Toxic-Induced Nonthyroidal Illness Syndrome Induced by Acute Low-Dose Pesticides Exposure—Preliminary In Vivo Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Blood Sample Analysis
2.3. Statistical Analysis
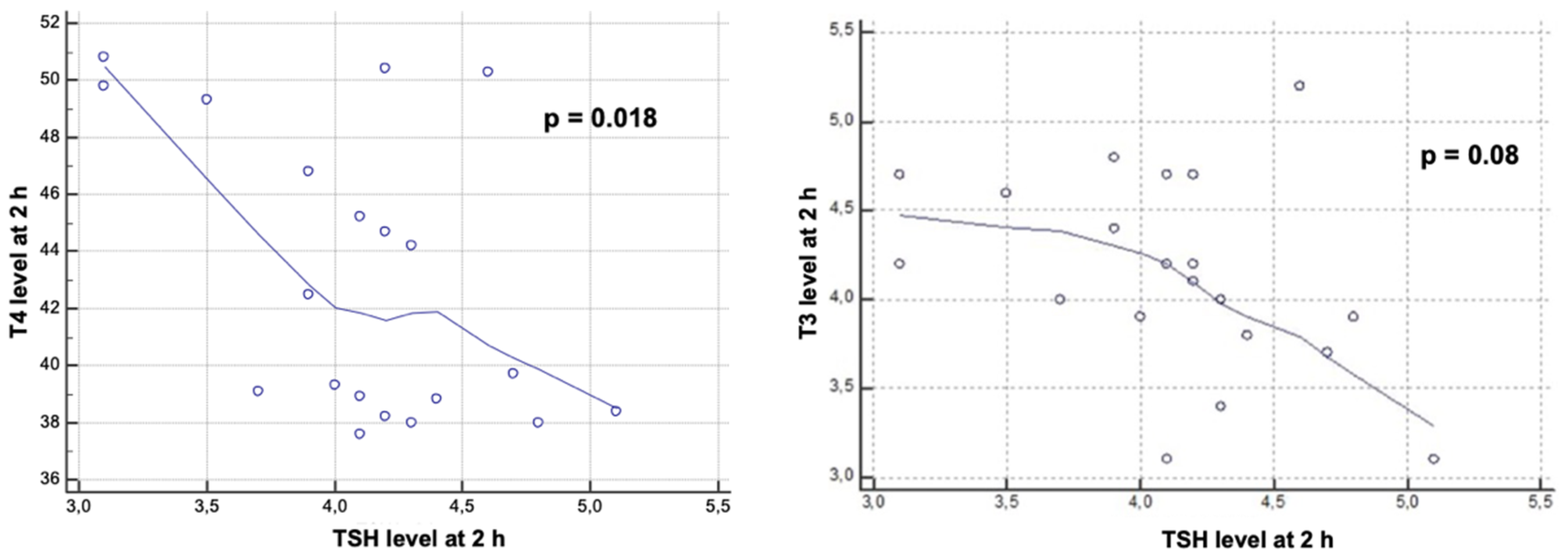
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ingels, C.; Gunst, J.; Van den Berghe, G. Endocrine and Metabolic Alterations in Sepsis and Implications for Treatment. Crit. Care Clin. 2018, 34, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.M.K.; Lekha, N.; Alegre, M.-L. Endocrine and metabolic considerations in critically ill patients. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Akbaș, T.; Șahin, E.I.; Öytürk, A. Alterations in thyroid hormones in brain-dead patients are related to non-thyroidal illness syndrome. Endokrynol. Pol. 2018, 69, 545–549. [Google Scholar] [PubMed]
- Warner, M.H.; Beckett, G.J. Mechanisms behind the non-thyroidal illness syndrome: An update. J. Endocrinol. 2009, 205, 1–13. [Google Scholar] [CrossRef]
- De Luca, R.; Davis, P.J.; Lin, H.Y.; Gionfra, F.; Percario, Z.A.; Affabris, E.; Pedersen, J.Z.; Marchese, C.; Trivedi, P.; Anastasiadou, E.; et al. Thyroid Hormones Interaction With Immune Response, Inflammation and Non-thyroidal Illness Syndrome. Front. Cell Dev. Biol. 2021, 8, 1–9. [Google Scholar] [CrossRef]
- Maiden, M.J.; Torpy, D.J. Thyroid Hormones in Critical Illness. Crit. Care Clin. 2019, 35, 375–388. [Google Scholar] [CrossRef]
- Grill, E.; Strong, M.; Sonnad, S.S.; Sarani, B.; Pascual, J.; Collins, H.; Sims, C.A. Altered thyroid function in severely injured patients. J. Surg. Res. 2013, 179, 132–137. [Google Scholar] [CrossRef][Green Version]
- Cobilinschi, C.; Tincu, R.C.; Baetu, A.; Deaconu, C.O.; Totan, A.; Rusu, A.; Neagu, P.T.; Grintescu, I.M. Endocrine Disturbances Induced by Low-Dose Organophosphate Exposure in Male Wistar Rats. Acta Endocrinol. 2021, 17, 177–185. [Google Scholar] [CrossRef]
- Dutta, P.; Kamath, S.S.; Bhalla, A.; Shah, V.N.; Srinivasan, A.; Gupta, P.; Singh, S. Effects of acute organophosphate poisoning on pituitary target gland hormones at admission, discharge and three months after poisoning: A hospital based pilot study. Indian J. Endocrinol. Metab. 2015, 19, 116–123. [Google Scholar] [CrossRef]
- Smallridge, R.C.; Carr, F.E.; Fein, H.G. Diisopropylfluorophosphate (DFP) reduces serum prolactin, thyrotropin, luteinizing hormone, and growth hormone and increases adrenocorticotropin and corticosterone in rats: Involvement of dopaminergic and somatostatinergic as well as cholinergic pathways. Toxicol. Appl. Pharmacol. 1991, 108, 284–295. [Google Scholar] [CrossRef]
- Gomez Tello, V.; Garcia De Lorenzo y Mateos, A.; Anon Elizalde, J.M.; Lopez Martinez, J. Patron hormonal hipofisario anterior y tiroideo en el paciente critico. Med. Intensiva 2000, 24, 307–315. [Google Scholar] [CrossRef]
- Leemans, M.; Couderq, S.; Demeneix, B.; Fini, J.B. Pesticides With Potential Thyroid Hormone-Disrupting Effects: A Review of Recent Data. Front. Endocrinol. 2019, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Lacasaña, M.; López-Flores, I.; Rodríguez-Barranco, M.; Aguilar-Garduño, C.; Blanco-Muñoz, J.; Pérez-Méndez, O.; Gamboa, R.; Gonzalez-Alzaga, B.; Bassol, S.; Cebrian, M.E. Interaction between organophosphate pesticide exposure and PON1 activity on thyroid function. Toxicol. Appl. Pharmacol. 2010, 249, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Garfitt, S.J.; Jones, K.; Mason, H.J.; Cocker, J. Exposure to the organophosphate diazinon: Data from a human volunteer study with oral and dermal doses. Toxicol. Lett. 2002, 134, 105–113. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Human Services Toxicological Profile for Chlorpyrifos. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp84.pdf (accessed on 13 May 2022).
- Lovick, T.A.; Zangrossi, H. Effect of Estrous Cycle on Behavior of Females in Rodent Tests of Anxiety. Front. Psychiatry 2021, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Vale, J.A. Toxicokinetic and toxicodynamic aspects of organophosphorus (OP) insecticide poisoning. Toxicol. Lett. 1998, 102, 649–652. [Google Scholar] [CrossRef]
- Eddleston, M.; Buckley, N.A.; Eyer, P.; Dawson, A.H. Management of acute organophosphorus pesticide poisoning. Lancet 2008, 371, 597–607. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, D.H.; Yoo, J.; Hwang, E.Y.; Shin, M.S.; Lee, N.T.; Cho, I.R.; Kang, H.G.; Kim, Y.J.; Park, S.; et al. Detection of organophosphate bound butyrylcholinesterase using a monoclonal antibody. Appl. Biol. Chem. 2017, 60, 233–240. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.K.; Kim, J.Y.; Lee, K.; Choi, J.R.; Chang, S.J.; Chung, C.H.; Park, K.S.; Oh, S.S.; Koh, S.B. Exposure to pesticides and the prevalence of diabetes in a rural population in Korea. Neurotoxicology 2019, 70, 12–18. [Google Scholar] [CrossRef]
- Eddleston, M.; Eyer, P.; Worek, F.; Rezvi Sheriff, M.H.; Buckley, N.A. Predicting outcome using butyrylcholinesterase activity in organophosphorus pesticide self-poisoning. QJM 2008, 101, 467–474. [Google Scholar] [CrossRef]
- Krenz, J.E.; Hofmann, J.N.; Smith, T.R.; Cunningham, R.N.; Fenske, R.A.; Simpson, C.D.; Keifer, M. Determinants of butyrylcholinesterase inhibition among agricultural pesticide handlers in Washington State: An Update. Ann. Occup. Hyg. 2015, 59, 25–40. [Google Scholar] [PubMed]
- Bomser, J.A.; Quistad, G.B.; Casida, J.E.; Kinase, S.; Activation, E.R.K.; Lipase, D.; Bomser, I.; Pharmacol, J.E.T.A. Chlorpyrifos Oxon Potentiates Diacylglycerol-Induced Extracellular Signal-Regulated Kinase (ERK 44/42) Activation, Possibly by Diacylglycerol Lipase Inhibition 1. Toxicol. Appl. Pharmacol. 2002, 178, 29–36. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, S.; Tassinari, R.; Maranghi, F.; Eusepi, A.; Di Virgilio, A.; Chiarotti, F.; Ricceri, L.; Pesciolini, A.V.; Gilardi, E.; Moracci, G.; et al. Developmental Exposure to Chlorpyrifos Induces Alterations in Thyroid and Thyroid Hormone Levels Without Other Toxicity Signs in Cd1 Mice. Toxicol. Sci. 2009, 108, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Ciloglu, F.; Peker, I.; Pehlivan, A.; Karacabey, K.; Ilhan, N.; Saygin, O.; Ozmerdivenli, R. Exercise intensity and its effects on thyroid hormones. Neuroendocrinol. Lett. 2005, 26, 830–834. [Google Scholar]
- Otênio, J.K.; Souza, K.D.; Alberton, O.; Alberton, L.R.; Moreno, K.G.T.; Gasparotto Junior, A.; Palozi, R.A.C.; Lourenço, E.L.B.; Jacomassi, E. Thyroid-disrupting effects of chlorpyrifos in female Wistar rats. Drug Chem. Toxicol. 2022, 45, 387–392. [Google Scholar] [CrossRef]
- Satar, S.; Ahmet, S.; Metin, T. Endocrine Effects of Organophosphate Antidotal Therapy. Adv. Ther. 2004, 21, 301–311. [Google Scholar] [CrossRef]
- Güven, M.; Bayram, F.; Ünlühizarci, K.; Kelestimur, F. Endocrine changes in patients with acute organophosphate poisoning. Hum. Exp. Toxicol. 1999, 18, 598–601. [Google Scholar] [CrossRef]
- Slotkin, T.A.; Cooper, E.M.; Stapleton, H.M.; Seidler, F.J. Does thyroid disruption contribute to the developmental neurotoxicity of chlorpyrifos? Environ. Toxicol. Pharmacol. 2013, 36, 284–287. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, B.Y.; Kang, H.G.; Ku, H.O.; Cho, J.H. Effect of chlorpyrifos-methyl on steroid and thyroid hormones in rat F0- and F1-generations. Toxicology 2006, 220, 189–202. [Google Scholar] [CrossRef]
- Wang, Y.F.; Heng, J.F.; Yan, J.; Dong, L. Relationship between disease severity and thyroid function in Chinese patients with euthyroid sick syndrome. Medicine 2018, 97, 1–5. [Google Scholar] [CrossRef]
- Bespalov, A.; Martin, M.; Steckler, T. Good Research Practice in Non-Clinical Pharmacology and Biomedicine; Springer Nature: Berlin/Heidelberg, Germany, 2020; ISBN 9783030336554. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cobilinschi, C.; Țincu, R.; Ungureanu, R.; Dumitru, I.; Băetu, A.; Isac, S.; Cobilinschi, C.O.; Grințescu, I.M.; Mirea, L. Toxic-Induced Nonthyroidal Illness Syndrome Induced by Acute Low-Dose Pesticides Exposure—Preliminary In Vivo Study. Toxics 2022, 10, 511. https://doi.org/10.3390/toxics10090511
Cobilinschi C, Țincu R, Ungureanu R, Dumitru I, Băetu A, Isac S, Cobilinschi CO, Grințescu IM, Mirea L. Toxic-Induced Nonthyroidal Illness Syndrome Induced by Acute Low-Dose Pesticides Exposure—Preliminary In Vivo Study. Toxics. 2022; 10(9):511. https://doi.org/10.3390/toxics10090511
Chicago/Turabian StyleCobilinschi, Cristian, Radu Țincu, Raluca Ungureanu, Ioana Dumitru, Alexandru Băetu, Sebastian Isac, Claudia Oana Cobilinschi, Ioana Marina Grințescu, and Liliana Mirea. 2022. "Toxic-Induced Nonthyroidal Illness Syndrome Induced by Acute Low-Dose Pesticides Exposure—Preliminary In Vivo Study" Toxics 10, no. 9: 511. https://doi.org/10.3390/toxics10090511
APA StyleCobilinschi, C., Țincu, R., Ungureanu, R., Dumitru, I., Băetu, A., Isac, S., Cobilinschi, C. O., Grințescu, I. M., & Mirea, L. (2022). Toxic-Induced Nonthyroidal Illness Syndrome Induced by Acute Low-Dose Pesticides Exposure—Preliminary In Vivo Study. Toxics, 10(9), 511. https://doi.org/10.3390/toxics10090511








