Zamzam Water Ameliorates Gentamicin-Induced Testicular Toxicity in a Rat Model via Targeting Sperm Parameters, Testicular Tissue Oxidative Insult, Inflammation, Apoptosis, and Pituitary-Gonadal Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Groups
2.3. Assessment of the Weight of the Testis and Specimen Collection
2.4. Assessment of Sperm Parameters (Motility and Morphology)
2.5. Hormonal Assay
2.6. Oxidative Stress Measurement
2.7. Microscopic Examination
2.8. Immunohistochemical Assay of Caspes3, NF-kβ and PCNA
2.9. Measurement of Cytokines in Testicular Tissue
2.10. Analysis of Testicular Tissue and Sperm Ultra-Structure by Electron Microscope
2.11. RT-PCR Assessment
2.12. Statistical Analysis
3. Results
3.1. The Outcome of Zamzam Water on Variations of Body and Testicular Induced by Administration of Gentamicin
3.2. Ameliorating Outcome of Zamzam Water upon Gentamicin-Induced Oxidative Insult and Variation of Pituitary-Gonadal Axis
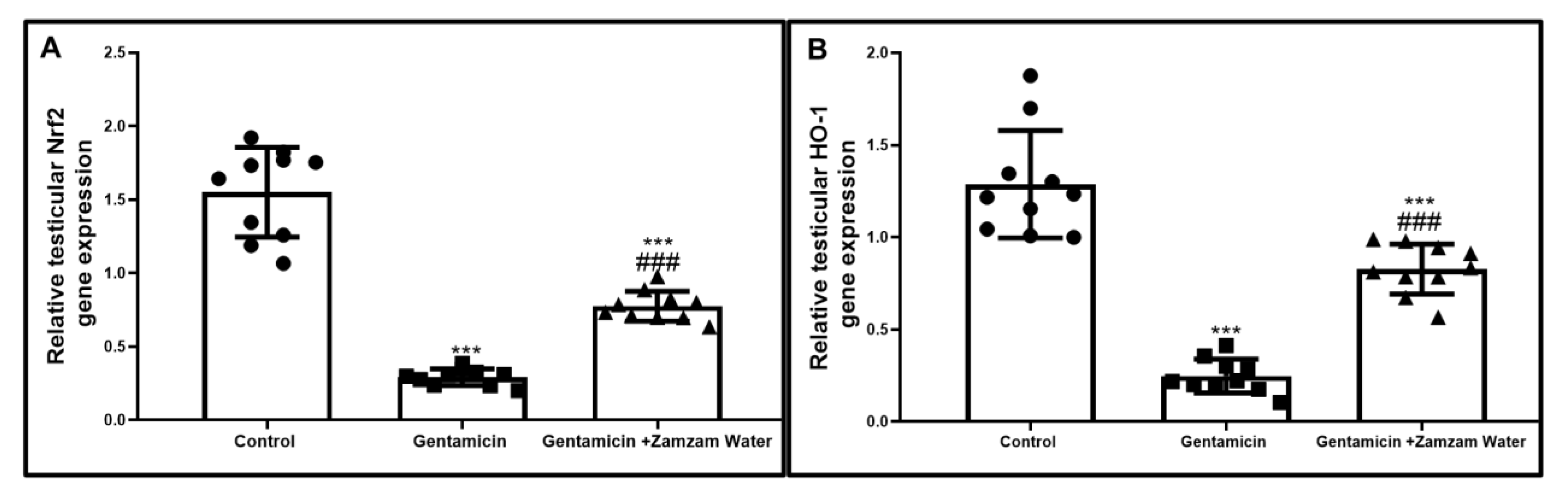
3.3. Zamzam Water Impact upon Nrf2/Ho_1 Path in Testicular Tissue
3.4. Zamzam Water Impact upon Spermatic Parameters
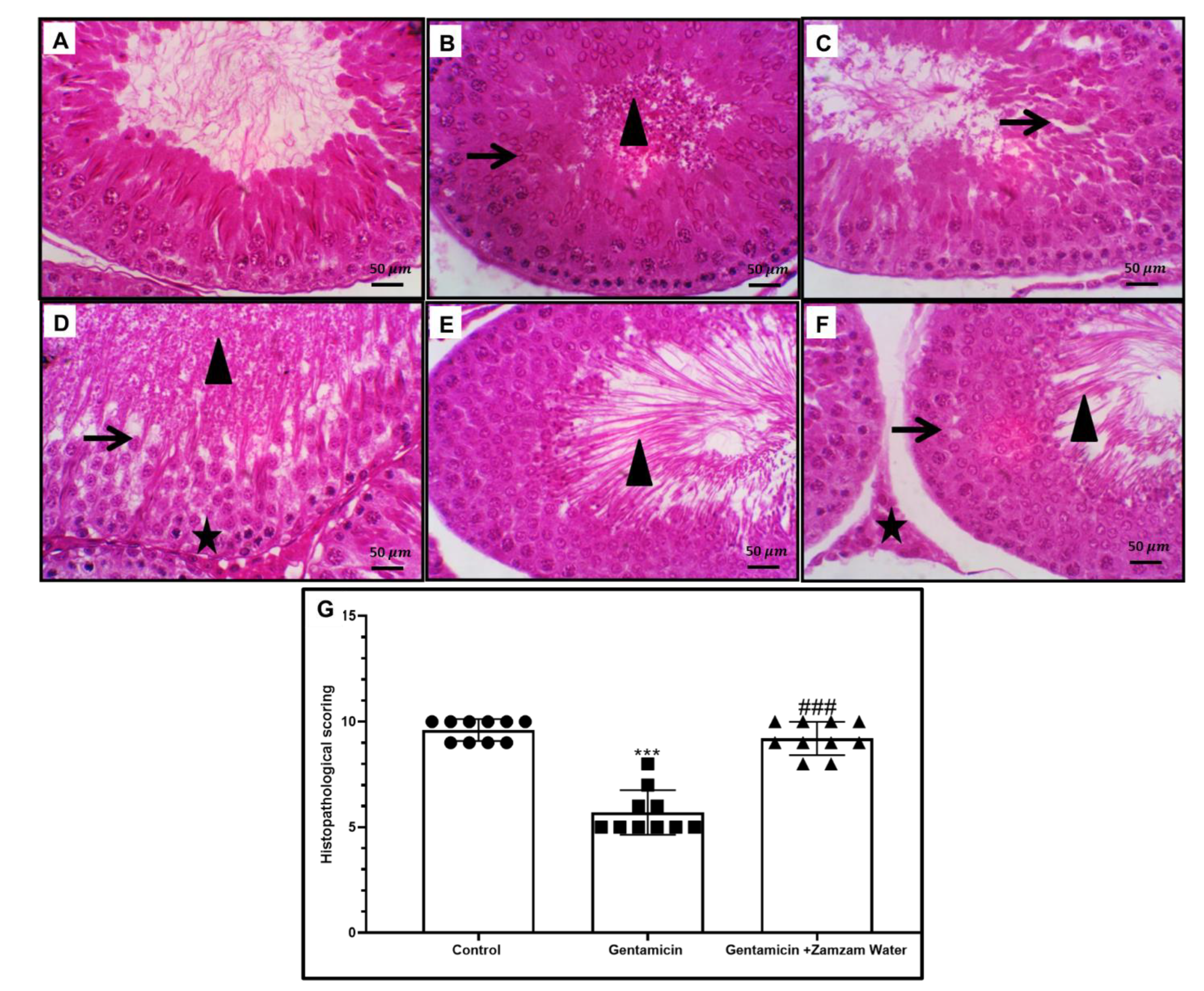
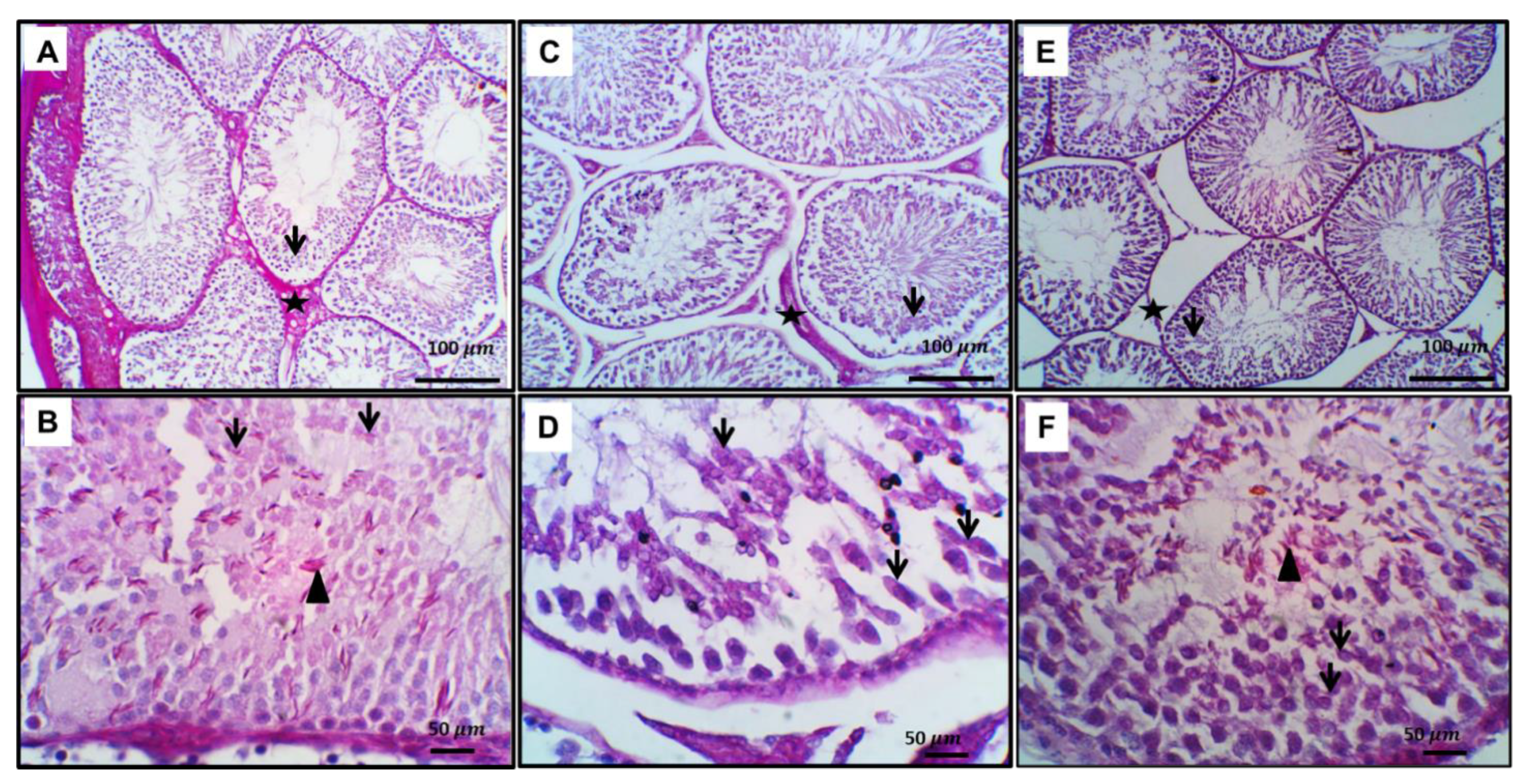
3.5. Ameliorative Effect of Zamzam Water on Gentamicin-Induced Testicular Tissue Damage and Glycogen Store Depletion
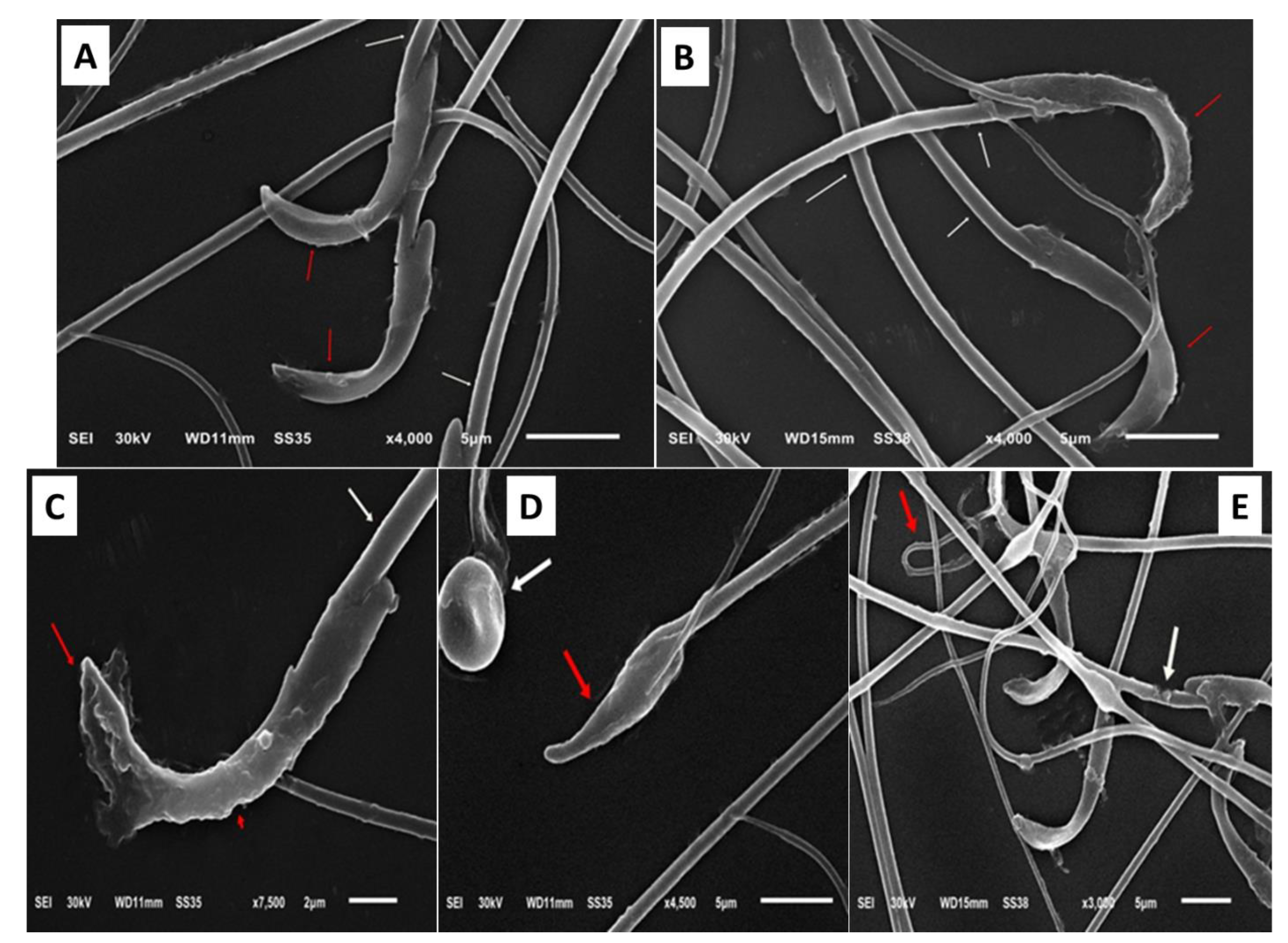
3.6. Impact of Zamzam Water on Gentamicin-Induced Testicular Ultrastructural Changes
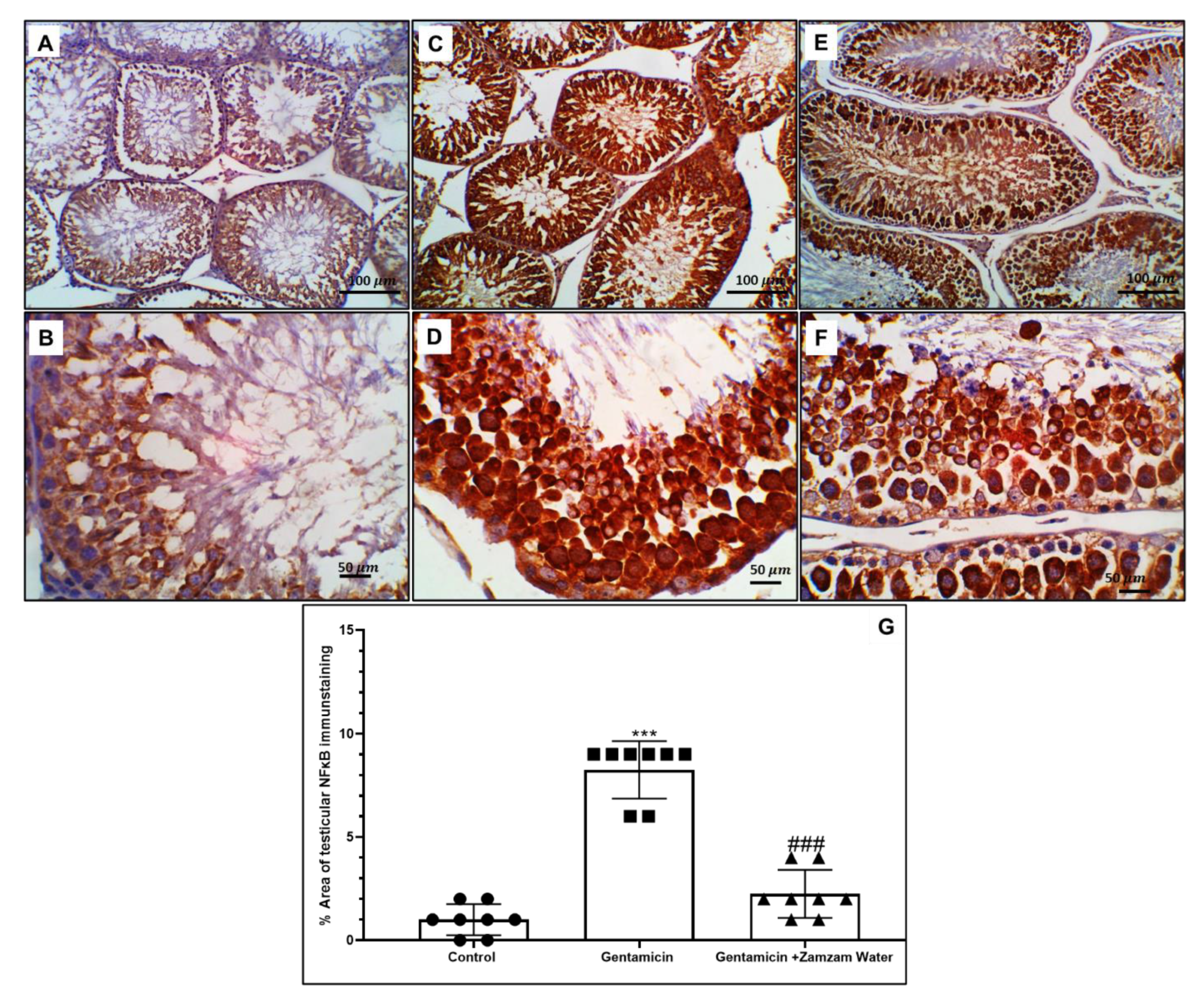
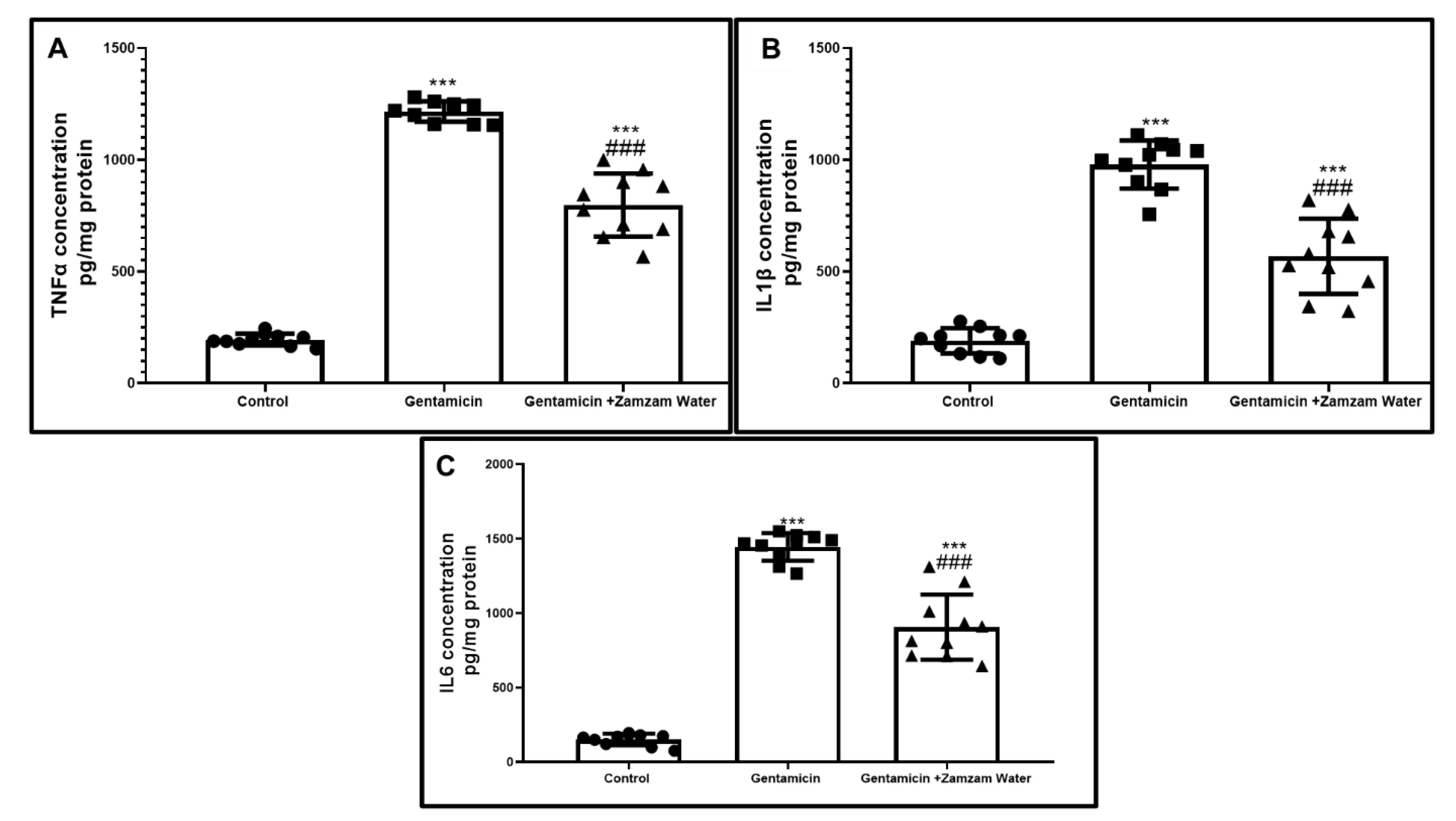
3.7. Ameliorative Effect of Zamzam Water on Gentamicin-Induced Testicular Tissue Inflammation
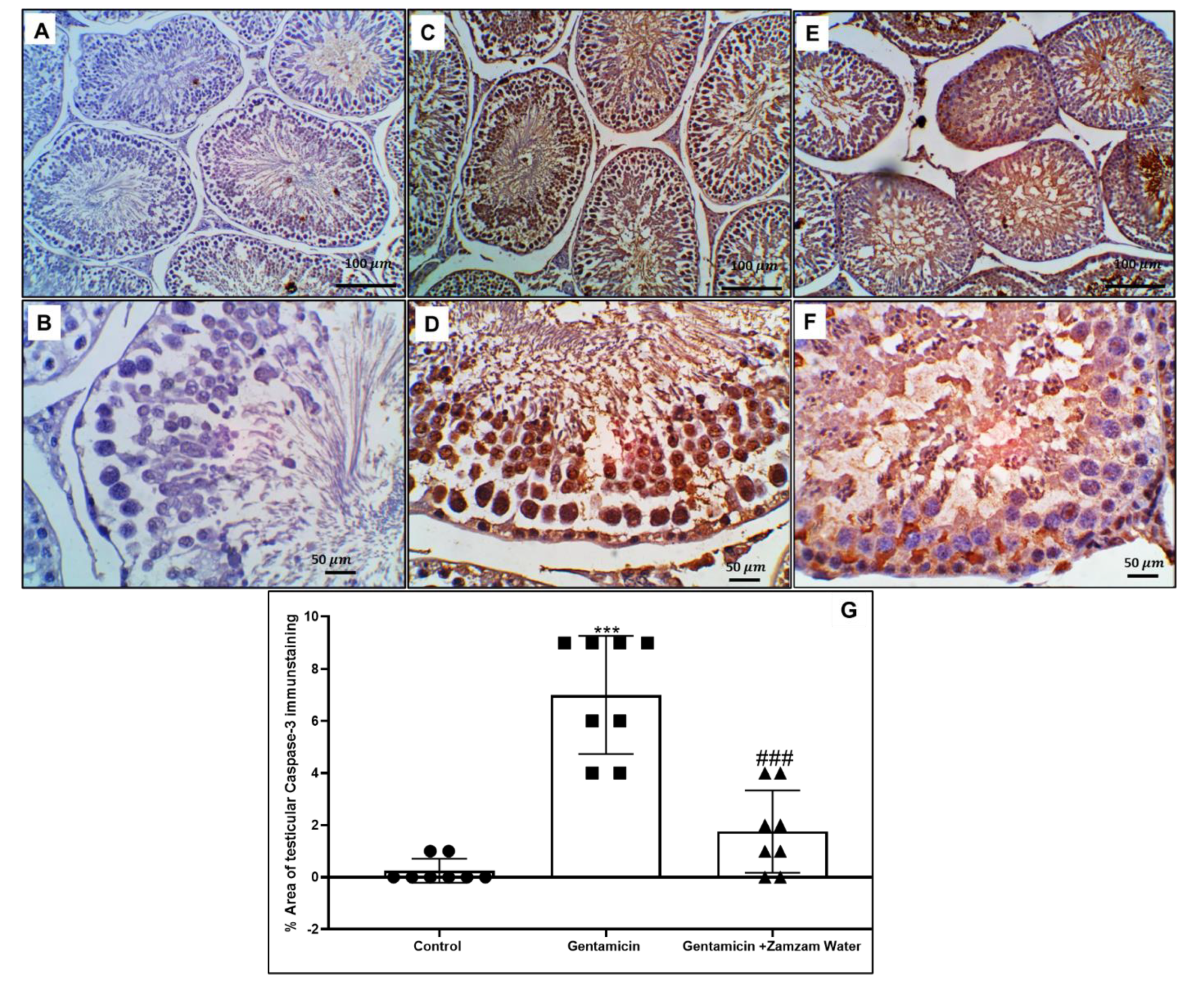
3.8. Zamzam Water Mitigates Gentamicin-Induced Testicular Apoptosis
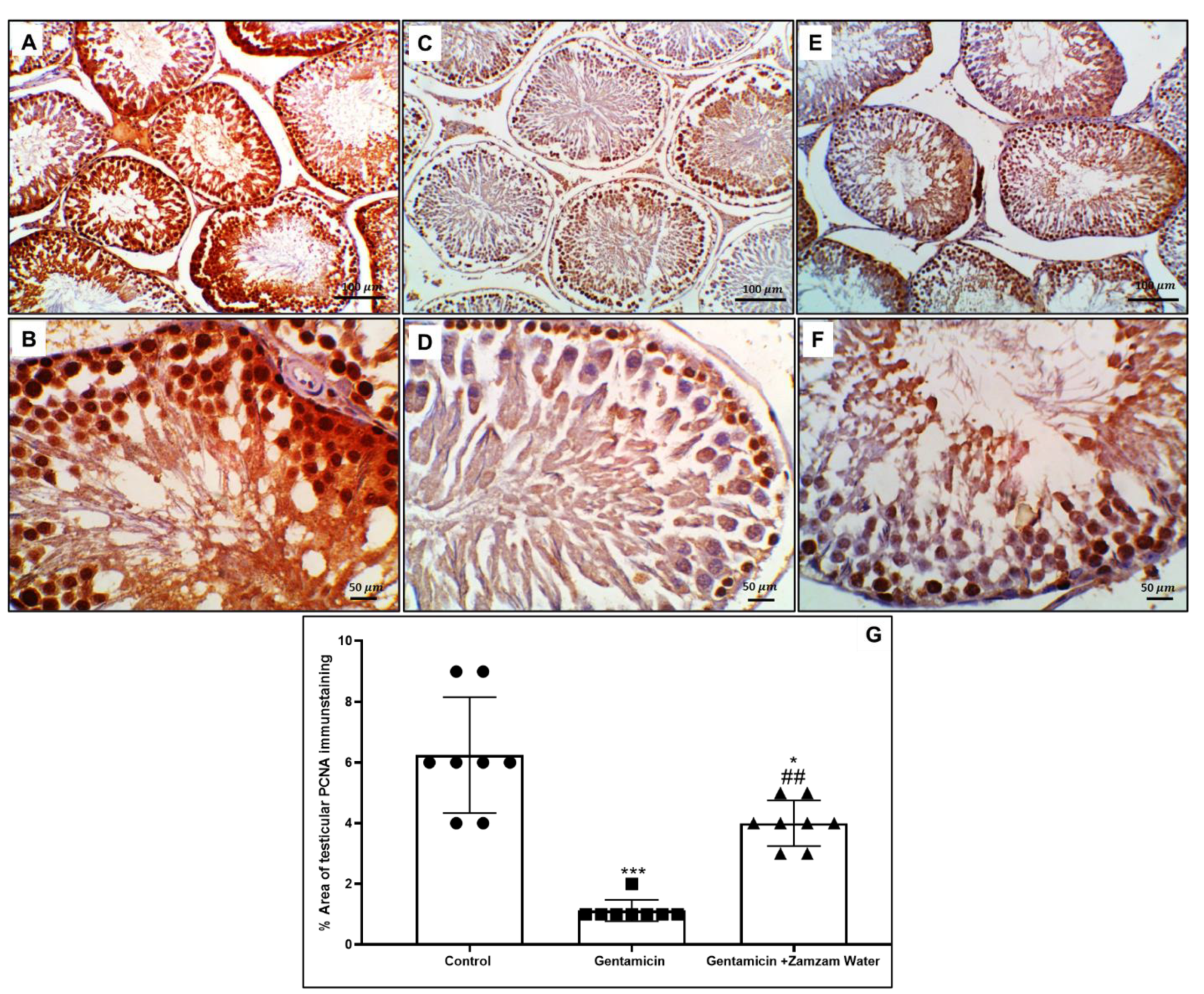
3.9. Ameliorative Effect of Zamzam Water on Gentamicin Impaired Spermatogenesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Apaf-1 | apoptotic protease activating factor 1 |
| Bax | Bcl-2-associated protein |
| BCL-2 | B-cell lymphoma 2 |
| CAT | catalase |
| ELISA | enzyme-linked immunosorbent assay |
| FSH | follicle-stimulating hormone |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GPx | glutathione peroxidase |
| IL-1β | interleukin-1β |
| IL-6 | interleukin 6 |
| LH | luteinizing hormone |
| NF-κB | nuclear factors kappa-β |
| Nrf2/HO-1 | the nuclear factor erythroid 2-related factor transcription factor/hemoxygenase 1 |
| HbA1c | hemoglobin A1C |
| PAS | periodic-acid-Schiff |
| PCNA | proliferating cell nuclear antigen |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| STZ | streptozotocin |
| Th1 | type 1 T helper (Th1) cells |
| Th2 | type 2 T helper (Th1) cells |
| TNF-α | tumor necrosis factor alpha |
References
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Ogundipe, D.J.; Akomolafe, R.O.; Sanusi, A.A.; Imafidon, C.E.; Olukiran, O.S.; Oladele, A.A. Ocimum gratissimum ameliorates gentamicin-induced kidney injury but decreases creatinine clearance following sub-chronic administration in rats. J. Evid. Based Complement. Altern. Med. 2017, 22, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Mingeot-Leclercq, M.P.; Glupczynski, Y.; Tulkens, P.M. Aminoglycosides: Activity and resistance. Antimicrob. Agents Chemother. 1999, 43, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Durante-Mangoni, E.; Grammatikos, A.; Utili, R.; Falagas, M.E. Do we still need the aminoglycosides? Int. J. Antimicrob. Agents 2009, 33, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, I.C.; Baek, H.S.; Shin, I.S.; Moon, C.; Kim, S.H.; Yun, W.K.; Nam, K.H.; Kim, H.C.; Kim, J.C. Melatonin prevents gentamicin-induced testicular toxicity and oxidative stress in rats. Andrologia 2014, 46, 1032–1040. [Google Scholar] [CrossRef]
- Aly, H.A.A.; Hassan, M.H. Potential testicular toxicity of gentamicin in adult rats. Biochem. Biophys. Res. Commun. 2018, 497, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Park, S.K.; Cho, Y.S.; Lee, H.S.; Kim, K.R.; Kim, M.G.; Chung, W.H. Gentamicin induced nitric oxide-related oxidative damages on vestibular afferents in the guinea pig. Hear Res. 2006, 211, 46–53. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, I.C.; Lim, J.H.; Moon, C.; Bae, C.S.; Kim, S.H.; Shin, D.H.; Kim, H.C.; Kim, J.C. Spermatotoxic effects of α-chlorohydrin in rats. Lab. Anim. Res. 2012, 2, 11–16. [Google Scholar] [CrossRef][Green Version]
- Aitken, R.J. The cell biology of fertilization. Adv. Exp. Med. Biol. 1997, 424, 291–299. [Google Scholar]
- Saradha, B.; Mathur, P.P. Induction of oxidative stress by lindane in epididymis of adult male rats. Environ. Toxicol. Pharmacol. 2006, 22, 90–96. [Google Scholar] [CrossRef]
- Padron, O.F.; Brackett, N.L.; Sharma, R.K.; Lynne, C.M.; Thomas, A.J.; Agarwal, A. Seminal reactive oxygen species and sperm motility and morphology in men with spinal cord injury. Fertil. Steril. 1997, 67, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Türk, G.; Ateşşahin, A.; Sönmez, M.; Yüce, A.; Ceribaşi, A.O. Lycopene protects against cyclosporine A-induced testicular toxicity in rats. Theriogenology 2007, 67, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, A.; Nasri, H.; Nematbakhsh, M.; Rafieian-Kopaei, M. Antioxidant activity and preventive effect of aqueous leaf extract of Aloe Vera on gentamicin-induced nephrotoxicity in male Wistar rats. Clin Ter. 2014, 165, 7–11. [Google Scholar] [PubMed]
- Matsushita, T.; Kusakabe, Y.; Kitamura, A.; Okada, S.; Murase, K. Protective effect of hydrogen-rich water against gentamicin-induced nephrotoxicity in rats using blood oxygenation level-dependent MR imaging. Magn. Reson. Med. Sci. 2011, 10, 169–176. [Google Scholar] [CrossRef]
- Nassini, R.; Andrè, E.; Gazzieri, D.; De Siena, G.; Zanasi, A.; Geppetti, P.; Materazzi, S. A bicarbonate-alkaline mineral water protects from ethanol-induced hemorrhagic gastric lesions in mice. Biol. Pharm. Bull. 2010, 33, 1319–1323. [Google Scholar] [CrossRef][Green Version]
- Huang, K.C.; Yang, C.C.; Lee, K.T.; Chien, C.T. Reduced hemodialysis-induced oxidative stress in end-stage renal disease patients by electrolyzed reduced water. Kidney Int. 2003, 64, 704–714. [Google Scholar] [CrossRef]
- Hofer, T.; Marzetti, E.; Xu, J.; Seo, A.Y.; Gulec, S.; Knutson, M.D.; Leeuwenburgh, C. Dupont-Versteegden EE. Increased iron content and RNA oxidative damage in skeletal muscle with aging and disuse atrophy. Exp. Gerontol. 2008, 43, 563–570. [Google Scholar] [CrossRef]
- Jin, D.; Ryu, S.H.; Kim, H.W.; Yang, E.J.; Lim, S.J.; Ryang, Y.S.; Chung, C.H.; Park, S.K.; Lee, K.J. Anti-diabetic effect of alkaline-reduced water on OLETF rats. Biosci. Biotechnol. Biochem. 2006, 7, 31–37. [Google Scholar] [CrossRef]
- WHO. Nutrients in Drinking Water; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Koshak, Y.H. Zamzam. Dar Alelm for Publications: Jeddah, Saudi Arabia, 1983. [Google Scholar]
- Mashat, B.H. The microbiological quality of sabil free drinking water in Makkah Al-Mukarramah. JKAU Met. Environ. Arid. Land Agric. Sci. 2012, 21, 87–100. [Google Scholar] [CrossRef]
- Al Zuhair, N.; Khounganian, R. A comparative study between the chemical composition of potable water and Zamzam water in Saudi Arabia. Saudi Dent. J. 2006, 18, 1–9. [Google Scholar]
- Bamosa, A.; Elnour, A.; Kaatabi, H.; Meheithif, A.; Aleissa, K.; Al-Almaie, S. Zamzam water ameliorates oxidative stress and reduces HemoglobinA1c in type 2 diabetic patients. J. Diabetes Metabol. 2013, 4, 1–5. [Google Scholar] [CrossRef]
- Al Meheithif, A.; Elnour, A.; Bamosa, A.; Aleissa, K. Antioxidant effects of Zamzam water in normal rats and those under induced-oxidative stress. J. Med. Plants Res. 2012, 6, 5507e5512. [Google Scholar]
- Badar, A.; Bamosa, A.O.; Salahuddin, M.; Al Meheithif, A. Effect of Zamzam water on blood methemoglobin level in young rats. J. Fam. Commun. Med. 2019, 26, 30e35. [Google Scholar]
- Zhou, J.; Chen, L.I.; Li, J.; Li, H.; Hong, Z.; Xie, M.; Chen, S.; Yao, B. The semen pH affects sperm motility and capacitation. PLoS ONE 2015, 10, e0132974. [Google Scholar] [CrossRef] [PubMed]
- LG, H.; WC, C.; QD, H.; TY, H. Semen pH and sperm morphological changes in less, weak sperm diseaseclinical significance. Contemp. Med. 2013, 19, 2. [Google Scholar]
- Holm, L.; Wishart, G.J. The effect of pH on the motility of spermatozoa from chicken, turkey and quail. Anim. Reprod. Sci. 1998, 54, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Mraisel, A.; Abu Ali, A. Protective effect of Zamzam water against kidneys damage induced in male rats: Immunohistochemistry evidence. J. Biosci. Appl. Res. 2017, 3, 42–47. [Google Scholar] [CrossRef]
- Hamzeh, M.; Hosseinimehr, S.J.; Karimpour, A.; Mohammadi, H.R.; Khalatbary, A.R.; Amiri, F.T. Cerium oxide nanoparticles protect cyclophosphamide-induced testicular toxicity in mice. Int. J. Prev. Med. 2019, 10, 5. [Google Scholar] [PubMed]
- Castro, A.; Berndtson, W.E.; Cardoso, F. Plasma and testicular testosterone levels, volume density and number of Leydig cells and spermatogenic efficiency of rabbits. Braz. J. Med. Biol. Res. 2002, 35, 493–498. [Google Scholar] [CrossRef]
- Johnsen, S.G. Testicular biopsy score count—A method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Horm. Res. Paediatr. 1970, 1, 2–25. [Google Scholar] [CrossRef]
- Hotchkiss, R.D. A microchemical reaction resulting in the staining of polysaccharide structures in fixed tissue preparations. Arch. Biochem. 1948, 16, 131. [Google Scholar] [PubMed]
- Saber, S.; Khalil, R.M.; Abdo, W.S.; Nassif, D.; El-Ahwany, E. Olmesartan ameliorates chemically-induced ulcerative colitis in rats via modulating NFκB and Nrf-2/HO-1 signaling crosstalk. Toxicol. Appl. Pharmacol. 2019, 364, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Khalil, R.; Shata, A.; Abd El-Kader, E.M.; Sharaf, H.; Abdo, W.S.; Amin, N.A.; Saber, S. Vildagliptin, a DPP-4 inhibitor, attenuates carbon tetrachloride-induced liver fibrosis by targeting ERK1/2, p38α, and NF-κB signaling. Toxicol. Appl. Pharmacol. 2020, 407, 115246. [Google Scholar] [CrossRef]
- Reynolds, E.S. The use of lead citrate of high pH as an electron opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208. [Google Scholar] [CrossRef]
- Ahmad, K.; Foote, R.H.; Kaproth, M. Antibiotics for bull semen frozen in milk and egg yolk extenders. J. Dairy Sci. 1987, 70, 2439–2443. [Google Scholar] [CrossRef]
- Timmermans, L.M. Modifications in spermatogenesis following antibiotic therapy. Acta Urol. Belg. 1989, 57, 35–46. [Google Scholar]
- Segovia, M.; Jenkins, J.A.; Paniagua-Chavez, C.; Tiersch, T.R. Flow cytometric evaluation of antibiotic effects on viability and mitochondrial function of refrigerated spermatozoa of Nile tilapia. Theriogenology 2000, 53, 1489–1499. [Google Scholar] [CrossRef]
- Khaki, A.; Novin, M.G.; Khaki, A.A.; Nouri, M.; Sanati, E.; Nikmanesh, M. Comparative study of the effects of gentamicin, neomycin, streptomycin and ofloxacin antibiotics on sperm parameters and testis apoptosis in rats. Pak. J. Biol. Sci. 2008, 11, 1683–1689. [Google Scholar] [CrossRef]
- Narayana, K. An aminoglycoside antibiotic gentamycin induces oxidative stress, reduces antioxidant reserve and impairs spermatogenesis in rats. J. Toxicol. Sci. 2008, 33, 85–96. [Google Scholar] [CrossRef]
- Nouri, M.; Khaki, A.; Azar, F.F.; Rashidi, M.R. The protective effects of carrot seed extract on spermatogenesis and cauda epididymal sperm reserves in gentamicin treated rats. Med. J. 2009, 11, 327–333. [Google Scholar]
- Zahedi, A.; Khaki, A.; Ahmadi-Ashtiani, H.R.; Rastegar, H.; Rezazadeh, S. Zingiber officinale protective effects on gentamicin’s toxicity on sperm in rats. J. Med. Plant 2010, 9, 93–98. [Google Scholar]
- Zahedi, A.; Fathiazad, F.; Khaki, A.; Ahmadnejad, B. Protective effect of ginger on gentamicin-induced apoptosis in testis of rats. Adv. Pharmaceut. Bull. 2012, 2, 197–200. [Google Scholar]
- Sayed, A.A.; Hassan, N.; Alhanout, F.K. Modulatory Effect of ZamZam Water on the Renal and Hepatic Function of Mice Challenged with Gentamicin. Indian J. Anim. Res. 2020, 1, 1–5. [Google Scholar]
- Kerr, J. Spontaneous degeneration of germ cells in normal rat testis: Assessment of cell types and frequency during the spermatogenic cycle. Reproduction 1992, 95, 825–830. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aly, H.A.; Khafagy, R.M. Taurine reverses endosulfan-induced oxidative stress and apoptosis in adult rat testis. Food Chem. Toxicol. 2014, 64, 1–9. [Google Scholar] [CrossRef]
- Chang, C.; Chen, Y.-T.; Yeh, S.-D.; Xu, Q.; Wang, R.-S.; Guillou, F.; Lardy, H.; Yeh, S. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc. Natl. Acad. Sci. USA 2004, 101, 6876–6881. [Google Scholar] [CrossRef]
- Holdcraft, R.W.; Braun, R.E. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development 2004, 131, 459–467. [Google Scholar] [CrossRef]
- Blanco-Rodriguez, J.; Martinez-Garcia, C. Apoptosis precedes detachment of germ cells from the seminiferous epithelium after hormone suppression by short-term oestradiol treatment of rats. Int. J. Androl. 1998, 21, 109–115. [Google Scholar] [CrossRef]
- Badri, S.; Vanithakumari, G.; Malini, T. Studies on methotrexate effects on testicular steroidogenesis in rats. Endocr. Res. 2000, 26, 247–262. [Google Scholar] [CrossRef]
- Elsawah, H.K.; Kandiel, M.M.; Amin, A.A.; Mokhimar, H.M.; El Mahmoudy, A.M. Gentamicin and amikacin adversely affect male infertility indicated by pharmacological, andrological and pathological evidence. J. Basic Clin. Physiol. Pharmacol. 2020, 9, 218. [Google Scholar] [CrossRef]
- Orr, J.W.; Price, D.E.; Stickland, L.H. The glycogen content of rat’s liver poisoning with large doses of dimethyl amino azobenzene. J. Pathol. Bacter. 1948, 60, 573–581. [Google Scholar] [CrossRef]
- Khaki, A.; Novin, M.G.; Khaki, A.; Fathiazad, F.; Khaberi, M.; Hossinchi, J.; Sehizadeh, R. Ultrastructural study of gentamicin and ofloxacin effect on testis tissue in rats: Light and transmission electron microscopy. Afr. J. Pharm. Pharmacol. 2009, 3, 105–109. [Google Scholar]
- Sharpe, R.; Maddocks, S.; Millar, M.; Kerr, J.; Saunders, P.; McKinnell, C. Testosterone and Spermatogenesis Identification of Stage-Specific, Androgen-Regulated Proteins Secreted by Adult Rat Seminiferous Tubules. J. Androl. 1992, 13, 172–184. [Google Scholar] [PubMed]
- Meligy, F.Y.; El-Deen Mohammed, H.S.; Mostafa, T.M.; Elfiky, M.M.; El-Sayed Mohamed Ashry, I.; Abd-Eldayem, A.M.; Rizk, N.I.; Sabry, D.; Abd Allah, E.S.H.; Ahmed, S.F. Therapeutic Potential of Mesenchymal Stem Cells versus Omega n—3 Polyunsaturated Fatty Acids on Gentamicin-Induced Cardiac Degeneration. Pharmaceutics 2022, 14, 1322. [Google Scholar] [CrossRef]
- Meseguer, M.; Martinez-Conejero, J.A.; O’Connor, J.E.; Pellicer, A.; Remohi, J.; Garrido, N. The significance of sperm DNA oxidation in embryo development and reproductive outcome in an oocyte donation program: A new model to study a male infertility prognostic factor. Fertil. Steril. 2008, 89, 1191–1199. [Google Scholar] [CrossRef]
- Pigeolet, E.; Corbisier, P.; Houbion, A.; Lambert, D.; Michiels, C.; Raes, M.; Zachary, M.D.; Remacle, J. Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech. Ageing Dev. 1990, 51, 283–297. [Google Scholar] [CrossRef]
- Ichikawa, T.; Oeda, T.; Ohmori, H.; Schill, W. Reactive oxygen species influence the acrosome reaction but not acrosin activity in human spermatozoa. Int. J. Androl. 1999, 22, 37–42. [Google Scholar] [CrossRef]
- Griveau, J.; Lannou, D.L. Reactive oxygen species and human spermatozoa: Physiology and pathology. Int. J. Androl. 1997, 20, 61–69. [Google Scholar] [CrossRef]
- Saif, A.; Sarhan, O.; Elmogy, M.; Mutwally, D.H. Hepatoprotective effects of Zamzam water against carbon tetrachloride induced liver damage in rats: Biochemical, histopathological, and molecular evidence. Life Sci. J. 2014, 11, 300–308. [Google Scholar]
- El Maleky, W.; Mahfoz, A.M.; Osman, A.O.; Abd El-Latif, H.A. Investigation of the impacts of Zamzam water on streptozotocin-induced diabetic nephropathy in rats. In-vivo and in-vitro study. Biomed. Pharmacother. 2021, 138, 111474. [Google Scholar] [CrossRef]
- Elnour, A.; Bamosa, A.; Al Meheithif Aleissa, K. Amelioration of Severe Carbon Tetrachloride Toxicity by Zamzam Water in Rats. J. Nutr. Food Sci. 2013, 3, 2. [Google Scholar]
- Althunibat, O.Y.; Abukhalil, M.H.; Aladaileh, S.H.; Qaralleh, H.; Al-Amarat, W.; Alfwuaires, M.A.; Algefare, A.I.; Namazi, N.I.; Melebary, S.J.; Babalghith, A.O.; et al. Formononetin Ameliorates Renal Dysfunction, Oxidative Stress, Inflammation, and Apoptosis and Upregulates Nrf2/HO-1 Signaling in a Rat Model of Gentamicin-Induced Nephrotoxicity. Front. Pharmacol. 2022, 13, 916732. [Google Scholar] [CrossRef] [PubMed]
- Satta, S.; Mahmoud, A.M.; Wilkinson, F.L.; Alexander, M.Y.; White, S.J. The role of Nrf2 in cardiovascular function and disease. Oxid. Med. Cell. Longev. 2017, 2017, 9237263. [Google Scholar] [CrossRef] [PubMed]
- Moni, S.S.; Sultan, M.H.; Alshahrani, S.; Tripathi, P.; Assiri, A.; Alqahtani, S.S.; Bakkari, M.A.; Madkhali, O.A.; Alam, M.F.; Alqahtani, A.H.; et al. Physical characterization, and wound healing properties of Zamzam water. Braz. J. Biol. 2022, 82, e262815. [Google Scholar] [CrossRef]
- Ambrosch, A.; Lobmann, R.; Pott, A.; Preissler, J. Interleukin-6 concentrations in wound fluids rather than serological markers are useful in assessing bacterial triggers of ulcer inflammation. Int. Wound J. 2008, 5, 99–106. [Google Scholar] [CrossRef]
- Yang, L.; Li, D.; Chen, S. Hydrogen water reduces NSE, IL-6, and TNF-α levels in hypoxic-ischemic encephalopathy. Open Med. 2016, 11, 399–406. [Google Scholar] [CrossRef]
- Shaha, C.; Tripathi, R.; Mishra, D.P. Male germ cell apoptosis: Regulation and biology. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1501–1515. [Google Scholar] [CrossRef]
- Kitazumi, I.; Tsukahara, M. Regulation of DNA fragmentation: The role of caspases and phosphorylation. FEBS J. 2011, 278, 427–441. [Google Scholar] [CrossRef]
- Singla, N.; Dhawan, D.K. Zinc Improves Cognitive and Neuronal Dysfunction During Aluminium-Induced Neurodegeneration. Mol. Neurobiol. 2016, 54, 406–422. [Google Scholar] [CrossRef]

| 10 | Complete spermatogenesis with many mature spermatozoa |
| 9 | Many spermatozoa, with a disorganized germinal epithelium that showed sloughing into lumen |
| 8 | Presence of few spermatozoa (<5 to 10/seminiferous tubule) |
| 7 | Absence of spermatozoa, but many spermatids are present |
| 6 | Absence of spermatozoa, with few spermatids (<5/seminiferous tubule) |
| 5 | Absence of spermatozoa, and spermatid, with the presence of several spermatocytes |
| 4 | Absence of spermatozoa, and spermatid, with the presence of few spermatocytes |
| 3 | Spermatogonia are the only cell present |
| 2 | Sertoli cells only present with absence of germ cells |
| 1 | No cells visualized in the tubular section |
| Forward Sequence | Reverse Sequence | Gene Accession Number | |
|---|---|---|---|
| Nrf2 | AGGACATGGAGCAAGTTTGG | TTGCCCTAAGCTCATCTCGT | NM_031789.2 |
| HO-1 | TCAGGTGTCCAGAGAAGGCTTT | CTCTTCCAGGGCCGTGTAGA | NM_012580.2 |
| GAPDH | CCTTCTCCATGGTGGTGAAGA | CACCATCTTCCAGGAGCGAG | NM_001394060.2 |
| Control Group | Gentamicin Group | Gentamicin + Zamzam Water | |
|---|---|---|---|
| Initial body weight (gm) | 142.2 ± 10.2 | 142.7 ± 12.05 | 146.9 ± 13.4 |
| Final body weight (gm) | 205 ± 12.57 | 189.7 ± 14.52 | 202.3 ± 16.6 |
| Weight of the testes (gm) | 3.1 ± 0.41 | 2.42 ± 0.38 ** | 2.9 ± 0.47 # |
| Group | Testicular Tissue | Serum | |||||
|---|---|---|---|---|---|---|---|
| GPx μmol/g | SOD U/g | MDA nmol/g | CAT U/g | Testosterone (nmol/L) | FSH (mIU/mL) | LH (mIU/mL) | |
| Control group | 3.02 ± 0.23 | 187.6 ± 8.05 | 9.5 ± 1.01 | 4.14 ± 0.22 | 5.01 ± 0.53 | 0.93 ± 0.07 | 2.37 ± 0.18 |
| Gentamicin group | 0.98 ± 0.12 *** | 88.9 ± 7.9 *** | 36.48 ± 3.09 *** | 1.23 ± 0.27 *** | 1.99 ± 0.33 *** | 0.40 ± 0.06 *** | 1.14 ± 0.09 *** |
| Gentamicin + Zamzam water group | 2.83 ± 0.42 ### | 144.9 ± 9.35 ### | 25.49 ± 2.77 ### | 3.06 ± 0.55 ### | 2.83 ± 0.26 ## 0.0004 | 0.77 ± 0.06 ### | 1.68 ± 0.13 ### |
| Percentage of Sperm Motility % | Percentage of Progressive Motility % | Percentage of Dead Sperm % | Percentage of Abnormal Sperm % | |
|---|---|---|---|---|
| Control group | 68.3 ± 2.49 | 42.8 ± 2.15 | 20.0 ± 1.94 | 12.1 ± 2.13 |
| Gentamicin group | 27.9 ± 5.62 *** | 12.6 ± 2.01 *** | 46.7 ± 4.21 *** | 29.3 ± 2.75 *** |
| Gentamicin + Zamzam water group | 49.8 ± 4.06 ### | 18.1 ± 4.86 # p = 0.0024 | 29.4 ± 2.87 ### | 21.5 ± 2.46 ### |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, M.; Elazab, S.T.; Saati, A.A.; Ahmed, G.S.; Baokbah, T.A.S.; Fathy, K.; El-Shenbaby, I.; Abdelbagi, O.; Hassan, M.A.E.; Ibrahim, M.M.; et al. Zamzam Water Ameliorates Gentamicin-Induced Testicular Toxicity in a Rat Model via Targeting Sperm Parameters, Testicular Tissue Oxidative Insult, Inflammation, Apoptosis, and Pituitary-Gonadal Axis. Toxics 2023, 11, 2. https://doi.org/10.3390/toxics11010002
Taha M, Elazab ST, Saati AA, Ahmed GS, Baokbah TAS, Fathy K, El-Shenbaby I, Abdelbagi O, Hassan MAE, Ibrahim MM, et al. Zamzam Water Ameliorates Gentamicin-Induced Testicular Toxicity in a Rat Model via Targeting Sperm Parameters, Testicular Tissue Oxidative Insult, Inflammation, Apoptosis, and Pituitary-Gonadal Axis. Toxics. 2023; 11(1):2. https://doi.org/10.3390/toxics11010002
Chicago/Turabian StyleTaha, Medhat, Sara T. Elazab, Abdullah A. Saati, Gomaa S. Ahmed, Tourki A. S. Baokbah, Khaled Fathy, Ibrahim El-Shenbaby, Omer Abdelbagi, Mahmoud A. E. Hassan, Mohie Mahmoud Ibrahim, and et al. 2023. "Zamzam Water Ameliorates Gentamicin-Induced Testicular Toxicity in a Rat Model via Targeting Sperm Parameters, Testicular Tissue Oxidative Insult, Inflammation, Apoptosis, and Pituitary-Gonadal Axis" Toxics 11, no. 1: 2. https://doi.org/10.3390/toxics11010002
APA StyleTaha, M., Elazab, S. T., Saati, A. A., Ahmed, G. S., Baokbah, T. A. S., Fathy, K., El-Shenbaby, I., Abdelbagi, O., Hassan, M. A. E., Ibrahim, M. M., & Badawy, A. M. (2023). Zamzam Water Ameliorates Gentamicin-Induced Testicular Toxicity in a Rat Model via Targeting Sperm Parameters, Testicular Tissue Oxidative Insult, Inflammation, Apoptosis, and Pituitary-Gonadal Axis. Toxics, 11(1), 2. https://doi.org/10.3390/toxics11010002









