on the traditional environmental pollutants such as PCBs and BPA, whereas few studies focus on newer or emerging environmental pollutants. With the development and application of new materials, the types of environmental pollutants in water and food are constantly increasing, and new drugs and EDC substitutes are on the rise. It is clear that several gaps exist in the toxicity studies and the molecular mechanisms triggered by these pollutants. Mechanistic studies provide a sound basis for the identification of key molecular toxicity targets of EDC and provide theoretical basis for its health risk assessment. Hence, foundational knowledge on the mechanism of action of traditional EDCs on kidney is imperative for more effective prevention strategies and/or treatment options.
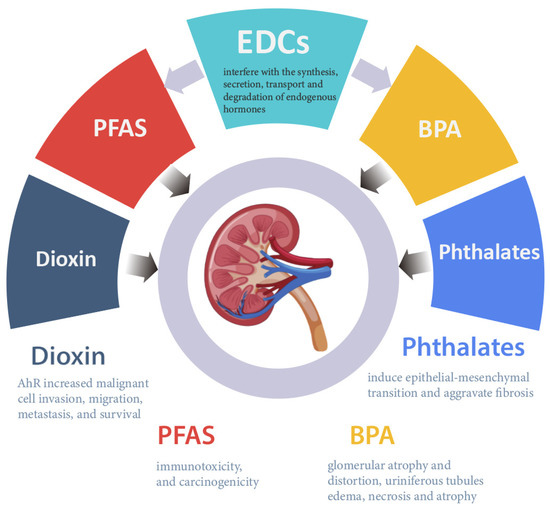
Figure 3.
Diseases/conditions triggered due to exposure to EDCs.
The toxicity of EDCs to organisms is not only caused by a single pollutant, but often comes from multiple pollutants. Therefore, future studies, could focus on chronic exposures from EDC mixtures at low concentrations.
Past work has shown that EDC isomers (example, dioxin) induce different biotoxicity. Hence, it is necessary to analyze major isomer components in human blood. Alternatively, the analysis of isomers can be used to distinguish the sources of different production modes and identify direct or indirect exposure routes in humans. Due to the differences in molecular structure, isomers may have different accumulation rates. Very few studies have focused on the composition of isomers in human blood and the potential harm to human body. Thus, these effects need to be addressed.
Author Contributions
Conceptualization, X.Z. and J.I.; methodology, X.Z. and J.I.; investigation, X.Z., J.I. and J.A.F.; resources, J.I.; writing—original draft preparation, X.Z.; writing—review and editing, X.Z, J.I., J.A.F. and M.J.S.; supervision, J.I.; project administration, J.I.; funding acquisition, J.I., J.A.F. and M.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the External Research Board, OVCRI, University of Illinois at Urbana-Champaign; NIEHS, grant number R01ES034112-01A1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Administrative support from the Biomedical Research Center at Carle Foundation Hospital is greatly appreciated.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Cabana, H.; Jones, J.P.; Agathos, S.N. Elimination of Endocrine Disrupting Chemicals using White Rot Fungi and their Lignin Modifying Enzymes: A Review. Eng. Life Sci. 2007, 7, 429–456. [Google Scholar] [CrossRef]
- Tri, T.M.; Anh, D.H.; Hoai, P.M.; Minh, N.H.; Nam, V.D.; Viet, P.H.; Minh, T.B. Emerging Endocrine Disrupting Chemicals and Pharmaceuticals in Vietnam: A Review of Environmental Occurrence and Fate in Aquatic and Indoor Environments. In Persistent Organic Chemicals in the Environment: Status and Trends in the Pacific Basin Countries II Temporal Trends; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2016; Volume 1244, pp. 223–253. [Google Scholar]
- Pinar, E.; Belma, K.-G. Environmental Effects of Endocrine-Disrupting Chemicals: A Special Focus on Phthalates and Bisphenol A. In Environmental Health Risk; Marcelo, L.L., Sonia, S., Eds.; IntechOpen: London, UK, 2016; pp. 155–190. [Google Scholar]
- Hsu, C.N.; Tain, Y.L. Adverse Impact of Environmental Chemicals on Developmental Origins of Kidney Disease and Hypertension. Front. Endocrinol. 2021, 12, 745716. [Google Scholar] [CrossRef]
- Singh, R.D.; Koshta, K.; Tiwari, R.; Khan, H.; Sharma, V.; Srivastava, V. Developmental Exposure to Endocrine Disrupting Chemicals and Its Impact on Cardio-Metabolic-Renal Health. Front. Toxicol. 2021, 28, 663372. [Google Scholar] [CrossRef]
- Rochman, C.M.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.K.; Rios-Mendoza, L.M.; Takada, H.; Teh, S.; Thompson, R.C. Classify plastic waste as hazardous. Nature 2013, 494, 169–171. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Anderson, S.J.; Harvey, G.R.; Miklas, H.P.; Peck, B.B. Polystyrene Spherules in Coastal Waters. Science 1972, 178, 749–750. [Google Scholar] [CrossRef]
- Hurley, R.; Woodward, J.; Rothwell, J.J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 2018, 11, 251–257. [Google Scholar] [CrossRef]
- Kwan, C.S.; Takada, H. Release of Additives and Monomers from Plastic Wastes. In Hazardous Chemicals Associated with Plastics in the Marine Environment; Takada, H., Karapanagioti, H.K., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 51–70. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Peng, X.; Zheng, K.; Liu, J.; Fan, Y.; Tang, C.; Xiong, S. Body size–dependent bioaccumulation, tissue distribution, and trophic and maternal transfer of phenolic endocrine-disrupting contaminants in a freshwater ecosystem. Environ. Toxicol. Chem. 2018, 37, 1811–1823. [Google Scholar] [CrossRef]
- Ruhí, A.; Acuña, V.; Barceló, D.; Huerta, B.; Mor, J.-R.; Rodríguez-Mozaz, S.; Sabater, S. Bioaccumulation and trophic magnification of pharmaceuticals and endocrine disruptors in a Mediterranean river food web. Sci. Total Environ. 2016, 540, 250–259. [Google Scholar] [CrossRef]
- Windsor, F.M.; Ormerod, S.J.; Tyler, C.R. Endocrine disruption in aquatic systems: Up-scaling research to address ecological consequences. Biol. Rev. 2018, 93, 626–641. [Google Scholar] [CrossRef]
- Li, Y.; Taggart, M.A.; McKenzie, C.; Zhang, Z.; Lu, Y.; Pap, S.; Gibb, S.W. A SPE-HPLC-MS/MS method for the simultaneous determination of prioritised pharmaceuticals and EDCs with high environmental risk potential in freshwater. J. Environ. Sci. 2021, 100, 18–27. [Google Scholar] [CrossRef]
- Sosa-Ferrera, Z.; Mahugo-Santana, C.; Santana-Rodríguez, J.J. Analytical Methodologies for the Determination of Endocrine Disrupting Compounds in Biological and Environmental Samples. BioMed. Res. Int. 2013, 2013, 674838. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, Y.; Mizuno, T.; Tsuno, H.; Zhu, W. A Pretreatment Method for GC–MS Determination of Endocrine Disrupting Chemicals in Mollusk Tissues. Chromatographia 2009, 69, 65–71. [Google Scholar] [CrossRef]
- Azzouz, A.; Rascón, A.J.; Ballesteros, E. Determination of free and conjugated forms of endocrine-disrupting chemicals in human biological fluids by GC−MS. Bioanalysis 2016, 8, 1145–1158. [Google Scholar] [CrossRef]
- Deng, Z.-H.; Li, N.; Jiang, H.-L.; Lin, J.-M.; Zhao, R.-S. Pretreatment techniques and analytical methods for phenolic endocrine disrupting chemicals in food and environmental samples. TrAC Trends Anal. Chem. 2019, 119, 115592. [Google Scholar] [CrossRef]
- Kitazawa, T.; Sato, T.; Nishiyama, K.; Asai, R.; Arima, Y.; Uchijima, Y.; Kurihara, Y.; Kurihara, H. Identification and developmental analysis of endothelin receptor type-A expressing cells in the mouse kidney. Gene Expr. Patterns 2011, 11, 371–377. [Google Scholar] [CrossRef]
- Lehrke, I.; Waldherr, R.; Ritz, E.; Wagner, J. Renal Endothelin-1 and Endothelin Receptor Type B Expression in Glomerular Diseases with Proteinuria. J. Am. Soc. Nephrol. 2001, 12, 2321. [Google Scholar] [CrossRef]
- Provenzano, M.; Andreucci, M.; Garofalo, C.; Minutolo, R.; Serra, R.; De Nicola, L. Selective endothelin A receptor antagonism in patients with proteinuric chronic kidney disease. Expert Opin. Investig. Drugs 2021, 30, 253–262. [Google Scholar] [CrossRef]
- Higginbotham, G.R.; Huang, A.; Firestone, D.; Verrett, J.; Ress, J.; Campbell, A.D. Chemical and Toxicological Evaluations of Isolated and Synthetic Chloro Derivatives of Dibenzo-p-dioxin. Nature 1968, 220, 702–703. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Crespo, J.G.; Afonso, C.A.M. Dioxins sources and current remediation technologies—A review. Environ. Int. 2008, 34, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Olie, K.; Addink, R.; Schoonenboom, M. Metals as Catalysts during the Formation and Decomposition of Chlorinated Dioxins and Furans in Incineration Processes. J. Air Waste Manag. Assoc. 1998, 48, 101–105. [Google Scholar] [CrossRef]
- Fernandes, A.; Mortimer, D.; Rose, M.; Gem, M. Dioxins (PCDD/Fs) and PCBs in offal: Occurrence and dietary exposure. Chemosphere 2010, 81, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.; Fernandes, A.; Foxall, C.; Dowding, A. Transfer and uptake of polychlorinated dibenzo-p-dioxins and furans (PCDD/Fs) and polychlorinated biphenyls (PCBs) into meat and organs of indoor and outdoor reared pigs. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2012, 29, 431–448. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernández-González, R.; Yebra-Pimentel, I.; Martínez-Carballo, E.; Simal-Gándara, J. A Critical Review about Human Exposure to Polychlorinated Dibenzo-p-Dioxins (PCDDs), Polychlorinated Dibenzofurans (PCDFs) and Polychlorinated Biphenyls (PCBs) through Foods. Crit. Rev. Food Sci. Nutr. 2015, 55, 1590–1617. [Google Scholar] [CrossRef]
- Chen, Y.P.; Liu, Q.; Ma, Q.Y.; Maltby, L.; Ellison, A.M.; Zhao, Y. Environmental toxicants impair liver and kidney function and sperm quality of captive pandas. Ecotoxicol. Environ. Saf. 2018, 162, 218–224. [Google Scholar] [CrossRef]
- Amutova, F.; Delannoy, M.; Baubekova, A.; Konuspayeva, G.; Jurjanz, S. Transfer of persistent organic pollutants in food of animal origin—Meta-analysis of published data. Chemosphere 2021, 262, 128351. [Google Scholar] [CrossRef]
- Driesen, C.; Zennegg, M.; Rothacher, M.; Dubois, S.; Wyss, U.; Nowack, B.; Lerch, S. Transgenerational mass balance and tissue distribution of PCBs and PCDD/Fs from grass silage and soil into cow-calf continuum. Chemosphere 2022, 307, 135745. [Google Scholar] [CrossRef]
- Roy, M.A.; Sant, K.E.; Venezia, O.L.; Shipman, A.B.; McCormick, S.D.; Saktrakulkla, P.; Hornbuckle, K.C.; Timme-Laragy, A.R. The emerging contaminant 3,3′-dichlorobiphenyl (PCB-11) impedes Ahr activation and Cyp1a activity to modify embryotoxicity of Ahr ligands in the zebrafish embryo model (Danio rerio). Environ. Pollut. 2019, 254, 113027. [Google Scholar] [CrossRef]
- Ji, C.; Yan, L.; Chen, Y.; Yue, S.; Dong, Q.; Chen, J.; Zhao, M. Evaluation of the developmental toxicity of 2,7-dibromocarbazole to zebrafish based on transcriptomics assay. J. Hazard. Mater. 2019, 368, 514–522. [Google Scholar] [CrossRef]
- Erdemli, M.E.; Yigitcan, B.; Erdemli, Z.; Gul, M.; Bag, H.G.; Gul, S. Thymoquinone protection against 2,3,7,8-tetrachlorodibenzo-p-dioxin induced nephrotoxicity in rats. Biotech. Histochem. 2020, 95, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Z.; Zhang, Q.H.; Schramm, K.W.; Xu, Y.; Kettrup, A. Distribution, transformation, and long-term accumulation of polychlorinated dibenzo-p-dioxins and dibenzofurans in different tissues of fish and piscivorous birds. Ecotoxicol. Environ. Saf. 2000, 46, 252–257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Henry, T.R.; Spitsbergen, J.M.; Hornung, M.W.; Abnet, C.C.; Peterson, R.E. Early Life Stage Toxicity of 2,3,7,8-Tetrachlorodibenzo-p-dioxin in Zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 1997, 142, 56–68. [Google Scholar] [CrossRef]
- Raldúa, D.; Padrós, F.; Solé, M.; Eljarrat, E.; Barceló, D.; Riva, M.C.; Barata, C. First evidence of polybrominated diphenyl ether (flame retardants) effects in feral barbel from the Ebro River basin (NE, Spain). Chemosphere 2008, 73, 56–64. [Google Scholar] [CrossRef]
- Albina, M.L.; Alonso, V.; Linares, V.; Bellés, M.; Sirvent, J.J.; Domingo, J.L.; Sánchez, D.J. Effects of exposure to BDE-99 on oxidative status of liver and kidney in adult rats. Toxicology 2010, 271, 51–56. [Google Scholar] [CrossRef]
- Ruan, F.; Liu, C.; Hu, W.; Ruan, J.; Ding, X.; Zhang, L.; Yang, C.; Zuo, Z.; He, C.; Huang, J. Early life PCB138 exposure induces kidney injury secondary to hyperuricemia in male mice. Environ. Pollut. 2022, 301, 118977. [Google Scholar] [CrossRef]
- Lu, C.-F.; Wang, Y.-M.; Peng, S.-Q.; Zou, L.-B.; Tan, D.-H.; Liu, G.; Fu, Z.; Wang, Q.-X.; Zhao, J. Combined Effects of Repeated Administration of 2,3,7,8-Tetrachlorodibenzo-p-dioxin and Polychlorinated Biphenyls on Kidneys of Male Rats. Arch. Environ. Contam. Toxicol. 2009, 57, 767–776. [Google Scholar] [CrossRef]
- Randerath, K.; Putman, K.L.; Randerath, E.; Mason, G.; Kelley, M.; Safe, S. Organ-specific effects of long term feeding of 2,3,7,8-tetrachlorodibenzo-p-dioxin and 1,2,3,7,8-pentachlorodibenzo-p-dioxin on I-compounds in hepatic and renal DNA of female Sprague-Dawley rats. Carcinogenesis 1988, 9, 2285–2289. [Google Scholar] [CrossRef]
- Shalat, S.L.; True, L.D.; Fleming, L.E.; Pace, P.E. Kidney cancer in utility workers exposed to polychlorinated biphenyls (PCBs). Br. J. Ind. Med. 1989, 46, 823–824. [Google Scholar] [CrossRef][Green Version]
- Xu, P.; Lou, X.; Ding, G.; Shen, H.; Wu, L.; Chen, Z.; Han, J.; Wang, X. Effects of PCBs and PBDEs on thyroid hormone, lymphocyte proliferation, hematology and kidney injury markers in residents of an e-waste dismantling area in Zhejiang, China. Sci. Total Environ. 2015, 536, 215–222. [Google Scholar] [CrossRef]
- Niu, S.; Tao, W.; Chen, R.; Hageman, K.J.; Zhu, C.; Zheng, R.; Dong, L. Using Polychlorinated Naphthalene Concentrations in the Soil from a Southeast China E-Waste Recycling Area in a Novel Screening-Level Multipathway Human Cancer Risk Assessment. Environ. Sci. Technol. 2021, 55, 6773–6782. [Google Scholar] [CrossRef] [PubMed]
- Rahmani Sani, A.; Abroudi, M.; Heydari, H.; Adli, A.; Miri, M.; Mehrabadi, S.; Pajohanfar, N.S.; Raoufinia, R.; Bazghandi, M.S.; Ghalenovi, M.; et al. Maternal exposure to ambient particulate matter and green spaces and fetal renal function. Environ. Res. 2020, 184, 109285. [Google Scholar] [CrossRef] [PubMed]
- De Tata, V. Association of Dioxin and Other Persistent Organic Pollutants (POPs) with Diabetes: Epidemiological Evidence and New Mechanisms of Beta Cell Dysfunction. Int. J. Mol. Sci. 2014, 15, 7787–7811. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Wu, C.L.; Wu, J.S.; Chang, J.W.; Cheng, Y.Y.; Kuo, Y.C.; Yang, Y.C.; Lee, C.C.; Guo, H.R. Association between Blood Dioxin Level and Chronic Kidney Disease in an Endemic Area of Exposure. PLoS ONE 2016, 11, e0150248. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.B. Trends in concentrations of selected dioxins and furans across various stages of kidney function for US adults. Environ. Sci. Pollut. Res. 2021, 28, 43763–43776. [Google Scholar] [CrossRef]
- Ishida, M.; Mikami, S.; Shinojima, T.; Kosaka, T.; Mizuno, R.; Kikuchi, E.; Miyajima, A.; Okada, Y.; Oya, M. Activation of aryl hydrocarbon receptor promotes invasion of clear cell renal cell carcinoma and is associated with poor prognosis and cigarette smoke. Int. J. Cancer 2015, 137, 299–310. [Google Scholar] [CrossRef]
- Wang, Z.; Snyder, M.; Kenison, J.E.; Yang, K.; Lara, B.; Lydell, E.; Bennani, K.; Novikov, O.; Federico, A.; Monti, S.; et al. How the AHR Became Important in Cancer: The Role of Chronically Active AHR in Cancer Aggression. Int. J. Mol. Sci. 2021, 22, 387. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, L.; Yang, T.; Feng, Y.-L.; Vaziri, N.D.; Liu, B.-L.; Liu, Q.-Q.; Guo, Y.; Zhao, Y.-Y. Aryl hydrocarbon receptor activation mediates kidney disease and renal cell carcinoma. J. Transl. Med. 2019, 17, 302. [Google Scholar] [CrossRef]
- Fiorito, F.; Ciarcia, R.; Granato, G.E.; Marfe, G.; Iovane, V.; Florio, S.; De Martino, L.; Pagnini, U. 2,3,7,8-tetrachlorodibenzo-p-dioxin induced autophagy in a bovine kidney cell line. Toxicology 2011, 290, 258–270. [Google Scholar] [CrossRef]
- Makhloufi, C.; Nicolas, F.; McKay, N.; Fernandez, S.; Hache, G.; Garrigue, P.; Brunet, P.; Guillet, B.; Burtey, S.; Poitevin, S. Female AhR Knockout Mice Develop a Minor Renal Insufficiency in an Adenine-Diet Model of Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 2483. [Google Scholar] [CrossRef]
- Esteban, J.; Sánchez-Pérez, I.; Hamscher, G.; Miettinen, H.M.; Korkalainen, M.; Viluksela, M.; Pohjanvirta, R.; Håkansson, H. Role of aryl hydrocarbon receptor (AHR) in overall retinoid metabolism: Response comparisons to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure between wild-type and AHR knockout mice. Reprod. Toxicol. 2021, 101, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Siddarth, M.; Datta, S.K.; Ahmed, R.S.; Banerjee, B.D.; Kalra, O.P.; Tripathi, A.K. Association of CYP1A1 gene polymorphism with chronic kidney disease: A case control study. Environ. Toxicol. Pharmacol. 2013, 36, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Nishimura, N.; Vogel, C.F.; Tohyama, C.; Matsumura, F. TCDD-induced cyclooxygenase-2 expression is mediated by the nongenomic pathway in mouse MMDD1 macula densa cells and kidneys. Biochem. Pharmacol. 2010, 79, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Nebert, D.W.; Dalton, T.P.; Okey, A.B.; Gonzalez, F.J. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J. Biol. Chem. 2004, 279, 23847–23850. [Google Scholar] [CrossRef]
- Yoshioka, W.; Tohyama, C. Mechanisms of Developmental Toxicity of Dioxins and Related Compounds. Int. J. Mol. Sci. 2019, 20, 617. [Google Scholar] [CrossRef]
- Harrill, J.A.; Hukkanen, R.R.; Lawson, M.; Martin, G.; Gilger, B.; Soldatow, V.; LeCluyse, E.L.; Budinsky, R.A.; Rowlands, J.C.; Thomas, R.S. Knockout of the aryl hydrocarbon receptor results in distinct hepatic and renal phenotypes in rats and mice. Toxicol. Appl. Pharmacol. 2013, 272, 503–518. [Google Scholar] [CrossRef]
- Moriguchi, T.; Motohashi, H.; Hosoya, T.; Nakajima, O.; Takahashi, S.; Ohsako, S.; Aoki, Y.; Nishimura, N.; Tohyama, C.; Fujii-Kuriyama, Y.; et al. Distinct response to dioxin in an arylhydrocarbon receptor (AHR)-humanized mouse. Proc. Natl. Acad. Sci. USA 2003, 100, 5652–5657. [Google Scholar] [CrossRef]
- Boutros, P.C.; Bielefeld, K.A.; Pohjanvirta, R.; Harper, P.A. Dioxin-Dependent and Dioxin-Independent Gene Batteries: Comparison of Liver and Kidney in AHR-Null Mice. Toxicol. Sci. 2009, 112, 245–256. [Google Scholar] [CrossRef]
- Evich, M.G.; Davis, M.J.B.; McCord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.U.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J.; et al. Per- and polyfluoroalkyl substances in the environment. Science 2022, 375, eabg9065. [Google Scholar] [CrossRef]
- Fauconier, G.; Groffen, T.; Wepener, V.; Bervoets, L. Perfluorinated compounds in the aquatic food chains of two subtropical estuaries. Sci. Total Environ. 2020, 719, 135047. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- Jha, G.; Kankarla, V.; McLennon, E.; Pal, S.; Sihi, D.; Dari, B.; Diaz, D.; Nocco, M. Per- and Polyfluoroalkyl Substances (PFAS) in Integrated Crop-Livestock Systems: Environmental Exposure and Human Health Risks. Int. J. Environ. Res. Public Health 2021, 18, 12550. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.W.D.; Rice, J.S.; Nelson, K.D.; Vernon, C.R.; McManamay, R.; Dickson, K.; Marston, L. Comparison of potential drinking water source contamination across one hundred U.S. cities. Nat. Commun. 2021, 12, 7254. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ng, C. Absorption, distribution, and toxicity of per- and polyfluoroalkyl substances (PFAS) in the brain: A review. Environ. Sci. Process. Impacts 2021, 23, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, E.D.; Kleinman, M.T.; Bai, X.; Barlaz, M.; Abraczinskas, M.; Guidry, V.; Watson, J.; Chow, J. Critical review on PFOA, kidney cancer, and testicular cancer. J. Air Waste Manag. Assoc. 2021, 71, 1265–1276. [Google Scholar] [CrossRef]
- Voulgaropoulos, A. Mitigation of PFAS in U.S. Public Water Systems: Future steps for ensuring safer drinking water. Environ. Prog. Sustain. Energy 2022, 41, e13800. [Google Scholar] [CrossRef]
- Kudo, N.; Katakura, M.; Sato, Y.; Kawashima, Y. Sex hormone-regulated renal transport of perfluorooctanoic acid. Chem. Biol. Interact. 2002, 139, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Butenhoff, J.L.; Kennedy, G.L., Jr.; Hinderliter, P.M.; Lieder, P.H.; Jung, R.; Hansen, K.J.; Gorman, G.S.; Noker, P.E.; Thomford, P.J. Pharmacokinetics of Perfluorooctanoate in Cynomolgus Monkeys. Toxicol. Sci. 2004, 82, 394–406. [Google Scholar] [CrossRef]
- Perez, F.; Llorca, M.; Farré, M.; Barceló, D. Automated analysis of perfluorinated compounds in human hair and urine samples by turbulent flow chromatography coupled to tandem mass spectrometry. Anal. Bioanal. Chem. 2012, 402, 2369–2378. [Google Scholar] [CrossRef]
- Harada, K.; Inoue, K.; Morikawa, A.; Yoshinaga, T.; Saito, N.; Koizumi, A. Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species-specific excretion. Environ. Res. 2005, 99, 253–261. [Google Scholar] [CrossRef]
- Zhang, Y.; Beesoon, S.; Zhu, L.; Martin, J.W. Biomonitoring of Perfluoroalkyl Acids in Human Urine and Estimates of Biological Half-Life. Environ. Sci. Technol. 2013, 47, 10619–10627. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.W.; Mabury, S.A.; Solomon, K.R.; Muir, D.C. Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 2003, 22, 196–204. [Google Scholar] [CrossRef]
- Sun, J.M.; Kelly, B.C.; Gobas, F.; Sunderland, E.M. A food web bioaccumulation model for the accumulation of per- and polyfluoroalkyl substances (PFAS) in fish: How important is renal elimination? Environ. Sci. Process. Impacts 2022, 24, 1152–1164. [Google Scholar] [CrossRef] [PubMed]
- Schultes, L.; van Noordenburg, C.; Spaan, K.M.; Plassmann, M.M.; Simon, M.; Roos, A.; Benskin, J.P. High Concentrations of Unidentified Extractable Organofluorine Observed in Blubber from a Greenland Killer Whale (Orcinus orca). Environ. Sci. Technol. Lett. 2020, 7, 909–915. [Google Scholar] [CrossRef]
- Van de Vijver, K.I.; Holsbeek, L.; Das, K.; Blust, R.; Joiris, C.; De Coen, W. Occurrence of perfluorooctane sulfonate and other perfluorinated alkylated substances in harbor porpoises from the Black Sea. Environ. Sci. Technol. 2007, 41, 315–320. [Google Scholar] [CrossRef]
- Van de Vijver, K.I.; Hoff, P.; Das, K.; Brasseur, S.; Van Dongen, W.; Esmans, E.; Reijnders, P.; Blust, R.; De Coen, W. Tissue distribution of perfluorinated chemicals in harbor seals (Phoca vitulina) from the Dutch Wadden Sea. Environ. Sci. Technol. 2005, 39, 6978–6984. [Google Scholar] [CrossRef]
- Dassuncao, C.; Pickard, H.; Pfohl, M.; Tokranov, A.K.; Li, M.; Mikkelsen, B.; Slitt, A.; Sunderland, E.M. Phospholipid Levels Predict the Tissue Distribution of Poly- and Perfluoroalkyl Substances in a Marine Mammal. Environ. Sci. Technol. Lett. 2019, 6, 119–125. [Google Scholar] [CrossRef]
- Holmström, K.E.; Berger, U. Tissue distribution of perfluorinated surfactants in common guillemot (Uria aalge) from the Baltic Sea. Environ. Sci. Technol. 2008, 42, 5879–5884. [Google Scholar] [CrossRef]
- Aas, C.B.; Fuglei, E.; Herzke, D.; Yoccoz, N.G.; Routti, H. Effect of body condition on tissue distribution of perfluoroalkyl substances (PFASs) in Arctic fox (Vulpes lagopus). Environ. Sci. Technol. 2014, 48, 11654–11661. [Google Scholar] [CrossRef]
- Abraham, K.; El-Khatib, A.H.; Schwerdtle, T.; Monien, B.H. Perfluorobutanoic acid (PFBA): No high-level accumulation in human lung and kidney tissue. Int. J. Hyg. Environ. Health 2021, 237, 113830. [Google Scholar] [CrossRef]
- Wang, L.-Q.; Liu, T.; Yang, S.; Sun, L.; Zhao, Z.-Y.; Li, L.-Y.; She, Y.-C.; Zheng, Y.-Y.; Ye, X.-Y.; Bao, Q.; et al. Perfluoroalkyl substance pollutants activate the innate immune system through the AIM2 inflammasome. Nat. Commun. 2021, 12, 2915. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jurkovic-Mlakar, S.; Li, Y.; Wahlberg, K.; Scott, K.; Pineda, D.; Lindh, C.H.; Jakobsson, K.; Engström, K. Association between serum concentrations of perfluoroalkyl substances (PFAS) and expression of serum microRNAs in a cohort highly exposed to PFAS from drinking water. Environ. Int. 2020, 136, 105446. [Google Scholar] [CrossRef] [PubMed]
- Rashid, F.; Ramakrishnan, A.; Fields, C.; Irudayaraj, J. Acute PFOA exposure promotes epigenomic alterations in mouse kidney tissues. Toxicol. Rep. 2020, 7, 125–132. [Google Scholar] [CrossRef]
- Wen, Y.; Mirji, N.; Irudayaraj, J. Epigenetic toxicity of PFOA and GenX in HepG2 cells and their role in lipid metabolism. Toxicol. In Vitro 2020, 65, 104797. [Google Scholar] [CrossRef]
- Jabeen, M.; Fayyaz, M.; Irudayaraj, J. Epigenetic Modifications, and Alterations in Cell Cycle and Apoptosis Pathway in A549 Lung Carcinoma Cell Line upon Exposure to Perfluoroalkyl Substances. Toxics 2020, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Wen, Y.; Irudayaraj, J.M.K. PFOA induces alteration in DNA methylation regulators and SARS-CoV-2 targets Ace2 and Tmprss2 in mouse lung tissues. Toxicol. Rep. 2021, 8, 1892–1898. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Wang, F.; Wang, R.; Zhang, S.; Zhang, Z.; Li, P.; Yao, J.; Bi, J.; He, J.; et al. Associations between serum PFOA and PFOS levels and incident chronic kidney disease risk in patients with type 2 diabetes. Ecotoxicol. Environ. Saf. 2022, 229, 113060. [Google Scholar] [CrossRef]
- Nielsen, C.; Andersson Hall, U.; Lindh, C.; Ekström, U.; Xu, Y.; Li, Y.; Holmäng, A.; Jakobsson, K. Pregnancy-induced changes in serum concentrations of perfluoroalkyl substances and the influence of kidney function. Environ. Health 2020, 19, 80. [Google Scholar] [CrossRef]
- Erdal, H.; Sungur, S.; Koroglu, M.; Turgut, F. Determination of Serum Perfluorooctanoic Acid and Perfluorooctanesulfonic Acid Levels with Different Stages of Chronic Kidney Disease. Saudi J. Kidney Dis. Transpl. 2021, 32, 1664–1670. [Google Scholar] [CrossRef]
- Watkins, D.J.; Josson, J.; Elston, B.; Bartell, S.M.; Shin, H.M.; Vieira, V.M.; Savitz, D.A.; Fletcher, T.; Wellenius, G.A. Exposure to perfluoroalkyl acids and markers of kidney function among children and adolescents living near a chemical plant. Environ. Health Perspect. 2013, 121, 625–630. [Google Scholar] [CrossRef]
- Blake, B.E.; Pinney, S.M.; Hines, E.P.; Fenton, S.E.; Ferguson, K.K. Associations between longitudinal serum perfluoroalkyl substance (PFAS) levels and measures of thyroid hormone, kidney function, and body mass index in the Fernald Community Cohort. Environ. Pollut. 2018, 242, 894–904. [Google Scholar] [CrossRef]
- Conway, B.N.; Badders, A.N.; Costacou, T.; Arthur, J.M.; Innes, K.E. Perfluoroalkyl substances and kidney function in chronic kidney disease, anemia, and diabetes. Diabetes Metab. Syndr. Obes. 2018, 11, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.B.; Ducatman, A. Dynamics of associations between perfluoroalkyl substances and uric acid across the various stages of glomerular function. Environ. Sci. Pollut. Res. Int. 2019, 26, 12425–12434. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.B. Impact of kidney hyperfiltration on concentrations of selected perfluoroalkyl acids among US adults for various disease groups. Environ. Sci. Pollut. Res. Int. 2021, 28, 21499–21515. [Google Scholar] [CrossRef]
- Park, S.K.; Wang, X.; Ding, N.; Karvonen-Gutierrez, C.A.; Calafat, A.M.; Herman, W.H.; Mukherjee, B.; Harlow, S.D. Per- and polyfluoroalkyl substances and incident diabetes in midlife women: The Study of Women’s Health Across the Nation (SWAN). Diabetologia 2022, 65, 1157–1168. [Google Scholar] [CrossRef]
- Chang, E.T.; Adami, H.O.; Boffetta, P.; Cole, P.; Starr, T.B.; Mandel, J.S. A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and cancer risk in humans. Crit. Rev. Toxicol. 2014, 44 (Suppl. 1), 1–81. [Google Scholar] [CrossRef]
- Fenner, A. Is PFOA a renal carcinogen? Nat. Rev. Urol. 2020, 17, 602. [Google Scholar] [CrossRef]
- Shearer, J.J.; Callahan, C.L.; Calafat, A.M.; Huang, W.Y.; Jones, R.R.; Sabbisetti, V.S.; Freedman, N.D.; Sampson, J.N.; Silverman, D.T.; Purdue, M.P.; et al. Serum Concentrations of Per- and Polyfluoroalkyl Substances and Risk of Renal Cell Carcinoma. J. Natl. Cancer Inst. 2021, 113, 580–587. [Google Scholar] [CrossRef]
- Steenland, K.; Hofmann, J.N.; Silverman, D.T.; Bartell, S.M. Risk assessment for PFOA and kidney cancer based on a pooled analysis of two studies. Environ. Int. 2022, 167, 107425. [Google Scholar] [CrossRef]
- Bartell, S.M.; Vieira, V.M. Critical review on PFOA, kidney cancer, and testicular cancer. J. Air Waste Manag. Assoc. 2021, 71, 663–679. [Google Scholar] [CrossRef]
- Boyd, R.I.; Ahmad, S.; Singh, R.; Fazal, Z.; Prins, G.S.; Madak Erdogan, Z.; Irudayaraj, J.; Spinella, M.J. Toward a Mechanistic Understanding of Poly- and Perfluoroalkylated Substances and Cancer. Cancers 2022, 14, 2919. [Google Scholar] [CrossRef]
- Hu, W.; Jones, P.D.; Upham, B.L.; Trosko, J.E.; Lau, C.; Giesy, J.P. Inhibition of gap junctional intercellular communication by perfluorinated compounds in rat liver and dolphin kidney epithelial cell lines in vitro and Sprague-Dawley rats in vivo. Toxicol. Sci. 2002, 68, 429–436. [Google Scholar] [CrossRef]
- Dzierlenga, A.L.; Robinson, V.G.; Waidyanatha, S.; DeVito, M.J.; Eifrid, M.A.; Gibbs, S.T.; Granville, C.A.; Blystone, C.R. Toxicokinetics of perfluorohexanoic acid (PFHxA), perfluorooctanoic acid (PFOA) and perfluorodecanoic acid (PFDA) in male and female Hsd:Sprague dawley SD rats following intravenous or gavage administration. Xenobiotica 2020, 50, 722–732. [Google Scholar] [CrossRef]
- Gwinn, W.M.; Auerbach, S.S.; Parham, F.; Stout, M.D.; Waidyanatha, S.; Mutlu, E.; Collins, B.; Paules, R.S.; Merrick, B.A.; Ferguson, S.; et al. Evaluation of 5-day In Vivo Rat Liver and Kidney With High-throughput Transcriptomics for Estimating Benchmark Doses of Apical Outcomes. Toxicol. Sci. 2020, 176, 343–354. [Google Scholar] [CrossRef]
- Stanifer, J.W.; Stapleton, H.M.; Souma, T.; Wittmer, A.; Zhao, X.; Boulware, L.E. Perfluorinated Chemicals as Emerging Environmental Threats to Kidney Health: A Scoping Review. Clin. J. Am. Soc. Nephrol. 2018, 13, 1479–1492. [Google Scholar] [CrossRef]
- Gorrochategui, E.; Lacorte, S.; Tauler, R.; Martin, F.L. Perfluoroalkylated Substance Effects in Xenopus laevis A6 Kidney Epithelial Cells Determined by ATR-FTIR Spectroscopy and Chemometric Analysis. Chem. Res. Toxicol. 2016, 29, 924–932. [Google Scholar] [CrossRef]
- Kemper, R.A.; Nabb, D.L. In vitro studies in microsomes from rat and human liver, kidney, and intestine suggest that perfluorooctanoic acid is not a substrate for microsomal UDP-glucuronosyltransferases. Drug Chem. Toxicol. 2005, 28, 281–287. [Google Scholar] [CrossRef]
- Wen, Y.; Rashid, F.; Fazal, Z.; Singh, R.; Spinella, M.J.; Irudayaraj, J. Nephrotoxicity of perfluorooctane sulfonate (PFOS)-effect on transcription and epigenetic factors. Environ. Epigenet. 2022, 8, dvac010. [Google Scholar] [CrossRef]
- Daniels, R.D.; Kubale, T.L.; Yiin, J.H.; Dahm, M.M.; Hales, T.R.; Baris, D.; Zahm, S.H.; Beaumont, J.J.; Waters, K.M.; Pinkerton, L.E. Mortality and cancer incidence in a pooled cohort of US firefighters from San Francisco, Chicago and Philadelphia (1950–2009). Occup. Environ. Med. 2014, 71, 388. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, K.; Li, X.; Tang, Z.; Xiang, L.; Zhao, H.; Fu, J.; Wang, L.; Zhu, N.; Cai, Z.; et al. Mass Spectrometry-Based Metabolomics Reveals Occupational Exposure to Per- and Polyfluoroalkyl Substances Relates to Oxidative Stress, Fatty Acid β-Oxidation Disorder, and Kidney Injury in a Manufactory in China. Environ. Sci. Technol. 2019, 53, 9800–9809. [Google Scholar] [CrossRef]
- Kafkoutsou, A.L.; Yang, Y.P.; Zeynaloo, E.; Deo, S.K.; Solle, N.S.; Kobetz, E.N.; Daunert, S.; Caban-Martinez, A.J. Impact of Firefighting Aqueous Film-Forming Foams on Human Cell Proliferation and Cellular Mortality. J. Occup. Environ. Med. 2022, 64, e340–e344. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.E.; Barnett, L.M.A.; Cummings, B.S. Nephrotoxicity of flame retardants: An understudied but critical toxic endpoint. Curr. Opin. Toxicol. 2022, 32, 100359. [Google Scholar] [CrossRef]
- LeMasters, G.K.; Genaidy, A.M.; Succop, P.; Deddens, J.; Sobeih, T.; Barriera-Viruet, H.; Dunning, K.; Lockey, J. Cancer Risk Among Firefighters: A Review and Meta-analysis of 32 Studies. J. Occup. Environ. Med. 2006, 48, 1189–1202. [Google Scholar] [CrossRef]
- Trowbridge, J.; Gerona, R.R.; Lin, T.; Rudel, R.A.; Bessonneau, V.; Buren, H.; Morello-Frosch, R. Exposure to Perfluoroalkyl Substances in a Cohort of Women Firefighters and Office Workers in San Francisco. Environ. Sci. Technol. 2020, 54, 3363–3374. [Google Scholar] [CrossRef]
- Hu, X.C.; Andrews, D.Q.; Lindstrom, A.B.; Bruton, T.A.; Schaider, L.A.; Grandjean, P.; Lohmann, R.; Carignan, C.C.; Blum, A.; Balan, S.A.; et al. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett. 2016, 3, 344–350. [Google Scholar] [CrossRef]
- İyİgÜndoĞdu, İ.; ÜstÜndaĞ, A.; Duydu, Y. Toxicological Evaluation of Bisphenol A and Its Analogues. Turk. J. Pharm. Sci. 2020, 17, 457–462. [Google Scholar] [CrossRef]
- Efsa Panel on Food Contact Materials, E.F.; Processing, A. Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- Loizou, G.; McNally, K.; Paini, A.; Hogg, A. Derivation of a Human In Vivo Benchmark Dose for Bisphenol A from ToxCast In Vitro Concentration Response Data Using a Computational Workflow for Probabilistic Quantitative In Vitro to In Vivo Extrapolation. Front. Pharm. 2021, 12, 754408. [Google Scholar] [CrossRef]
- Moreno-Gómez-Toledano, R. Relationship between emergent BPA-substitutes and renal and cardiovascular diseases in adult population. Environ. Pollut. 2022, 313, 120106. [Google Scholar] [CrossRef]
- Völkel, W.; Colnot, T.; Csanády, G.A.; Filser, J.G.; Dekant, W. Metabolism and Kinetics of Bisphenol A in Humans at Low Doses Following Oral Administration. Chem. Res. Toxicol. 2002, 15, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Doerge, D.R.; Twaddle, N.C.; Vanlandingham, M.; Brown, R.P.; Fisher, J.W. Distribution of bisphenol A into tissues of adult, neonatal, and fetal Sprague–Dawley rats. Toxicol. Appl. Pharmacol. 2011, 255, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Doerge, D.R.; Twaddle, N.C.; Woodling, K.A.; Fisher, J.W. Pharmacokinetics of bisphenol A in neonatal and adult rhesus monkeys. Toxicol. Appl. Pharmacol. 2010, 248, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gómez-Toledano, R.; Arenas, M.I.; Vélez-Vélez, E.; Saura, M.; Bosch, R.J. New Evidence of Renal and Cardiovascular Alterations Promoted by Bisphenol A. Biomolecules 2021, 11, 1649. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zhu, X.; Shrubsole, M.J.; Fan, H.; Chen, J.; Dong, J.; Hao, C.M.; Dai, Q. Renal function, bisphenol A, and alkylphenols: Results from the National Health and Nutrition Examination Survey (NHANES 2003-2006). Environ. Health Perspect. 2011, 119, 527–533. [Google Scholar] [CrossRef]
- Krieter, D.H.; Canaud, B.; Lemke, H.D.; Rodriguez, A.; Morgenroth, A.; von Appen, K.; Dragoun, G.P.; Wanner, C. Bisphenol A in chronic kidney disease. Artif. Organs 2013, 37, 283–290. [Google Scholar] [CrossRef]
- Nicholson, T.M.; Nguyen, J.L.; Leverson, G.E.; Taylor, J.A.; Vom Saal, F.S.; Wood, R.W.; Ricke, W.A. Endocrine disruptor bisphenol A is implicated in urinary voiding dysfunction in male mice. Am. J. Physiol. Ren. Physiol. 2018, 315, F1208–F1216. [Google Scholar] [CrossRef]
- Jacobson, M.H.; Wu, Y.; Liu, M.; Attina, T.M.; Naidu, M.; Karthikraj, R.; Kannan, K.; Warady, B.A.; Furth, S.; Vento, S.; et al. Serially assessed bisphenol A and phthalate exposure and association with kidney function in children with chronic kidney disease in the US and Canada: A longitudinal cohort study. PLoS Med. 2020, 17, e1003384. [Google Scholar] [CrossRef]
- Abbas, M.A.M.; Elmetwally, S.A.F.; Mokhtar Abo-Elfotoh, M.A. Effect of Oral Exposure to Bisphenol A on the Liver and Kidney of Adult Male Albino Rats. Int. J. Med. Arts 2021, 3, 930–937. [Google Scholar] [CrossRef]
- Hussein, A. Histopathological study of lung, kidney, spleen and prostate in adult male rats treated with Bisphenol A. Basrah J. Vet. Res. 2015, 14, 74–86. [Google Scholar]
- Hoque, E.; Sujan, K.M.; Mia, M.S.; Haque, M.I.; Mustari, A.; Miah, M.A.; Islam, M.K. Effects of bisphenol-A (BPA) on body weight, hematological parameters and histo-texture of kidney in swiss albino mice. Asian J. Med. Biol. Res. 2021, 6, 635–640. [Google Scholar] [CrossRef]
- Shaimaa, H.A.; Marwa, M.A. Histopathological Changes Produced by Bispenol A in the Renal Cortex of Adult Male Albino Rats. Med. J. Cairo Univ. 2019, 87, 2045–2058. [Google Scholar] [CrossRef]
- Yıldız, N.; Barlas, N. Hepatic and renal functions in growing male rats after bisphenol A and octylphenol exposure. Hum. Exp. Toxicol. 2013, 32, 675–686. [Google Scholar] [CrossRef]
- Taylor, J.A.; Jones, M.B.; Besch-Williford, C.L.; Berendzen, A.F.; Ricke, W.A.; Vom Saal, F.S. Interactive Effects of Perinatal BPA or DES and Adult Testosterone and Estradiol Exposure on Adult Urethral Obstruction and Bladder, Kidney, and Prostate Pathology in Male Mice. Int. J. Mol. Sci. 2020, 21, 3902. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, P.; Fernandez, T.; García-Arévalo, M.; Alonso-Magdalena, P.; Nadal, A.; Perillan, C.; Arguelles, J. Effects of bisphenol A treatment during pregnancy on kidney development in mice: A stereological and histopathological study. J. Dev. Orig. Health Dis. 2018, 9, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Elshaer, F.; Abu-Shaeir, W.; Bakry, S. Histopathological changes in the Kidney of mosquito fish, Gambusia affinis and guppy fish, Poecilia reticulata exposed to Bisphenol A. Egypt. J. Aquat. Biol. Fish. 2013, 17, 83–93. [Google Scholar] [CrossRef][Green Version]
- Moreno-Gómez-Toledano, R.; Arenas, M.I.; Vélez-Vélez, E.; Coll, E.; Quiroga, B.; Bover, J.; Bosch, R.J. Bisphenol a Exposure and Kidney Diseases: Systematic Review, Meta-Analysis, and NHANES 03-16 Study. Biomolecules 2021, 11, 1046. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Xiang, X.; Peng, C.; Gao, R.; Goswami, R.; Zhou, H.; Zhang, Y.; Zhen, Q.; Cheng, Q.; et al. Serum bisphenol A as a predictor of chronic kidney disease progression in primary hypertension: A 6-year prospective study. J. Hypertens. 2016, 34, 332–337. [Google Scholar] [CrossRef]
- Jain, R.B. Concentrations of bisphenol A and its associations with urinary albumin creatinine ratios across the various stages of renal function. Environ. Sci. Pollut. Res. Int. 2021, 28, 9946–9953. [Google Scholar] [CrossRef]
- Mahfouz, N.; Salah, E.; Armaneous, A.; Youssef, M.M.; Abu Shady, M.M.; Sallam, S.; Anwar, M.; Morsy, S.; Hussein, J. Association between Bisphenol A Urine Level with Low-Grade Albuminuria in Egyptian Children and Adolescents. Open Access Maced. J. Med. Sci. 2021, 9, 1092–1097. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, T.; Shi, Y.; Zhuang, F.; Lu, J.; Zhu, Q.; Ding, F. Bisphenol A analogs in patients with chronic kidney disease and dialysis therapy. Ecotoxicol. Environ. Saf. 2019, 185, 109684. [Google Scholar] [CrossRef]
- Kundakovic, M.; Gudsnuk, K.; Franks, B.; Madrid, J.; Miller, R.L.; Perera, F.P.; Champagne, F.A. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc. Natl. Acad. Sci. USA 2013, 110, 9956–9961. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Wang, F.; Zhang, Y.; Zhang, S.; Han, X.; Zhang, X.; Guo, H.; He, M. Associations of serum bisphenol A levels with incident chronic kidney disease risk. Sci. Total Environ. 2021, 771, 145401. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lim, Y.H.; Shin, C.H.; Kim, B.N.; Kim, J.I.; Hong, Y.C.; Cho, Y.M.; Lee, Y.A. Relationship between bisphenol A, bisphenol S, and bisphenol F and serum uric acid concentrations among school-aged children. PLoS ONE 2022, 17, e0268503. [Google Scholar] [CrossRef] [PubMed]
- Malits, J.; Attina, T.M.; Karthikraj, R.; Kannan, K.; Naidu, M.; Furth, S.; Warady, B.A.; Vento, S.; Trachtman, H.; Trasande, L. Renal Function and exposure to Bisphenol A and phthalates in children with Chronic Kidney Disease. Environ. Res. 2018, 167, 575–582. [Google Scholar] [CrossRef]
- Gowder, S.J. Nephrotoxicity of bisphenol A (BPA)—An updated review. Curr. Mol. Pharm. 2013, 6, 163–172. [Google Scholar] [CrossRef]
- Yuan, J.; Kong, Y.; Ommati, M.M.; Tang, Z.; Li, H.; Li, L.; Zhao, C.; Shi, Z.; Wang, J. Bisphenol A-induced apoptosis, oxidative stress and DNA damage in cultured rhesus monkey embryo renal epithelial Marc-145 cells. Chemosphere 2019, 234, 682–689. [Google Scholar] [CrossRef]
- Mourad, I.M.; Khadrawy, Y.A. The sensetivity of liver, kidney andtestis of rats to oxidative stress induced by different doses of bisphenol A. Life 2012, 50, 19. [Google Scholar]
- Zhang, R.; Liu, R.; Zong, W. Bisphenol S Interacts with Catalase and Induces Oxidative Stress in Mouse Liver and Renal Cells. J. Agric. Food Chem. 2016, 64, 6630–6640. [Google Scholar] [CrossRef]
- Kobroob, A.; Peerapanyasut, W.; Chattipakorn, N.; Wongmekiat, O. Damaging Effects of Bisphenol A on the Kidney and the Protection by Melatonin: Emerging Evidences from In Vivo and In Vitro Studies. Oxid. Med. Cell. Longev. 2018, 2018, 3082438. [Google Scholar] [CrossRef]
- Shi, R.; Liu, Z.; Liu, T. The antagonistic effect of bisphenol A and nonylphenol on liver and kidney injury in rats. Immunopharmacol. Immunotoxicol. 2021, 43, 527–535. [Google Scholar] [CrossRef]
- Dökmeci, A.H.; Karaboğa, İ.; Güzel, S.; Erboğa, Z.F.; Yılmaz, A. Toxicological assessment of low-dose bisphenol A, lead and endosulfan combination: Chronic toxicity study in male rats. Environ. Sci. Pollut. Res. Int. 2022, 29, 10558–10574. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.C.; Huang, J.K.; Chou, C.T.; Cheng, J.S.; Tsai, J.Y.; Fang, Y.C.; Hsu, S.S.; Liao, W.C.; Chang, H.T.; Ho, C.M.; et al. Effect of bisphenol A on Ca2+ fluxes and viability in Madin-Darby canine renal tubular cells. Drug Chem. Toxicol. 2011, 34, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, M.; Andresen, A.M.; Gjøen, T. Short-term effect of bisphenol-a on oxidative stress responses in Atlantic salmon kidney cell line: A transcriptional study. Toxicol. Mech. Methods 2016, 26, 295–300. [Google Scholar] [CrossRef]
- Moreno-Gómez-Toledano, R.; Arenas, M.I.; Muñoz-Moreno, C.; Olea-Herrero, N.; Reventun, P.; Izquierdo-Lahuerta, A.; Antón-Cornejo, A.; González-Santander, M.; Zaragoza, C.; Saura, M.; et al. Comparison of the renal effects of bisphenol A in mice with and without experimental diabetes. Role of sexual dimorphism. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166296. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Yang, S.; Li, T.; Gao, R.; Hu, J.; Luo, T.; Qing, H.; Zhen, Q.; Hu, R.; Li, X.; et al. Role of neutrophil extracellular traps in chronic kidney injury induced by bisphenol-A. J. Endocrinol. 2019, 241, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Nahar, M.S.; Liao, C.; Kannan, K.; Harris, C.; Dolinoy, D.C. In utero bisphenol A concentration, metabolism, and global DNA methylation across matched placenta, kidney, and liver in the human fetus. Chemosphere 2015, 124, 54–60. [Google Scholar] [CrossRef]
- TuzcuoĞlu, P.; Özden, S. Global DNA Hypomethylation and Rassf1a and c-myc Promoter Hypermethylation in Rat Kidney Cells after Bisphenol A Exposure. Turk. J. Pharm. Sci. 2020, 17, 337–342. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Z.; Liu, H.; Jia, L.; Qin, M.; Wang, X. Exacerbating lupus nephritis following BPA exposure is associated with abnormal autophagy in MRL/lpr mice. Am. J. Transl. Res. 2020, 12, 649–659. [Google Scholar]
- Priego, A.R.; Parra, E.G.; Mas, S.; Morgado-Pascual, J.L.; Ruiz-Ortega, M.; Rayego-Mateos, S. Bisphenol A Modulates Autophagy and Exacerbates Chronic Kidney Damage in Mice. Int. J. Mol. Sci. 2021, 22, 7189. [Google Scholar] [CrossRef]
- Charaya, A.; Sahu, C.; Singla, S.; Jena, G. Zinc Deficiency Exacerbates Bisphenol A-Induced Hepatic and Renal Damage: Delineation of Molecular Mechanisms. Biol. Trace Elem. Res. 2022, 1–16. [Google Scholar] [CrossRef]
- Yoo, M.H.; Lee, S.J.; Kim, W.; Kim, Y.; Kim, Y.B.; Moon, K.S.; Lee, B.S. Bisphenol A impairs renal function by reducing Na(+)/K(+)-ATPase and F-actin expression, kidney tubule formation in vitro and in vivo. Ecotoxicol. Environ. Saf. 2022, 246, 114141. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, Y.; Qi, X.; Shi, X.; Huang, X.; Xu, S.W. Selenium deficiency aggravates bisphenol A-induced autophagy in chicken kidney through regulation of nitric oxide and adenosine monophosphate activated protein kinase/mammalian target of rapamycin signaling pathway. Environ. Toxicol. 2022, 37, 2503–2514. [Google Scholar] [CrossRef] [PubMed]
- Peerapanyasut, W.; Kobroob, A.; Palee, S.; Chattipakorn, N.; Wongmekiat, O. Bisphenol A aggravates renal ischemia-reperfusion injury by disrupting mitochondrial homeostasis and N-acetylcysteine mitigates the injurious outcomes. IUBMB Life 2020, 72, 758–770. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Zou, M.; Qi, X.; Xu, S. Bisphenol A aggravates renal apoptosis and necroptosis in selenium-deficient chickens via oxidative stress and PI3K/AKT pathway. J. Cell Physiol. 2022, 237, 3292–3304. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Zhao, C.; Feng, L.; Zhao, Y.; Duan, S.; Qiu, M.; Wu, K.; Zhang, N.; Hu, X.; Fu, Y. Ferritinophagy is involved in Bisphenol A-induced ferroptosis of renal tubular epithelial cells through the activation of the AMPK-mTOR-ULK1 pathway. Food Chem. Toxicol. 2022, 163, 112909. [Google Scholar] [CrossRef]
- Esplugas, R.; MI, L.L.; Bellés, M.; Serra, N.; Vallvé, J.C.; Domingo, J.L.; Linares, V. Renal and hepatic effects following neonatal exposure to low doses of Bisphenol-A and (137)Cs. Food Chem. Toxicol. 2018, 114, 270–277. [Google Scholar] [CrossRef]
- Huber, W.W.; Grasl-kraupp, B.; Schulte-hermann, R. Hepatocarcinogenic Potential of Di(2-Ethylhexyl)phthalate in Rodents and its Implications on Human Risk. Crit. Rev. Toxicol. 1996, 26, 365–481. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, H. Phthalates and Their Impacts on Human Health. Healthcare 2021, 9, 603. [Google Scholar] [CrossRef]
- Chang, W.-H.; Herianto, S.; Lee, C.-C.; Hung, H.; Chen, H.-L. The effects of phthalate ester exposure on human health: A review. Sci. Total Environ. 2021, 786, 147371. [Google Scholar] [CrossRef]
- Praveena, S.M.; Siok Fong, C.; Amaruddin, A.F. Phthalates in children toys available in Malaysian market: Quantification and potential human health risk. J. Steroid Biochem. Mol. Biol. 2021, 213, 105955. [Google Scholar] [CrossRef] [PubMed]
- Hannon, P.R.; Brannick, K.E.; Wang, W.; Flaws, J.A. Mono(2-Ethylhexyl) Phthalate Accelerates Early Folliculogenesis and Inhibits Steroidogenesis in Cultured Mouse Whole Ovaries and Antral Follicles1. Biol. Reprod. 2015, 92, 120; 121–111. [Google Scholar] [CrossRef] [PubMed]
- Hannon, P.R.; Brannick, K.E.; Wang, W.; Gupta, R.K.; Flaws, J.A. Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol. Appl. Pharmacol. 2015, 284, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.J.; Wu, P.Y.; Huang, J.C.; Chen, S.C. Environmental Pollution and Chronic Kidney Disease. Int. J. Med. Sci. 2021, 18, 1121–1129. [Google Scholar] [CrossRef]
- Crocker, J.F.S.; Safe, S.H.; Acott, P. Effects of chronic phthalate exposure on the kidney. J. Toxicol. Environ. Health 1988, 23, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Gao, M.; Zhang, W.; Yan, L.; Shao, F.; Zhou, J. Exposure to phthalates DEHP and DINP May lead to oxidative damage and lipidomic disruptions in mouse kidney. Chemosphere 2021, 271, 129740. [Google Scholar] [CrossRef]
- Zeng, Q.; Wei, C.; Wu, Y.; Li, K.; Ding, S.; Yuan, J.; Yang, X.; Chen, M. Approach to distribution and accumulation of dibutyl phthalate in rats by immunoassay. Food Chem. Toxicol. 2013, 56, 18–27. [Google Scholar] [CrossRef]
- McKee, R.H.; El-Hawari, M.; Stoltz, M.; Pallas, F.; Lington, A.W. Absorption, disposition and metabolism of di-isononyl phthalate (DINP) in F-344 rats. J. Appl. Toxicol. 2002, 22, 293–302. [Google Scholar] [CrossRef]
- Camacho, L.; Latendresse, J.R.; Muskhelishvili, L.; Law, C.D.; Delclos, K.B. Effects of intravenous and oral di(2-ethylhexyl) phthalate (DEHP) and 20% Intralipid vehicle on neonatal rat testis, lung, liver, and kidney. Food Chem. Toxicol. 2020, 144, 111497. [Google Scholar] [CrossRef]
- Arenas, I.A. Invited Perspective: Phthalates and Blood Pressure: The Unknowns of Dietary Factors. Environ. Health Perspect. 2021, 129, 121303. [Google Scholar] [CrossRef]
- Melnick, R.L.; Ward, J.M.; Huff, J. War on Carcinogens: Industry disputes human relevance of chemicals causing cancer in laboratory animals based on unproven hypotheses, using kidney tumors as an example. Int. J. Occup. Environ. Health 2013, 19, 255–260. [Google Scholar] [CrossRef][Green Version]
- Pugh, G., Jr.; Isenberg, J.S.; Kamendulis, L.M.; Ackley, D.C.; Clare, L.J.; Brown, R.; Lington, A.W.; Smith, J.H.; Klaunig, J.E. Effects of Di-isononyl Phthalate, Di-2-ethylhexyl Phthalate, and Clofibrate in Cynomolgus Monkeys. Toxicol. Sci. 2000, 56, 181–188. [Google Scholar] [CrossRef]
- Nagao, T.; Ohta, R.; Marumo, H.; Shindo, T.; Yoshimura, S.; Ono, H. Effect of butyl benzyl phthalate in Sprague-Dawley rats after gavage administration: A two-generation reproductive study. Reprod. Toxicol. 2000, 14, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Jiang, J.-T.; Li, D.; Zhu, Y.-P.; Xia, S.-J.; Han, B.-M. Maternal exposure to di-n-butyl phthalate promotes Snail1-mediated epithelial-mesenchymal transition of renal tubular epithelial cells via upregulation of TGF-β1 during renal fibrosis in rat offspring. Ecotoxicol. Environ. Saf. 2019, 169, 266–272. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.; Zhang, X.; Li, A.; Cheng, S. Metabolic profiles in serum of mouse after chronic exposure to drinking water. Hum. Exp. Toxicol. 2011, 30, 1088–1095. [Google Scholar] [CrossRef]
- Tsai, H.J.; Kuo, F.C.; Wu, C.F.; Sun, C.W.; Hsieh, C.J.; Wang, S.L.; Chen, M.L.; Hsieh, H.M.; Chuang, Y.S.; Wu, M.T. Association between two common environmental toxicants (phthalates and melamine) and urinary markers of renal injury in the third trimester of pregnant women: The Taiwan Maternal and Infant Cohort Study (TMICS). Chemosphere 2021, 272, 129925. [Google Scholar] [CrossRef]
- Chen, C.C.; Wang, Y.H.; Wu, C.F.; Hsieh, C.J.; Wang, S.L.; Chen, M.L.; Tsai, H.J.; Li, S.S.; Liu, C.C.; Tsai, Y.C.; et al. Benchmark dose in the presence of coexposure to melamine and diethylhexyl phthalate and urinary renal injury markers in pregnant women. Environ. Res. 2022, 215, 114187. [Google Scholar] [CrossRef]
- Kang, H.; Kim, S.; Lee, G.; Lee, I.; Lee, J.P.; Lee, J.; Park, H.; Moon, H.B.; Park, J.; Kim, S.; et al. Urinary metabolites of dibutyl phthalate and benzophenone-3 are potential chemical risk factors of chronic kidney function markers among healthy women. Environ. Int. 2019, 124, 354–360. [Google Scholar] [CrossRef]
- Chen, J.; Shi, X.; Zhou, X.; Dong, R.; Yuan, Y.; Wu, M.; Chen, W.; Liu, X.; Jia, F.; Li, S.; et al. Renal function and the exposure to melamine and phthalates in Shanghai adults. Chemosphere 2020, 246, 125820. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, L.; Liu, L.; Guo, W.; Yang, H.; Chen, S.; Yu, J.; Li, M.; Fang, Q.; Lai, X.; et al. Urinary phthalate metabolites mixture, serum cytokines and renal function in children: A panel study. J. Hazard. Mater. 2022, 422, 126963. [Google Scholar] [CrossRef]
- Liu, S.; Yang, R.; Yang, Q.; He, G.; Chen, B.; Dong, R. The independent and interactive effects of phthalates exposure and hypertension on the indicators of early renal injury in US adults: Evidence from NHANES 2001-2004. Environ. Res. 2022, 213, 113733. [Google Scholar] [CrossRef]
- Cheng, L.; Li, J.; Cheng, J.; Wu, Z. Dibutyl phthalate-induced activation of ROS and ERK1/2 causes hepatic and renal damage in Kunming mice. Hum. Exp. Toxicol. 2019, 38, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Mengozzi, A.; Carli, F.; Biancalana, E.; Della Latta, V.; Seghieri, M.; Gastaldelli, A.; Solini, A. Phthalates Exposure as Determinant of Albuminuria in Subjects with Type 2 Diabetes: A Cross-Sectional Study. J. Clin. Endocrinol. Metab. 2019, 104, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Kataria, A.; Trasande, L.; Trachtman, H. The effects of environmental chemicals on renal function. Nat. Rev. Nephrol. 2015, 11, 610–625. [Google Scholar] [CrossRef] [PubMed]
- Erkekoglu, P.; Giray, B.K.; Kızilgün, M.; Rachidi, W.; Hininger-Favier, I.; Roussel, A.-M.; Favier, A.; Hincal, F. Di(2-ethylhexyl)phthalate-induced renal oxidative stress in rats and protective effect of selenium. Toxicol. Mech. Methods 2012, 22, 415–423. [Google Scholar] [CrossRef]
- Ling, J.; Lopez-Dee, Z.P.; Cottell, C.; Wolfe, L.; Nye, D. Regulation of mRNA Translation Is a Novel Mechanism for Phthalate Toxicity. PLoS ONE 2016, 11, e0167914. [Google Scholar] [CrossRef]
- Liang, F.; Yan, B. Oxidative damage in the liver and kidney induced by dermal exposure to diisononyl phthalate in Balb/c mice. Toxicol. Ind. Health 2020, 36, 30–40. [Google Scholar] [CrossRef]
- Amara, I.; Salah, A.; Timoumi, R.; Annabi, E.; Scuto, M.; Trovato, A.; Neffati, F.; Calabrese, V.; Abid-Essefi, S. Effect of di(2-ethylhexyl) phthalate on Nrf2-regulated glutathione homeostasis in mouse kidney. Cell Stress Chaperones 2020, 25, 919–928. [Google Scholar] [CrossRef]
- Liang, F.; Xi, J.; Chen, X.; Huang, J.; Jin, D.; Zhu, X. Curcumin decreases dibutyl phthalate-induced renal dysfunction in Kunming mice via inhibiting oxidative stress and apoptosis. Hum. Exp. Toxicol. 2021, 40, 1528–1536. [Google Scholar] [CrossRef]
- Zhao, S.; Pan, L.; Chen, M.; Zhu, Y.P.; Han, B.M.; Xia, S.J.; Jiang, J.T. In utero di-n-butyl phthalate exposure induced abnormal autophagy in renal tubular cells via hedgehog signaling in newborn rats. Chem. Biol. Interact. 2020, 328, 109189. [Google Scholar] [CrossRef]
- Ye, Q.; Zhao, S.; Zhang, Y.; Su, Y.M.; Chen, M.; Zhao, J.; Jia, G.Z.; Han, B.M.; Jiang, J.T. Activation of the RhoA/ROCK pathway contributes to renal fibrosis in offspring rats induced by maternal exposure to di-n-butyl phthalate. Toxicology 2020, 443, 152573. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-T.; Wang, C.-C.; Huang, L.-C.; Liu, S.-H.; Chiang, C.-K. Plasticizer Di-(2-Ethylhexyl)Phthalate Induces Epithelial-to-Mesenchymal Transition and Renal Fibrosis In Vitro and In Vivo. Toxicol. Sci. 2018, 164, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Z.; Zhao, Y.; Wang, H.R.; Talukder, M.; Li, J.L. Lycopene Preventing DEHP-Induced Renal Cell Damage Is Targeted by Aryl Hydrocarbon Receptor. J. Agric. Food Chem. 2021, 69, 12853–12861. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).