Adsorption Characteristics of Indigenous Chromium-Resistant Aspergillus niger Strain Isolated from Red Soil for Remediation of Toxic Chromium in Red Soil Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Medium and Reagents
2.2. Preparation of Chromium-Contaminated Red Soil
2.3. Isolation of Chromium-Resistant Strains
2.4. Selection of Strains with Chromium Adsorption Capacity
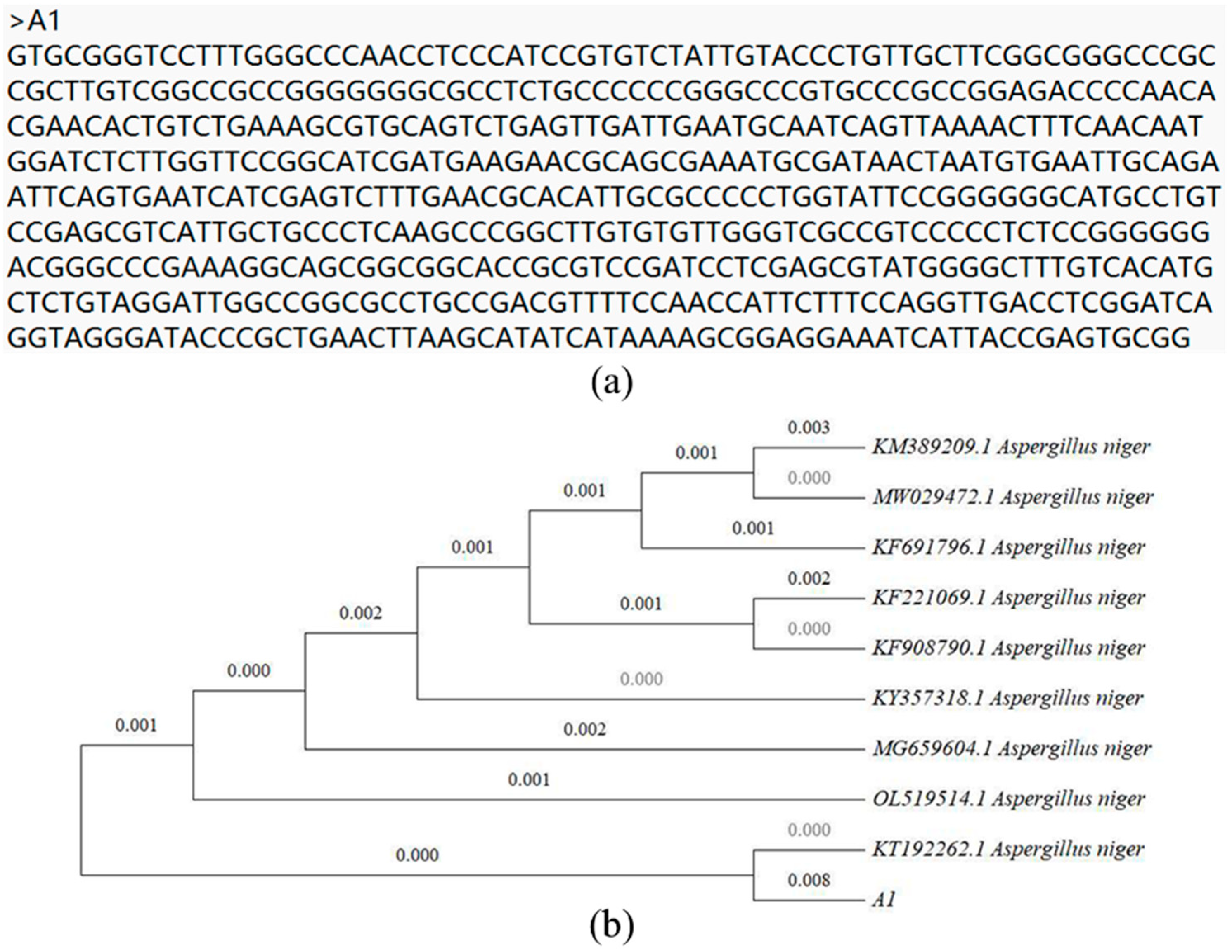
2.5. Identification and Phylogenetic Analysis of Strains
2.6. A. niger Colony Characteristics under Chromium Stress
2.7. Optimization Studies
2.8. Kinetic and Isotherm Validation
2.9. Effects of A. niger Inoculation Amount on Chromium State Transformation
2.10. Analysis of Red Soil Fungus Diversity after A. niger Inoculation
3. Results
3.1. Isolation and Identification of Chromium-Resistant Strains
3.2. A. niger Colony Characteristics under Chromium Stress
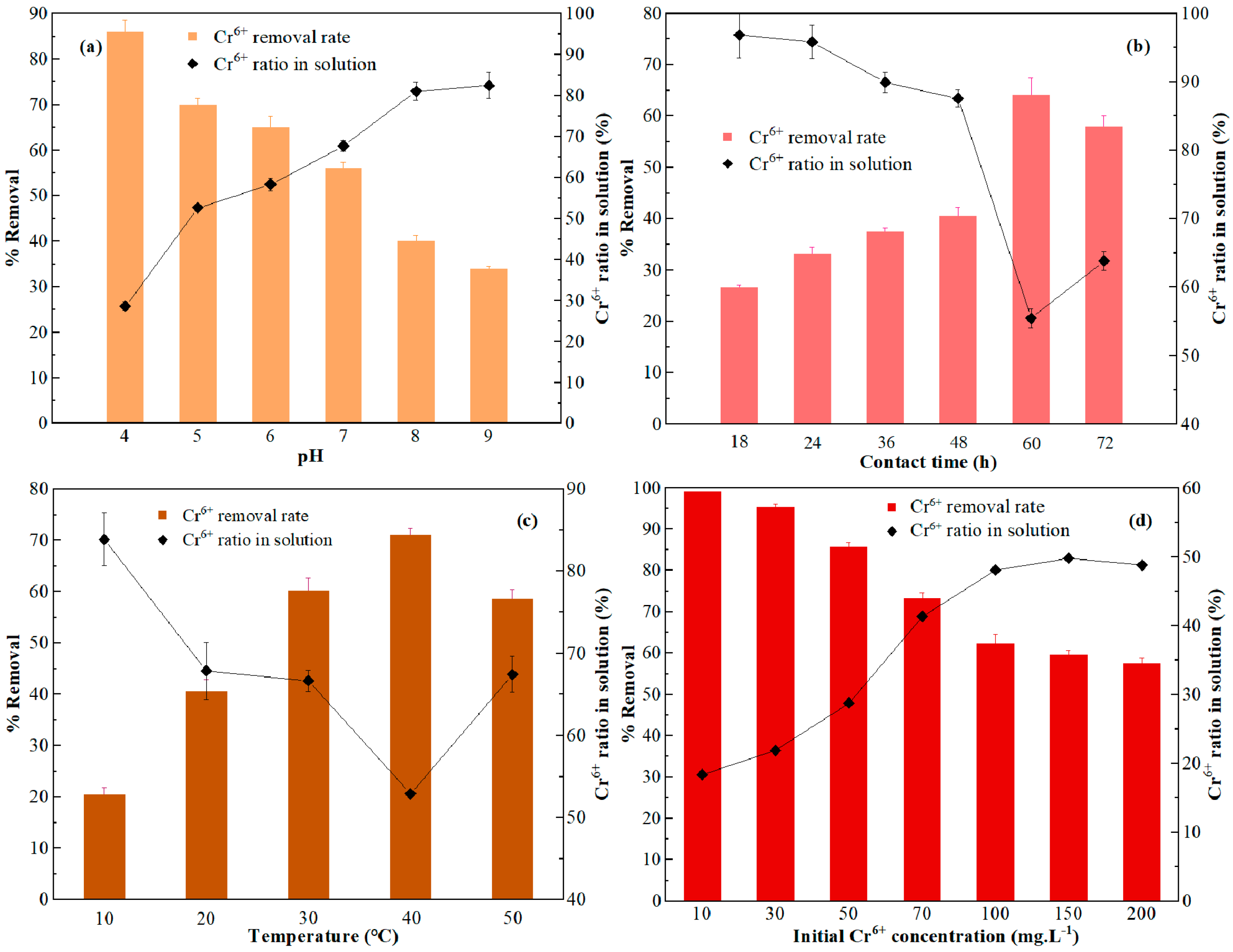
3.3. Optimization of Adsorption Conditions
3.4. Adsorption Kinetics and Isotherm Analyses of A. niger in Chromium Solution
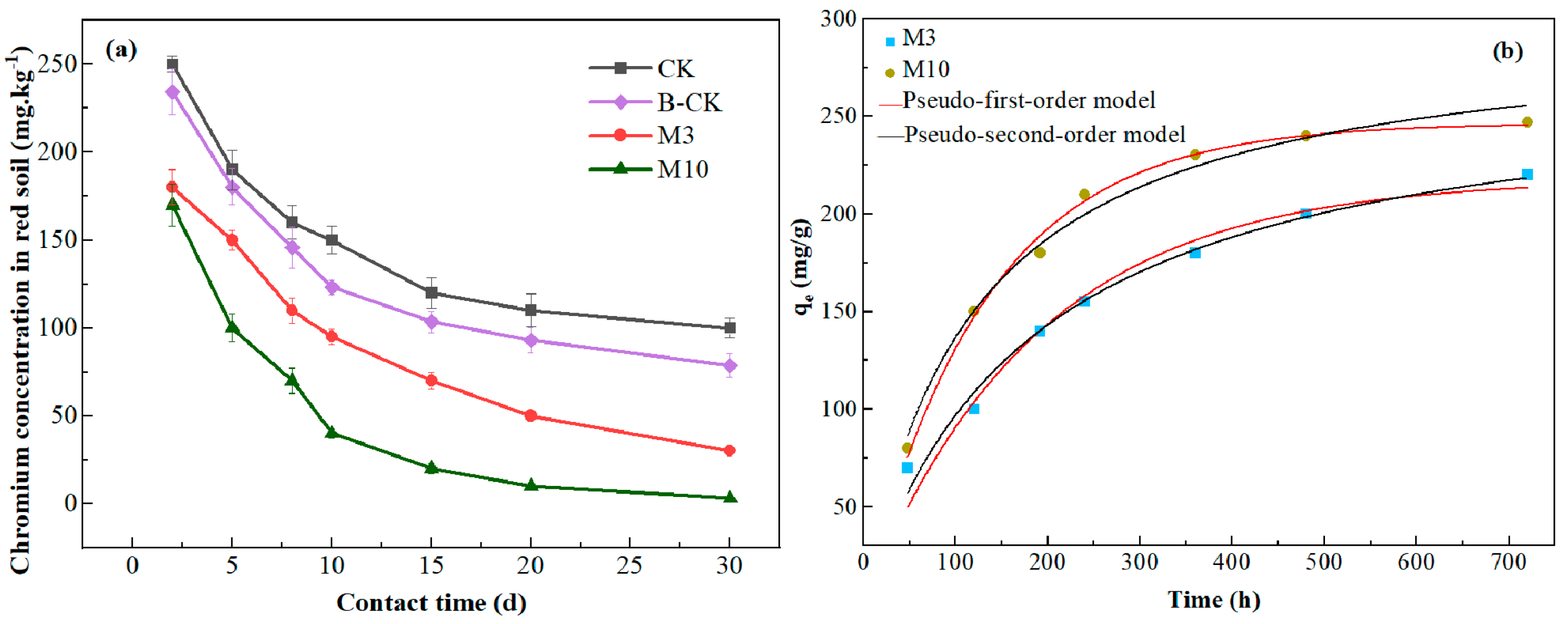
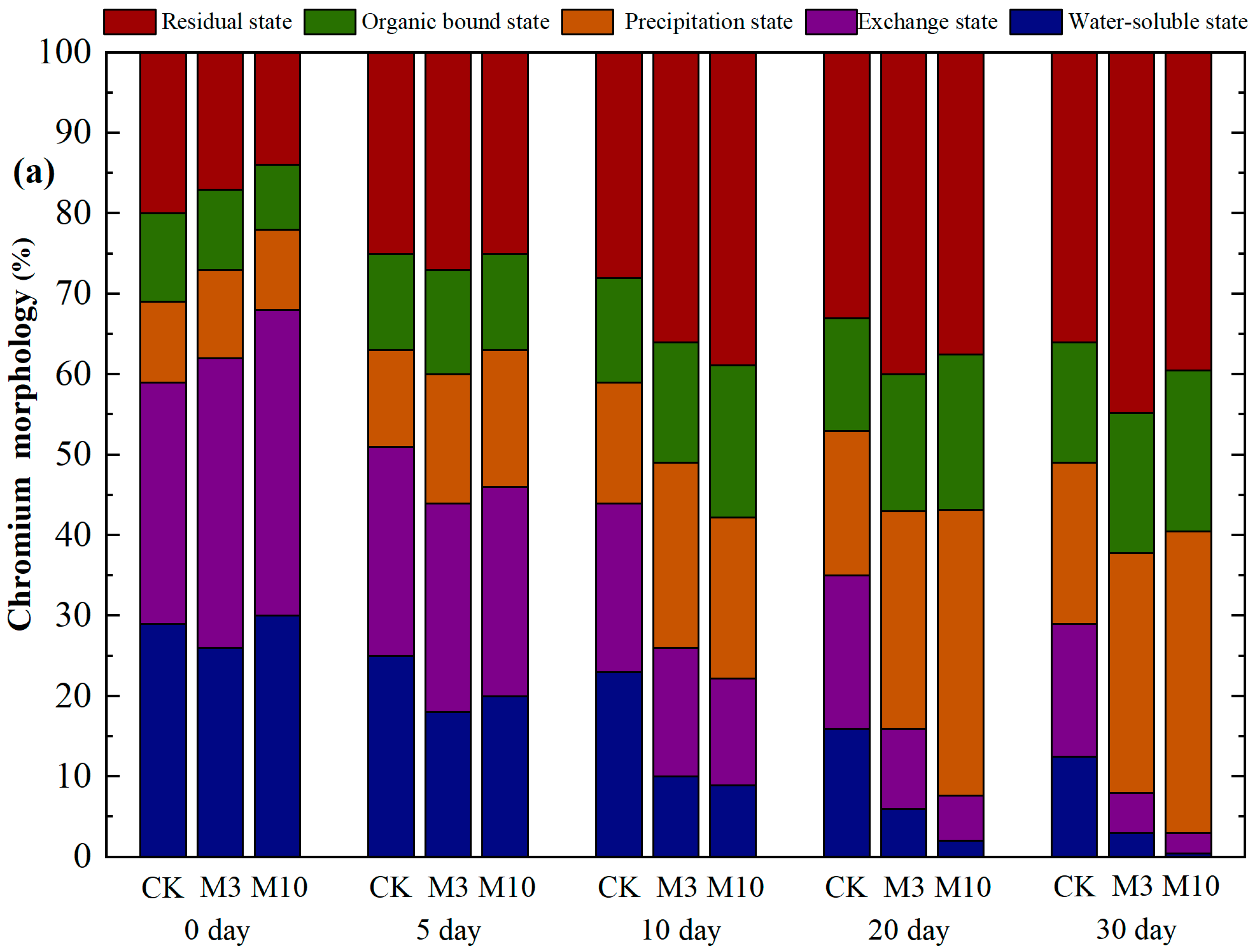
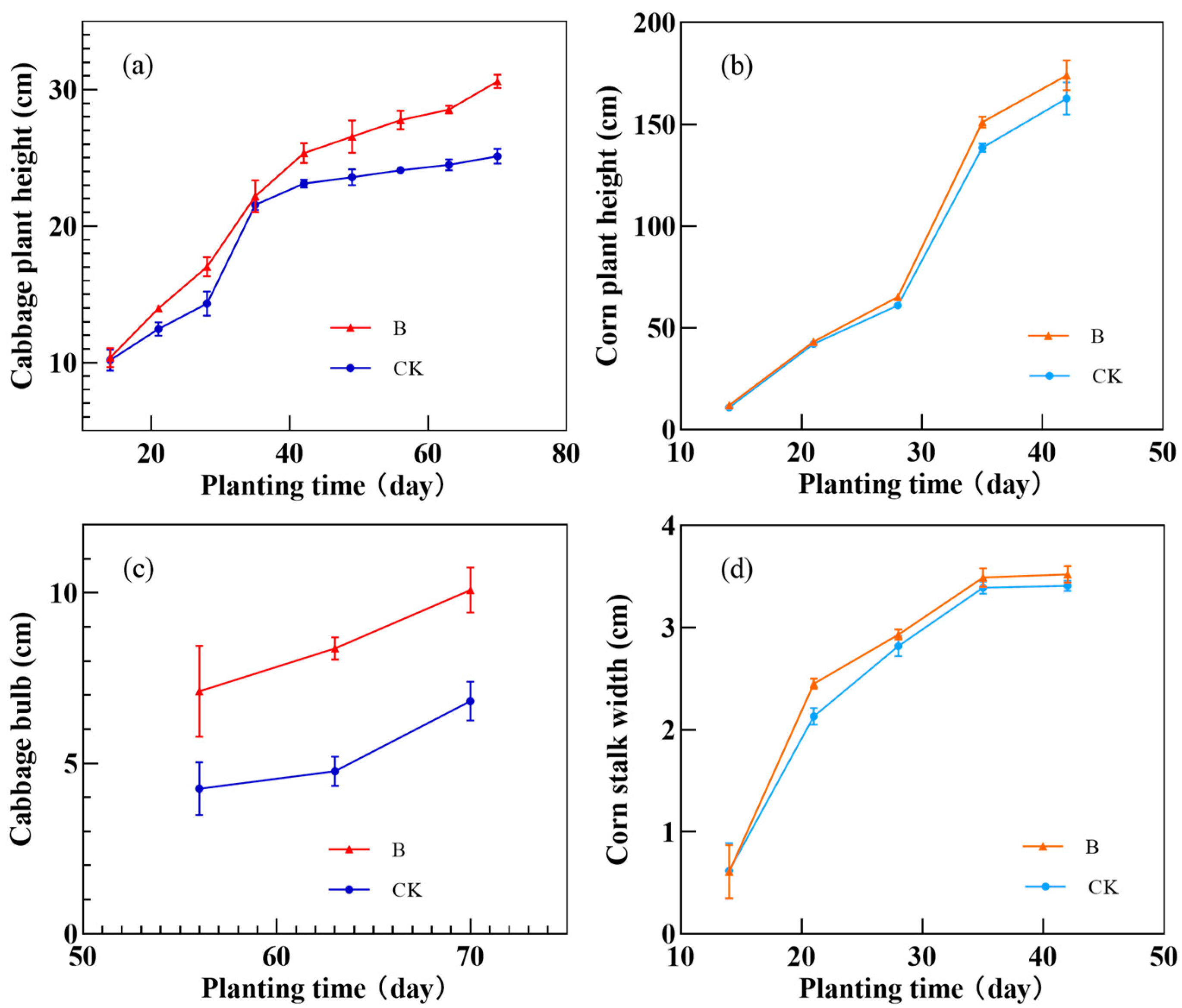
3.5. Remediation of Chromium Pollution in Red Soil by A. niger (Strain A1)
4. Discussion
4.1. Significance of Screening Indigenous Chromium-Tolerant Microorganisms to Treat Heavy Metal Pollution in Red Soil
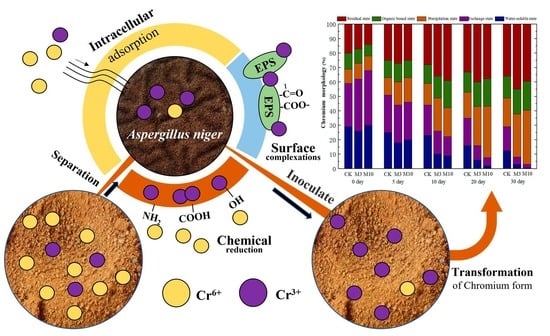
4.2. Potential of Indigenous A. niger to Reduce Chromium Pollution in Red Soil Environments and Its Adsorption Mechanism
4.3. Contributions and Limitations of Current Research and Future Development Directions
4.4. Virulence Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hu, G.; Bi, S.; Xu, G.; Zhang, Y.; Mei, X.; Li, A. Distribution and assessment of heavy metals off the Changjiang River mouth and adjacent area during the past century and the relationship of the heavy metals with anthropogenic activity. Mar. Pollut. Bull. 2015, 96, 434–440. [Google Scholar] [CrossRef]
- Yao, Y.; Li, J.; He, C.; Hu, X.; Yin, L.; Zhang, Y.; Zhang, J.; Huang, H.; Yang, S.; He, H.; et al. Distribution Characteristics and Relevance of Heavy Metals in Soils and Colloids Around a Mining Area in Nanjing, China. Bull. Environ. Contam. Toxicol. 2021, 107, 996–1003. [Google Scholar] [CrossRef]
- Jeevanantham, S.; Saravanan, A.; Hemavathy, R.; Kumar, P.S.; Yaashikaa, P.; Yuvaraj, D. Removal of toxic pollutants from water environment by phytoremediation: A survey on application and future prospects. Environ. Technol. Innov. 2019, 13, 264–276. [Google Scholar] [CrossRef]
- Jobby, R.; Jha, P.; Yadav, A.K.; Desai, N. Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: A comprehensive review. Chemosphere 2018, 207, 255–266. [Google Scholar] [CrossRef]
- Yuan, X.; Xiong, T.; Wang, H.; Wu, Z.; Jiang, L.; Zeng, G.; Li, Y. Immobilization of heavy metals in two contaminated soils using a modified magnesium silicate stabilizer. Environ. Sci. Pollut. Res. 2018, 25, 32562–32571. [Google Scholar] [CrossRef]
- Gu, C.; Wilson, S.G.; Margenot, A.J. Lithological and bioclimatic impacts on soil phosphatase activities in California temperate forests. Soil Biol. Biochem. 2019, 141, 107633. [Google Scholar] [CrossRef]
- Zhao, K.; Cai, Z.; Wang, B.; Wen, S.; Zhou, X.; Sun, N. Changes in pH and exchangeable acidity at depths of red soils derived from 4 parent materials under 3 vegetations. Sci. Agric. Sin. 2015, 48, 4818–4826. [Google Scholar] [CrossRef]
- Fan, X.; Ding, S.; Chen, M.; Gao, S.; Fu, Z.; Gong, M.; Wang, Y.; Zhang, C. Mobility of chromium in sediments dominated by macrophytes and cyanobacteria in different zones of Lake Taihu. Sci. Total Environ. 2019, 666, 994–1002. [Google Scholar] [CrossRef]
- Yin, L.; Liao, Y.; Liu, L.; Song, Y.; He, G. Screening identification and tolerance characteristics of Fe/Al/Cu tolerant bacteria in acidic groundwater in red soil area. Fresenius Environ. Bull. 2022, 31, 2109–2116. [Google Scholar]
- Garg, U.K.; Kaur, M.; Garg, V.; Sud, D. Removal of hexavalent chromium from aqueous solution by agricultural waste biomass. J. Hazard. Mater. 2007, 140, 60–68. [Google Scholar] [CrossRef]
- Liang, B.; Han, G.; Zeng, J.; Qu, R.; Liu, M.; Liu, J. Source and Risk Assessment of Trace Metals in Red Soils from Yunnan Province, Southwest China. CLEAN Soil Air Water 2021, 49, 2000288. [Google Scholar] [CrossRef]
- Wang, X.-F.; Deng, C.-B.; Sunahara, G.; Yin, J.; Xu, G.-P.; Zhu, K.-X. Risk Assessments of Heavy Metals to Children Following Non-dietary Exposures and Sugarcane Consumption in a Rural Area in Southern China. Expo. Health 2018, 12, 1–8. [Google Scholar] [CrossRef]
- Pradhan, D.; Sukla, L.B.; Sawyer, M.; Rahman, P.K. Recent bioreduction of hexavalent chromium in wastewater treatment: A review. J. Ind. Eng. Chem. 2017, 55, 1–20. [Google Scholar] [CrossRef]
- Malaviya, P.; Singh, A. Bioremediation of chromium solutions and chromium containing wastewaters. Crit. Rev. Microbiol. 2014, 42, 607–633. [Google Scholar] [CrossRef] [PubMed]
- Kalidhasan, S.; Kumar, A.S.K.; Rajesh, V.; Rajesh, N. The journey traversed in the remediation of hexavalent chromium and the road ahead toward greener alternatives—A perspective. Co-ord. Chem. Rev. 2016, 317, 157–166. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, X.; Liu, J.; You, S.; Zhang, H.; Lin, H. Reduction of Cr (VI) to Cr (III): A detoxification mechanism in hyperaccumulator leersia hexandra swartz. Fresenius Environ. Bull. 2016, 25, 959–968. [Google Scholar]
- Saravanan, A.; Kumar, P.S.; Preetha, B. Optimization of process parameters for the removal of chromium(VI) and nickel(II) from aqueous solutions by mixed biosorbents (custard apple seeds and Aspergillus niger) using response surface methodology. Desalination Water Treat. 2015, 57, 14530–14543. [Google Scholar] [CrossRef]
- Chang, X.; Li, M.; Liu, Q.; Liu, Q.; Yao, J. Adsorption–reduction of chromium(vi) from aqueous solution by phenol–formaldehyde resin microspheres. RSC Adv. 2016, 6, 46879–46888. [Google Scholar] [CrossRef]
- Bao, Z.; Feng, H.; Tu, W.; Li, L.; Li, Q. Method and mechanism of chromium removal from soil: A systematic review. Environ. Sci. Pollut. Res. 2022, 29, 35501–35517. [Google Scholar] [CrossRef]
- Elahi, A.; Arooj, I.; Bukhari, D.A.; Rehman, A. Successive use of microorganisms to remove chromium from wastewater. Appl. Microbiol. Biotechnol. 2020, 104, 3729–3743. [Google Scholar] [CrossRef]
- Wang, R.; Shafi, M.; Ma, J.; Zhong, B.; Guo, J.; Hu, X.; Xu, W.; Yang, Y.; Ruan, Z.; Wang, Y.; et al. Effect of amendments on contaminated soil of multiple heavy metals and accumulation of heavy metals in plants. Environ. Sci. Pollut. Res. 2018, 25, 28695–28704. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, J.; Luan, Y.; Dai, W. Application of algae for heavy metal adsorption: A 20-year meta-analysis. Ecotoxicol. Environ. Saf. 2019, 190, 110089. [Google Scholar] [CrossRef] [PubMed]
- Kavamura, V.N.; Esposito, E. Biotechnological strategies applied to the decontamination of soils polluted with heavy metals. Biotechnol. Adv. 2010, 28, 61–69. [Google Scholar] [CrossRef]
- da Costa Waite, C.C.; da Silva, G.O.A.; Bitencourt, J.A.P.; Sabadini-Santos, E.; Crapez, M.A.C. Copper and lead removal from aqueous solutions by bacterial consortia acting as biosorbents. Marine Pollut. Bull. 2016, 109, 386–392. [Google Scholar] [CrossRef]
- Li, P.; Tao, H.-C. Cell surface engineering of microorganisms towards adsorption of heavy metals. Crit. Rev. Microbiol. 2013, 41, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Wiatrowski, H.A.; Barkay, T. Monitoring of microbial metal transformations in the environment. Curr. Opin. Biotechnol. 2005, 16, 261–268. [Google Scholar] [CrossRef]
- Jorquera, M.A.; de la Luz Mora, M. Bacillus-like phosphobacteria in agronomic volcanic soils from Chile. In Proceedings of the 19th World Congress of Soil Science: Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; pp. 1–6. [Google Scholar]
- Ghiglione, J.-F.; Gourbiere, F.; Potier, P.; Philippot, L.; Lensi, R.; Bouterige, S.; Robert, R.; Bouchara, J.P.; Marot-Leblond, A.; Molinero, V.; et al. Role of Respiratory Nitrate Reductase in Ability of Pseudomonas fluorescens YT101 To Colonize the Rhizosphere of Maize. Appl. Environ. Microbiol. 2000, 66, 3277–3282. [Google Scholar] [CrossRef]
- Pachamuthu, P.; Kamble, S.T. In Vivo Study on Combined Toxicity of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) Strain ESC-1 with Sublethal Doses of Chlorpyrifos, Propetamphos, and Cyfluthrin Against German Cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 2000, 93, 60–70. [Google Scholar] [CrossRef]
- Huang, G.; Zhao, Q. Initial exploration of red soil ecology. Acta Ecol. Sin. 2014, 34, 5173–5181. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Tao, J.; Feng, X.; Zou, K.; Xiao, Y.; Liang, Y.; Yin, H.; Liu, X. Bioleaching of the mixed oxide-sulfide copper ore by artificial indigenous and exogenous microbial community. Hydrometallurgy 2017, 169, 41–46. [Google Scholar] [CrossRef]
- Pérez-Palacios, P.; Funes-Pinter, I.; Agostini, E.; Talano, M.A.; Ibáñez, S.G.; Humphry, M.; Edwards, K.; Rodríguez-Llorente, I.D.; Caviedes, M.A.; Pajuelo, E. Targeting Acr3 from Ensifer medicae to the plasma membrane or to the tonoplast of tobacco hairy roots allows arsenic extrusion or improved accumulation. Effect of acr3 expression on the root transcriptome. Metallomics 2019, 11, 1864–1886. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Fan, L.; Li, Z.; Wang, J.; Chen, R.; Zhang, Y.; Cheng, J.; Wu, X.; Li, J.; Yin, H.; et al. Characterization of extracellular polymeric substances from Synechocystis sp. PCC6803 under Cd (II), Pb (II) and Cr (VI) stress. J. Environ. Chem. Eng. 2021, 9, 105347. [Google Scholar] [CrossRef]
- Cavet, J.; Borrelly, G.P.; Robinson, N.J. Zn, Cu and Co in cyanobacteria: Selective control of metal availability. FEMS Microbiol. Rev. 2003, 27, 165–181. [Google Scholar] [CrossRef]
- Guimarães-Soares, L.; Felícia, H.; Bebianno, M.J.; Cássio, F. Metal-binding proteins and peptides in the aquatic fungi Fontanospora fusiramosa and Flagellospora curta exposed to severe metal stress. Sci. Total Environ. 2006, 372, 148–156. [Google Scholar] [CrossRef]
- Gu, Y.; Xu, W.; Liu, Y.; Zeng, G.; Huang, J.; Tan, X.; Jian, H.; Hu, X.; Li, F.; Wang, D. Mechanism of Cr(VI) reduction by Aspergillus niger: Enzymatic characteristic, oxidative stress response, and reduction product. Environ. Sci. Pollut. Res. 2014, 22, 6271–6279. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2015, 46, 406–433. [Google Scholar] [CrossRef]
- Chaudhary, P.; Beniwal, V.; Kaur, R.; Kumar, R.; Kumar, A.; Chhokar, V. Efficacy of Aspergillus fumigatus MCC 1175 for Bioremediation of Tannery Wastewater. CLEAN Soil Air Water 2019, 47, 1900131. [Google Scholar] [CrossRef]
- Chhikara, S.; Hooda, A.; Rana, L.; Dhankhar, R. Chromium (VI) biosorption by immobilized Aspergillus niger in continuous flow system with special reference to FTIR analysis. J. Environ. Biol. 2010, 31, 561–566. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Hu, C.; Wang, X.; Lyu, L.; Sheng, G. Enhanced degradation of organic pollutants over Cu-doped LaAlO3 perovskite through heterogeneous Fenton-like reactions. Chem. Eng. J. 2018, 332, 572–581. [Google Scholar] [CrossRef]
- Persson, J.; Fink, P.; Goto, A.; Hood, J.M.; Jonas, J.; Kato, S. To be or not to be what you eat: Regulation of stoichiometric homeostasis among autotrophs and heterotrophs. Oikos 2010, 119, 741–751. [Google Scholar] [CrossRef]




| Strain | Initial Cr6+ Concentration: 50 mg·L−1 | Initial Cr6+ Concentration: 500 mg·L−1 | ||||
|---|---|---|---|---|---|---|
| Cr6+ Concentration after Culture (mg/L) | Adsorption Capacity (mg/L) | Adsorption Rate (%) | Cr6+ Concentration after Culture (mg/L) | Adsorption Capacity (mg/L) | Adsorption Rate (%) | |
| CK | 48.9 | —— | —— | 477.25 | —— | —— |
| A1 | 10.93 | 37.97 ± 0.73 h | 77.64 | 265.8 | 211.46 ± 0.94 f | 44.31 |
| A2 | 25.51 | 23.39 ± 0.44 g | 47.83 | 353.05 | 124.29 ± 0.26 e | 26.02 |
| A3 | 34.05 | 14.85 ± 0 f | 30.36 | 446.75 | 30.28 ± 0.18 d | 6.39 |
| A4 | 37.42 | 11.48 ± 0.11 d | 23.47 | 453.5 | 23.81 ± 0.12 c | 4.98 |
| A5 | 43.17 | 5.73 ± 0.29 b | 11.71 | 477.22 | —— | —— |
| A6 | 36.31 | 12.59 ± 0.12 e | 25.74 | 457 | 20.82 ± 0.26 b | 4.24 |
| A7 | 44.99 | 3.91 ± 0.09 a | 7.99 | 477.3 | —— | —— |
| B1 | 36.57 | 12.33 ± 0.08 e | 25.21 | 460 | 17.35 ± 0.88 a | 3.61 |
| B1 | 34.15 | 14.75 ± 0.11 f | 30.16 | 447 | 30.29 ± 0.11 d | 6.33 |
| B3 | 40.65 | 8.25 ± 0.13 c | 16.87 | 458.85 | 18.50 ± 0.35 a | 3.86 |
| Initial Chromium Concentration (mg/L) | Single Colony Diameter (mm) | |||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | |
| CK | 4.50 ± 0.29 b | 17.16 ± 0.17 d | 30.08 ± 0.08 e | 70.13 ± 0.08 e |
| 50 | 3.83 ± 0.16 ab | 16.16 ± 0.16 c | 27.25 ± 0.14 d | 65.18 ± 0.09 d |
| 100 | 3.91 ± 0.08 ab | 15.25 ± 0.14 b | 24.13 ± 0.08 c | 58.56 ± 0.29 c |
| 200 | 4.50 ± 0.22 a | 15.00 ± 0.14 b | 22.21 ± 0.06 b | 50.80 ± 0.60 b |
| 500 | 3.66 ± 0.22 a | 14.00 ± 0.00 a | 19.51 ± 0.13 a | 41.03 ± 1.18 a |
| Temperature (°C) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| qemax(mg/L) | KL(L/mg) | R2 | KF((mg/g)/(L/mg)(1/n)) | n | R2 | |
| 20 | 95.13 | 0.0085 | 0.9929 | 1.7261 | 1.3288 | 0.9810 |
| 30 | 104.80 | 0.0083 | 0.9882 | 1.9242 | 1.3129 | 0.9736 |
| 40 | 117.71 | 0.0076 | 0.9879 | 1.9676 | 1.2878 | 0.9757 |
| Initial Cr6+ | qeexp (mg/g) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | qecal (mg/g) | K1 (h−1) | R2 | qecal (mg/g) | K2 (g/mg/h) | R2 | |
| 25 | 21.2 | 19.83 | 0.0258 | 0.9927 | 22.941 | 0.00165 | 0.9974 |
| 50 | 35.1 | 32.25 | 0.0188 | 0.9825 | 38.353 | 0.00057 | 0.9934 |
| 100 | 54.0 | 49.45 | 0.0212 | 0.9745 | 57.282 | 0.00046 | 0.9892 |
| Initial Cr6+ | Intra-Particle Diffusion Model | |||
|---|---|---|---|---|
| Concentration (mg/L) | Ki1 (g/mg/min1/2) | R2 | Ki2 (g/mg/min1/2) | R2 |
| 25 | 0.79366 | 0.78666 | 0.61527 | 0.98419 |
| 50 | 1.43346 | 0.9141 | 1.60844 | 0.95323 |
| 100 | 1.87709 | 0.98207 | 2.04 | 0.86863 |
| Process Mode | qeexp (mg/g) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| qecal (mg/g) | K1 (h−1) | R2 | qecal (mg/g) | K2 (g/mg/h) | R2 | ||
| M3 | 220 | 208.14 | 0.00539 | 0.96344 | 259.66 | 0.0002 | 0.98527 |
| M10 | 247 | 242.03 | 0.0076 | 0.99297 | 284.04 | 0.00028 | 0.98461 |
| Process Mode | Intra-Particle Diffusion Model | |||
|---|---|---|---|---|
| Ki1 (g/mg/min1/2) | R2 | Ki2 (g/mg/min1/2) | R2 | |
| M3 | 12.30598 | 0.9819 | 5.7385 | 0.96802 |
| M10 | 12. 87473 | 0.94838 | 3.17912 | 0.84897 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Li, L.; Wang, H.; Gao, Z.; Wang, C.; Sun, R.; Zhang, Y.; Xu, W.; Hou, X.; Xu, R. Adsorption Characteristics of Indigenous Chromium-Resistant Aspergillus niger Strain Isolated from Red Soil for Remediation of Toxic Chromium in Red Soil Environments. Toxics 2023, 11, 31. https://doi.org/10.3390/toxics11010031
Xu J, Li L, Wang H, Gao Z, Wang C, Sun R, Zhang Y, Xu W, Hou X, Xu R. Adsorption Characteristics of Indigenous Chromium-Resistant Aspergillus niger Strain Isolated from Red Soil for Remediation of Toxic Chromium in Red Soil Environments. Toxics. 2023; 11(1):31. https://doi.org/10.3390/toxics11010031
Chicago/Turabian StyleXu, Jiwei, Lumeng Li, Huabin Wang, Zhanyuan Gao, Chuanshu Wang, Rong Sun, Yong Zhang, Wumei Xu, Xiying Hou, and Rui Xu. 2023. "Adsorption Characteristics of Indigenous Chromium-Resistant Aspergillus niger Strain Isolated from Red Soil for Remediation of Toxic Chromium in Red Soil Environments" Toxics 11, no. 1: 31. https://doi.org/10.3390/toxics11010031
APA StyleXu, J., Li, L., Wang, H., Gao, Z., Wang, C., Sun, R., Zhang, Y., Xu, W., Hou, X., & Xu, R. (2023). Adsorption Characteristics of Indigenous Chromium-Resistant Aspergillus niger Strain Isolated from Red Soil for Remediation of Toxic Chromium in Red Soil Environments. Toxics, 11(1), 31. https://doi.org/10.3390/toxics11010031