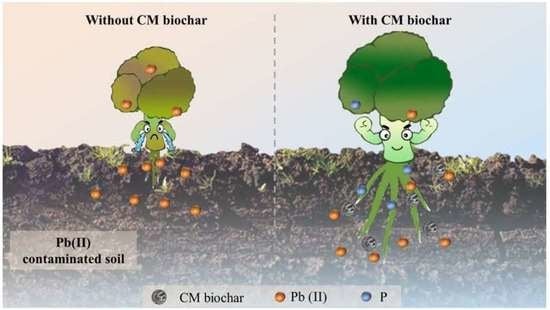
Rapid and Effective Lead Elimination Using Cow Manure Derived Biochar: Balance between Inherent Phosphorus Release and Pollutants Immobilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
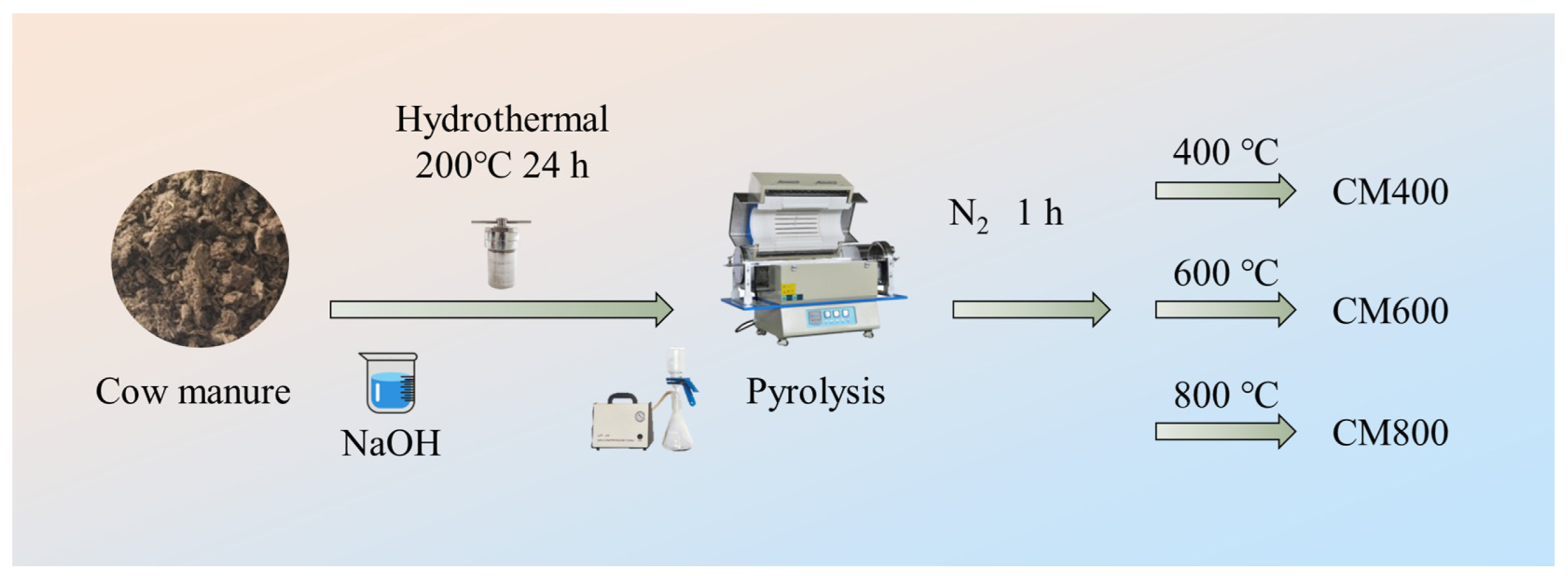
2.2. Preparation of CMBC
2.3. Analysis Methods
2.4. Adsorption Experiment
2.5. Pot Experiments
3. Results and Discussion
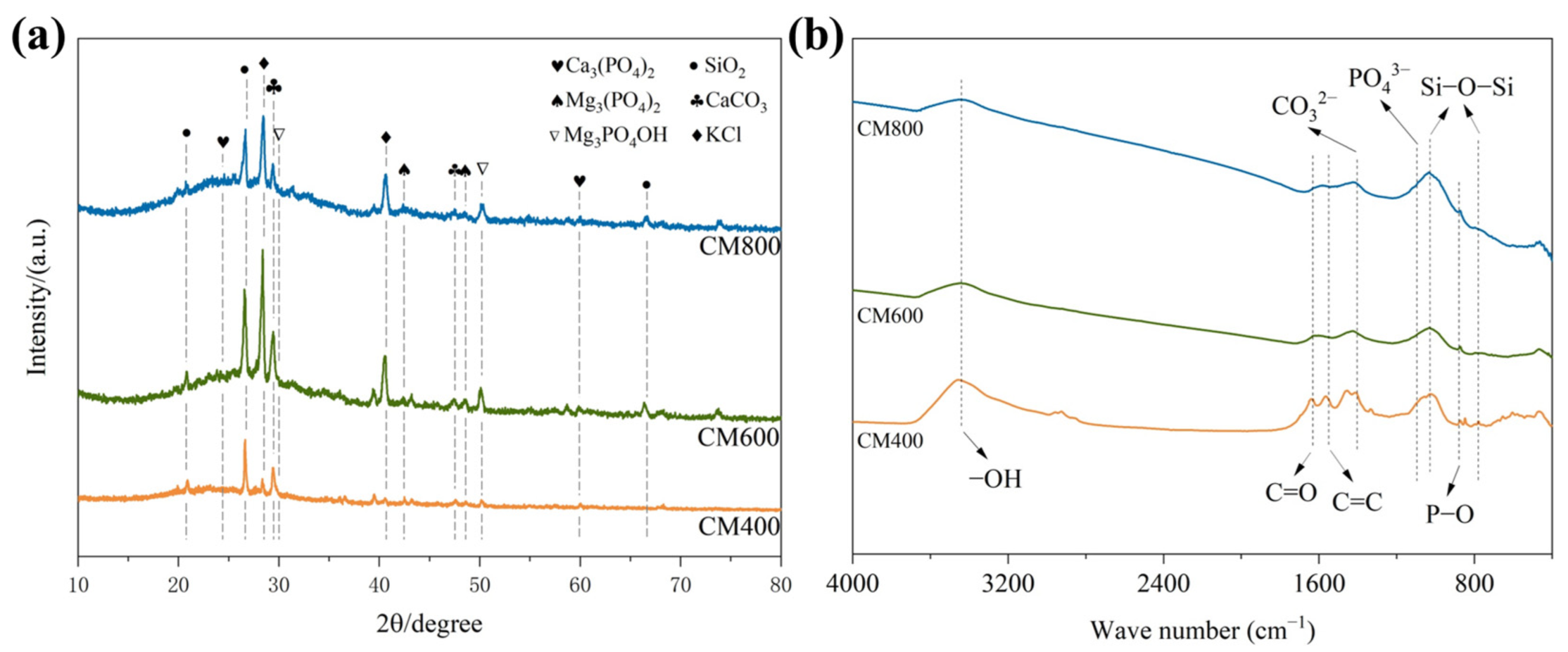
3.1. Characterization
3.2. Pb(II) Adsorption
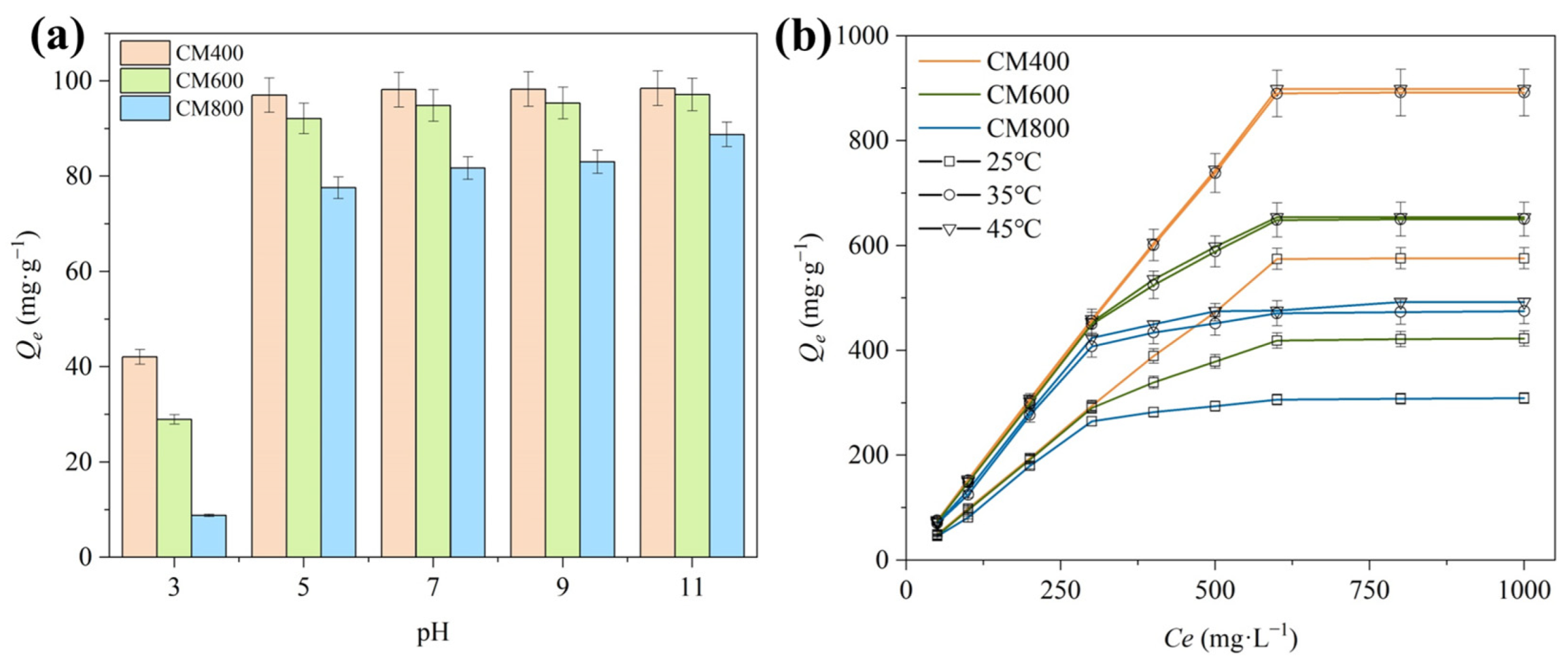
3.2.1. Effect of pH
3.2.2. Effect of Temperature
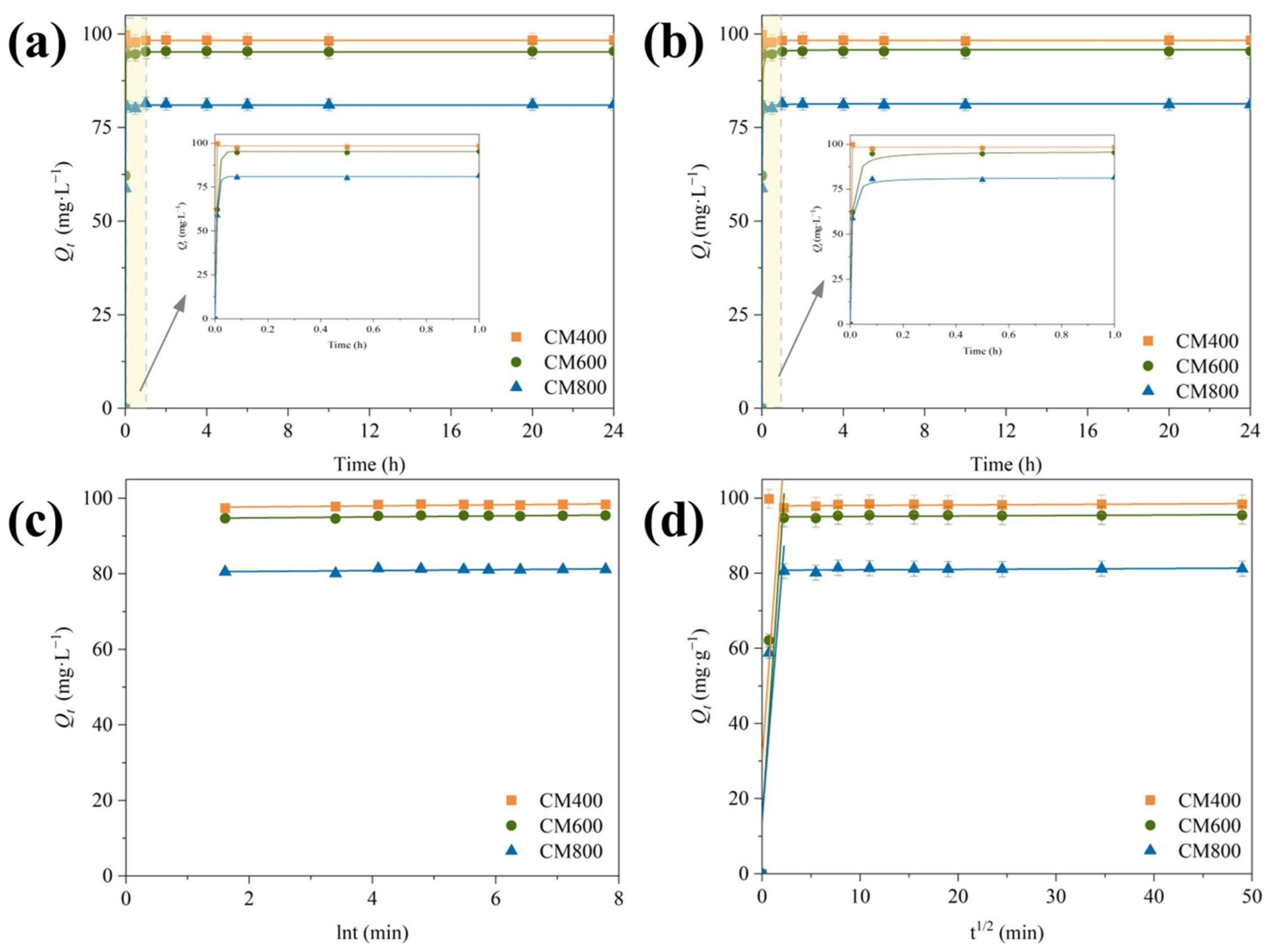
3.2.3. Adsorption Kinetics
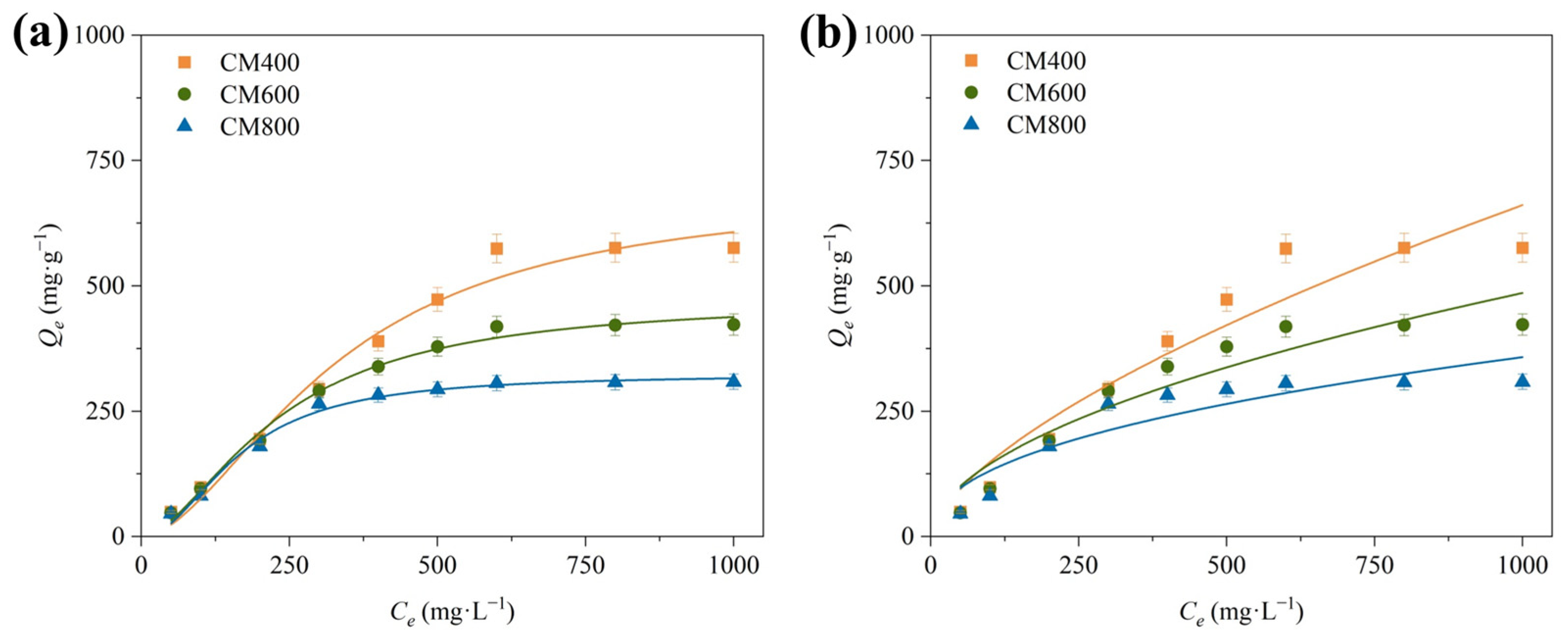
3.2.4. Isothermal Adsorption
| Adsorbent | Biomass | Pyrolyzation (°C) | Balance Time (min) | Qmax (mg·g–1) | Refs. |
|---|---|---|---|---|---|
| Nitrogen- and phosphorus-enriched biochar | C. oleifera shells | 550 | 200 | 723.6 | [41] |
| Biochar-supported phosphate-doped ferrihydrite | Corn stalks | 400 | 360 | 247 | [42] |
| Phosphorous-laden biochar | Sawdust | 500 | 1440 | 568.22 | [43] |
| Dairy manure-derived biochar | Cow manure | 200 | 180 | 140.76 | [44] |
| Biochar-supported nanoscale ferrous sulfide composite | Peanut shells | 250 | 150 | 88.06 | [45] |
| Sugarcane bagasse biochar | Sugarcane bagasse | 600 | 360 | 2.5 | [46] |
| Iron-sulfur co-doped biochar composite | rice straw | 300 | 240 | 631.7 | [3] |
| Cow manure biochar | Cow manure | 400 | 0.5 | 691.34 | This work |
| 600 | 5 | 473.36 | |||
| 800 | 5 | 323.83 |
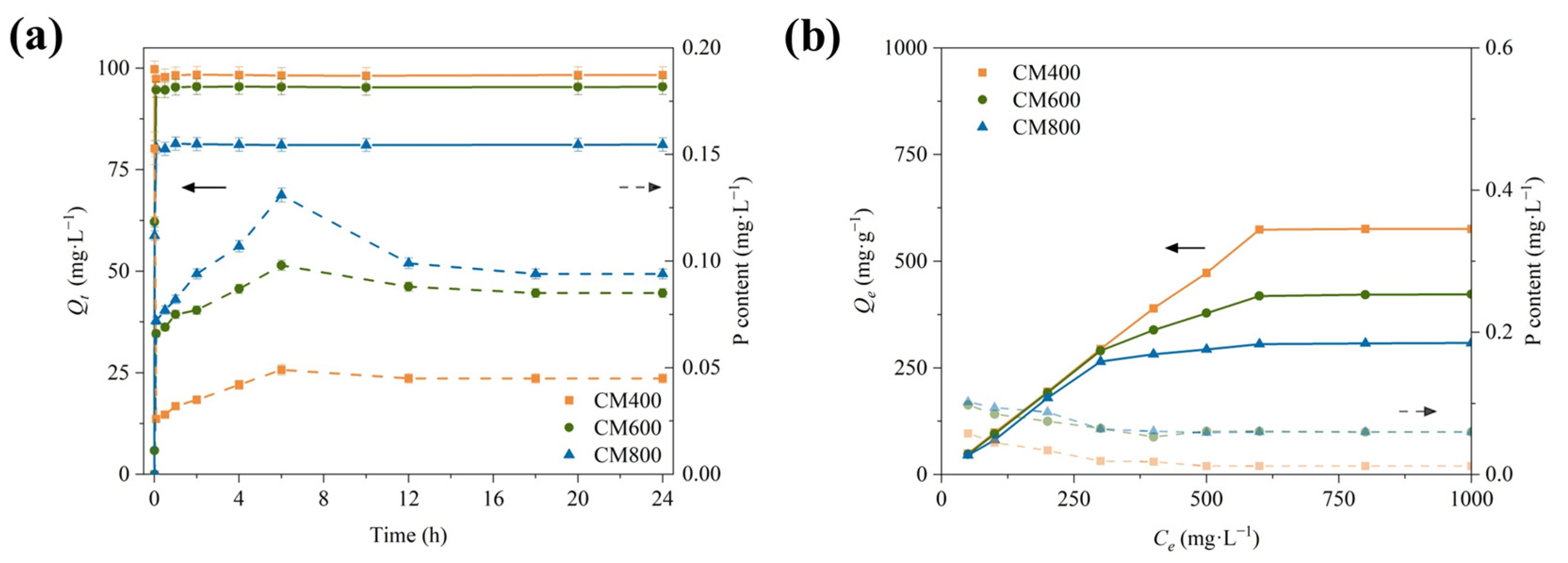
3.3. Inherent Phosphorus Release
3.4. Adsorption Mechanism Analysis
3.5. Analysis of Pot Experiment Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.H.; Tian, S.C.; Zhu, Y.; Zhong, W.; Qiu, R.; Han, L. Insight into the adsorption isotherms and kinetics of Pb (II) on pellet biochar via in-situ non-destructive 3D visualization using micro-computed tomography. Bioresour. Technol. 2022, 358, 127406. [Google Scholar] [CrossRef]
- Luo, X.; Wu, C.; Lin, Y.; Li, W.; Deng, M.; Tan, J.; Xue, S. Soil heavy metal pollution from Pb/Zn smelting regions in China and the remediation potential of biomineralization. J. Environ. Sci. 2023, 125, 662–677. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zhao, S.; Guo, H.; Xing, B.; Liu, Y.; Zhang, C.; Ma, M. High-efficiency removal of lead/cadmium from wastewater by MgO modified biochar derived from crofton weed. Bioresour. Technol. 2021, 343, 126081. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Chen, Z.; Zhou, X.; Wang, J.; Chen, Z. Engineered biochar with anisotropic layered double hydroxide nanosheets to simultaneously and efficiently capture Pb2+ and CrO42− from electroplating wastewater. Bioresour. Technol. 2020, 306, 123118. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Wang, L.; O’Connor, D.; Rinklebe, J.; Hou, D. Natural field freeze-thaw process leads to different performances of soil amendments towards Cd immobilization and enrichment. Sci. Total Environ. 2022, 831, 154880. [Google Scholar] [CrossRef] [PubMed]
- Soudani, A.; Youcef, L.; Bulgariu, L.; Youcef, S.; Toumi, K.; Soudani, N. Characterizing and modeling of oak fruit shells biochar as an adsorbent for the removal of Cu, Cd, and Zn in single and in competitive systems. Chem. Eng. Res. Des. 2022, 188, 972–987. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Wang, J.; Tang, Y.; Zhang, Z. Selective adsorption of Pb2+ and Cu2+ on amino-modified attapulgite: Kinetic, thermal dynamic and DFT studies. J. Hazard. Mater. 2020, 404, 124140. [Google Scholar] [CrossRef]
- Zhi, B.; Xiang, S.; Wang, Y.; Dai, Z.; Du, P.; Wang, R.; Li, X.; Yang, G.; Feng, Y.; Ren, G.; et al. Redeploy manure resources to enhance the agro-pastoral cycle. Sci. Total Environ. 2022, 846, 157439. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, Y.; Gu, X.; Zheng, M.; Fan, Z.; Luo, D.; Luo, K.; Liu, B. Spatiotemporal variation characteristics of livestock manure nutrient in the soil environment of the Yangtze River Delta from 1980 to 2018. Sci. Rep. 2022, 12, 7353. [Google Scholar] [CrossRef]
- Behjat, M.; Svanström, M.; Peters, G. A meta-analysis of LCAs for environmental assessment of a conceptual system: Phosphorus recovery from dairy wastewater. J. Clean. Prod. 2022, 369, 133307. [Google Scholar] [CrossRef]
- Fajobi, M.O.; Lasode, O.A.; Adeleke, A.A.; Ikubanni, P.P.; Balogun, A.O. Investigation of physicochemical characteristics of selected lignocellulose biomass. Sci. Rep. 2022, 12, 2918. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Wang, B.; Wang, H.; Liu, H.; Xie, H.; Han, L.; Wang, N.; Sun, X.; Feng, Y.; Xue, L. Assessment of livestock manure-derived hydrochar as cleaner products: Insights into basic properties, nutrient composition, and heavy metal content. J. Clean. Prod. 2021, 330, 129820. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Sasaki, Y.; Katahira, M.; Singh, D. Cow Manure Application Cuts Chemical Phosphorus Fertilizer Need in Silage Rice in Japan. Agronomy 2021, 11, 1483. [Google Scholar] [CrossRef]
- Chen, G.; Wang, J.; Yu, F.; Wang, X.; Xiao, H.; Yan, B.; Cui, X. A review on the production of P-enriched hydro/bio-char from solid waste: Transformation of P and applications of hydro/bio-char. Chemosphere 2022, 301, 134646. [Google Scholar] [CrossRef] [PubMed]
- Holatko, J.; Hammerschmiedt, T.; Mustafa, A.; Kintl, A.; Radziemska, M.; Baltazar, T.; Jaskulska, I.; Malicek, O.; Latal, O.; Brtnicky, M. Carbon-enriched organic amendments differently affect the soil chemical, biological properties and plant biomass in a cultivation time-dependent manner. Chem. Biol. Technol. Agric. 2022, 9, 52. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, X.; Ke, S.; Shao, J.; Yang, H.; Zhang, S.; Chen, H. Effect of different biomass species and pyrolysis temperatures on heavy metal adsorption, stability and economy of biochar. Ind. Crop. Prod. 2022, 186, 115238. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Li, Y.; Han, L. Influence of pyrolysis temperature on chemical speciation, leaching ability, and environmental risk of heavy metals in biochar derived from cow manure. Bioresour. Technol. 2020, 302, 122850. [Google Scholar] [CrossRef]
- Mulyani, O.; Joy, B.; Kurnia, D. The Various Forms of Cow Manure Waste as Adsorbents of Heavy Metals. Appl. Sci. 2022, 12, 5763. [Google Scholar] [CrossRef]
- Sarmah, M.; Borgohain, A.; Gogoi, B.B.; Yeasin, M.; Paul, R.K.; Malakar, H.; Handique, J.G.; Saikia, J.; Deka, D.; Khare, P.; et al. Insights into the effects of tea pruning litter biochar on major micronutrients (Cu, Mn, and Zn) pathway from soil to tea plant: An environmental armour. J. Hazard. Mater. 2023, 442, 129970. [Google Scholar] [CrossRef]
- Naeem, M.A.; Imran, M.; Amjad, M.; Abbas, G.; Tahir, M.; Murtaza, B.; Zakir, A.; Shahid, M.; Bulgariu, L.; Ahmad, I. Batch and Column Scale Removal of Cadmium from Water Using Raw and Acid Activated Wheat Straw Biochar. Water 2019, 11, 1438. [Google Scholar] [CrossRef]
- Goldfarb, J.L.; Hubble, A.H.; Ma, Q.; Volpe, M.; Severini, G.; Andreottola, G.; Fiori, L. Valorization of cow manure via hydrothermal carbonization for phosphorus recovery and adsorbents for water treatment. J. Environ. Manag. 2022, 308, 114561. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Chen, H.; Lin, D.; Xu, H.; Wang, J.; Zhang, J.; Hu, Z.; Deng, J.; Gao, J.; Li, H.; et al. A field study of nano-FeS loaded lignin hydrogel application for Cd reduction, nutrient enhancement, and microbiological shift in a polluted paddy soil. Chem. Eng. J. 2023, 451, 138647. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, F.; Duan, L.; Wu, H.; Zhang, H. The Industrial Pollution History Inferred by Stable Pb Isotope in the Chronological Sedimentary Record of a Plateau Lake, Yunnan Province, Southwestern of China. J. Coast. Res. 2020, 115, 641–647. [Google Scholar] [CrossRef]
- He, J.; Yang, Z.; Guo, M.; Gu, L.; Zhang, L.; Yan, Y.; Ran, J. Experimental study on the key factors affecting the gasification performance between different biomass: Compare citrus peel with pine sawdust. Int. J. Hydrogen Energy 2022, 47, 30428–30439. [Google Scholar] [CrossRef]
- Yan, C.; Jin, J.; Wang, J.; Zhang, F.; Tian, Y.; Liu, C.; Zhang, F.; Cao, L.; Zhou, Y.; Han, Q. Metal-organic frameworks (MOFs) for the efficient removal of contaminants from water: Underlying mechanisms, recent advances, challenges, and future prospects. Coord. Chem. Rev. 2022, 468, 214595. [Google Scholar] [CrossRef]
- Ahmad, S.; Gao, F.; Lyu, H.; Ma, J.; Zhao, B.; Xu, S.; Ri, C.; Tang, J. Temperature-dependent carbothermally reduced iron and nitrogen doped biochar composites for removal of hexavalent chromium and nitrobenzene. Chem. Eng. J. 2022, 450, 138006. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Zong, Y.; Yu, J.; Ding, H.; Kong, Y.; Ma, J.; Ding, L. Efficient removal of cadmium by salts modified-biochar: Performance assessment, theoretical calculation, and quantitative mechanism analysis. Bioresour. Technol. 2022, 361, 127717. [Google Scholar] [CrossRef]
- Shakya, A.; Vithanage, M.; Agarwal, T. Influence of pyrolysis temperature on biochar properties and Cr(VI) adsorption from water with groundnut shell biochars: Mechanistic approach. Environ. Res. 2022, 215, 114243. [Google Scholar] [CrossRef]
- Anacleto TMOliveira, H.R.; Diniz, V.L.; de Oliveira, V.P.; Abreu, F.; Enrich-Prast, A. Boosting manure biogas production with the application of pretreatments: A meta-analysis. J. Clean. Prod. 2022, 362, 132292. [Google Scholar] [CrossRef]
- Adhikari, S.; Timms, W.; Mahmud, M.P. Optimising water holding capacity and hydrophobicity of biochar for soil amendment—A review. Sci. Total. Environ. 2022, 851, 158043. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Huang, D.; Liu, X.; Tang, C.; Parikh, S.J.; Xu, J. A novel calcium-based magnetic biochar is effective in stabilization of arsenic and cadmium co-contamination in aerobic soils. J. Hazard. Mater. 2020, 387, 122010. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, D.; Liu, X.; Meng, J.; Tang, C.; Xu, J. Remediation of As(III) and Cd(II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar. J. Hazard. Mater. 2018, 348, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, J.; Su, Y.; Li, Q.; Gao, B.; Yue, Q.; Zhou, W. Adsorption and recycling of Cd(II) from wastewater using straw cellulose hydrogel beads. J. Ind. Eng. Chem. 2019, 80, 361–369. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Xia, S.; Zhao, J. Treatment of Pb(II) pollution in livestock wastewater by MgFe2O4 modified manure-biochar derived from livestock itself: Special role of endogenous dissolved organic matter and P species. Chem. Eng. J. 2022, 446, 137068. [Google Scholar] [CrossRef]
- Kumaraswamy, R.V.; Saharan, V.; Kumari, S.; Choudhary, R.C.; Pal, A.; Sharma, S.S.; Rakshit, S.; Raliya, R.; Biswas, P. Chitosan-silicon nanofertilizer to enhance plant growth and yield in maize (Zea mays L.). Plant Physiol. Biochem. 2021, 159, 53–66. [Google Scholar] [CrossRef]
- Ul Ain, Q.; Zhang, H.; Yaseen, M.; Rasheed, U.; Liu, K.; Subhan, S.; Tong, Z. Facile fabrication of hydroxyapatite-magnetite-bentonite composite for efficient adsorption of Pb(II), Cd(II), and crystal violet from aqueous solution. J. Clean. Prod. 2020, 247, 119088. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, B.; Zhang, X.; Xu, H.; Cheng, N.; Feng, Q.; Zhao, R.; Gao, Y.; Wei, M. Release characteristics of phosphate from ball-milled biochar and its potential effects on plant growth. Sci. Total. Environ. 2022, 821, 153256. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Cao, B.; Yin, H.; Al-Tabbaa, A. Simultaneous removal of Pb and MTBE by mixed zeolites in fixed-bed column tests. J. Environ. Sci. 2022, 122, 41–49. [Google Scholar] [CrossRef]
- Chowdhury, I.R.; Chowdhury, S.; Mazumder, M.A.J.; Al-Ahmed, A. Removal of lead ions (Pb2+) from water and wastewater: A review on the low-cost adsorbents. Appl. Water Sci. 2022, 12, 185. [Google Scholar] [CrossRef]
- Xu, J.; Hu, C.; Wang, M.; Zhao, Z.; Zhao, X.; Cao, L.; Lu, Y.; Cai, X. Changeable effects of coexisting heavy metals on transfer of cadmium from soils to wheat grains. J. Hazard. Mater. 2021, 423, 127182. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, H.; Deng, L.; Wang, Y.; Kang, D.; Li, C.; Chen, H. Enhanced adsorption of Pb(II) by nitrogen and phosphorus co-doped biochar derived from Camellia oleifera shells. Environ. Res. 2020, 191, 110030. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, Q.; Zhang, J.; Wang, Y.; Zhang, Y. Synchronization adsorption of Pb(II) and Ce(III) by biochar supported phosphate-doped ferrihydrite in aqueous solution: Adsorption efficiency and mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129230. [Google Scholar] [CrossRef]
- Pei, L.; Yang, F.; Xu, X.; Nan, H.; Gui, X.; Zhao, L.; Cao, X. Further reuse of phosphorus-laden biochar for lead sorption from aqueous solution: Isotherm, kinetics, and mechanism. Sci. Total Environ. 2021, 792, 148550. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Liu, M.; Chen, Z. Dairy manure biochar modified with sodium hydroxide and its effect on lead removal in aqueous solution. In Proceedings of the 4th International Conference on Sensors, Measurement and Intelligent Materials (ICSMIM), Shenzhen, China, 27–28 December 2015. [Google Scholar]
- Chen, C.G.; Qiu, M.Q. High efficiency removal of Pb(II) in aqueous solution by a biochar-supported nanoscale ferrous sulfide composite. RSC Adv. 2021, 11, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.; Gao, B.; Zimmerman, A.; Zhou, Y.; Cao, X. Sorption and cosorption of lead and sulfapyridine on carbon nanotube-modified biochars. Environ. Sci. Pollut. Res. 2014, 22, 1868–1876. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Q.; Li, D.; Li, J.; Guo, W. Performance comparison of phosphorus recovery from different sludges in sewage treatment plants through pyrolysis. J. Clean. Prod. 2022, 372, 133728. [Google Scholar] [CrossRef]
- Cui, H.; Dong, T.; Hu, L.; Xia, R.; Zhou, J.; Zhou, J. Adsorption and immobilization of soil lead by two phosphate-based biochars and phosphorus release risk assessment. Sci. Total Environ. 2022, 824, 153957. [Google Scholar] [CrossRef]
- Bruun, S.; Harmer, S.L.; Bekiaris, G.; Christel, W.; Zuin, L.; Hu, Y.; Jensen, L.S.; Lombi, E. The effect of different pyrolysis temperatures on the speciation and availability in soil of P in biochar produced from the solid fraction of manure. Chemosphere 2017, 169, 377–386. [Google Scholar] [CrossRef]
- Liu, Z.; Ou, T.; Su, M.; Peng, H.; Song, G.; Kong, L.; Chen, D. U(VI) sequestration by Al-rich minerals: Mechanism on phase dependence and the influence of natural organic matter. Chem. Eng. J. 2021, 415, 128858. [Google Scholar] [CrossRef]
- Liu, M.; Hu, B.; Zhang, C.; Wang, Q.; Sun, Z.; He, P.; Chen, Y.; Chen, D.; Zhu, J. Effect of sodium silicate on the flotation separation of chalcopyrite and galena using sodium sulfite and sulfonated lignin as depressant. Miner. Eng. 2022, 182, 107563. [Google Scholar] [CrossRef]
- Li, J.; Zheng, L.; Wang, S.-L.; Wu, Z.; Wu, W.; Niazi, N.K.; Shaheen, S.M.; Rinklebe, J.; Bolan, N.; Ok, Y.S.; et al. Sorption mechanisms of lead on silicon-rich biochar in aqueous solution: Spectroscopic investigation. Sci. Total. Environ. 2019, 672, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Wei, S.; Liu, Y.; Zhang, X.; Jiang, Z.; Tao, Y.; Zhang, G.; Zhang, B.; Wang, L.; Zhang, Y. Effective lead passivation in soil by bone char/CMC-stabilized FeS composite loading with phosphate-solubilizing bacteria. J. Hazard. Mater. 2021, 423, 127043. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cai, J.; Liao, Z.; Jawad, A.; Ifthikar, J.; Chen, Z.; Chen, Z. Black liquor as biomass feedstock to prepare zero-valent iron embedded biochar with red mud for Cr(VI) removal: Mechanisms insights and engineering practicality. Bioresour. Technol. 2020, 311, 123553. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Huang, M.; Huang, C.-P. Specific chemical adsorption of selected divalent heavy metal ions onto hydrous γ-Fe2O3-biochar from dilute aqueous solutions with pH as a master variable. Chem. Eng. J. 2023, 451, 138921. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Ifthikar, J.; Shi, L.; Khan, A.; Chen, Z.; Chen, Z. Towards a better understanding on mercury adsorption by magnetic bio-adsorbents with gamma-Fe2O3 from pinewood sawdust derived hydrochar: Influence of atmosphere in heat treatment. Bioresour. Technol. 2018, 256, 269–276. [Google Scholar] [CrossRef]
- Ali, A.; Shaheen, S.M.; Guo, D.; Li, Y.; Xiao, R.; Wahid, F.; Azeem, M.; Sohail, K.; Zhang, T.; Rinklebe, J.; et al. Apricot shell- and apple tree-derived biochar affect the fractionation and bioavailability of Zn and Cd as well as the microbial activity in smelter contaminated soil. Environ. Pollut. 2020, 264, 114773. [Google Scholar] [CrossRef]
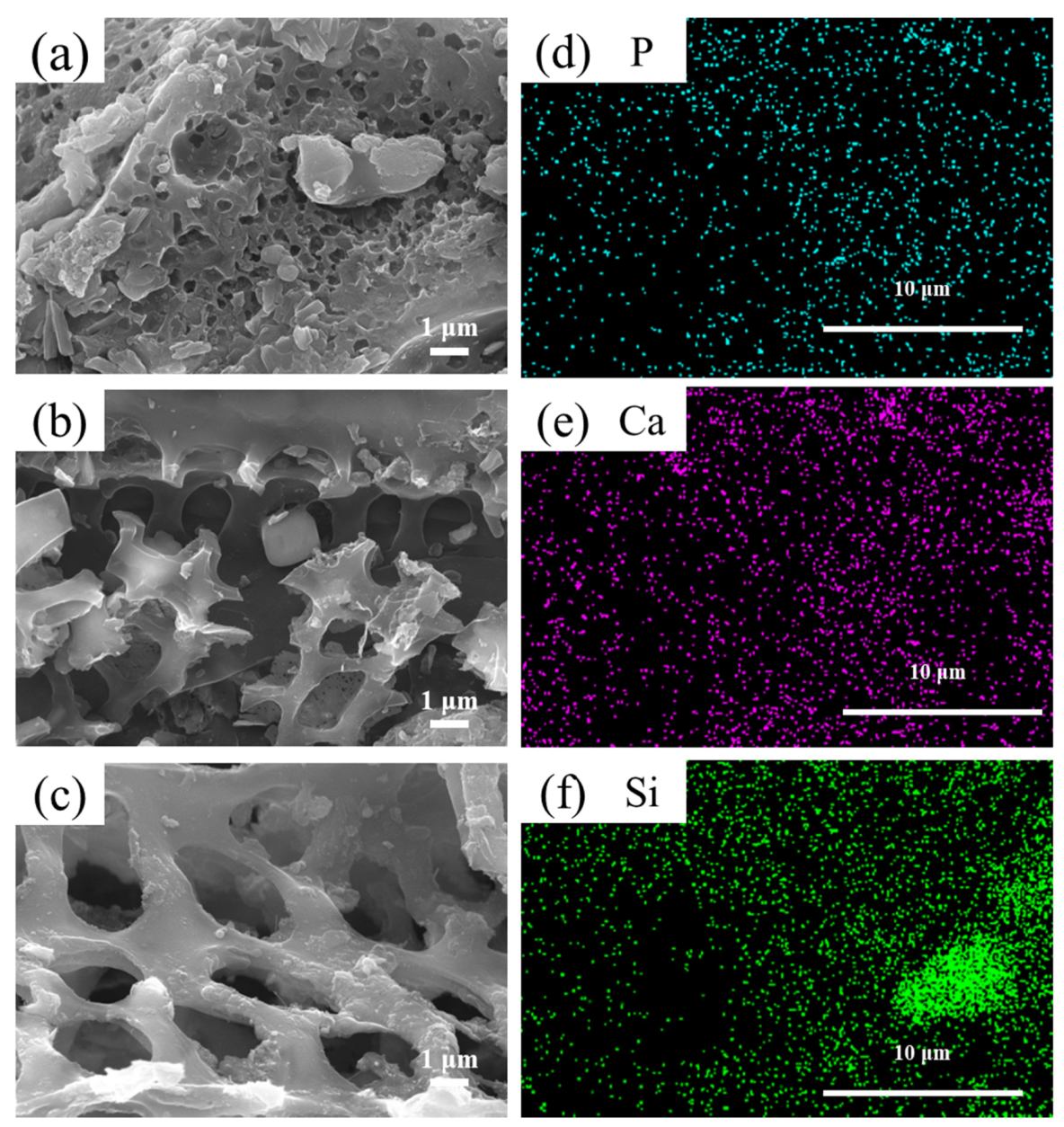


| CM400 | CM600 | CM800 | |
|---|---|---|---|
| C (%) | 28.11 | 37.14 | 39.21 |
| H (%) | 5.05 | 2.53 | 1.63 |
| O (%) | 41.18 | 40.34 | 37.89 |
| P (%) | 0.61 | 0.34 | 0.27 |
| O/C | 1.48 | 1.09 | 0.97 |
| H/C | 0.18 | 0.07 | 0.04 |
| BET (m2·g−1) | 2.51 | 11.78 | 12.93 |
| Vtotal (m3·g−1) | 0.05 | 0.03 | 0.03 |
| Average pore size (Å) | 837.94 | 90.31 | 108.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wen, Y.; Ding, Y.; Yue, Z.; Xu, D.; Liu, Y.; Zhang, Y.; Xu, R.; Zeng, W. Rapid and Effective Lead Elimination Using Cow Manure Derived Biochar: Balance between Inherent Phosphorus Release and Pollutants Immobilization. Toxics 2023, 11, 1. https://doi.org/10.3390/toxics11010001
Wang H, Wen Y, Ding Y, Yue Z, Xu D, Liu Y, Zhang Y, Xu R, Zeng W. Rapid and Effective Lead Elimination Using Cow Manure Derived Biochar: Balance between Inherent Phosphorus Release and Pollutants Immobilization. Toxics. 2023; 11(1):1. https://doi.org/10.3390/toxics11010001
Chicago/Turabian StyleWang, Huabin, Yi Wen, Yu Ding, Zhiqiang Yue, Dan Xu, Ying Liu, Yong Zhang, Rui Xu, and Weiqing Zeng. 2023. "Rapid and Effective Lead Elimination Using Cow Manure Derived Biochar: Balance between Inherent Phosphorus Release and Pollutants Immobilization" Toxics 11, no. 1: 1. https://doi.org/10.3390/toxics11010001
APA StyleWang, H., Wen, Y., Ding, Y., Yue, Z., Xu, D., Liu, Y., Zhang, Y., Xu, R., & Zeng, W. (2023). Rapid and Effective Lead Elimination Using Cow Manure Derived Biochar: Balance between Inherent Phosphorus Release and Pollutants Immobilization. Toxics, 11(1), 1. https://doi.org/10.3390/toxics11010001