Mineralogical Properties of a Refractory Tantalum-Niobium Slag and the Effect of Roasting on the Leaching of Uranium-Thorium
Abstract
:1. Introduction
2. Experiments and Methods
2.1. Instrumental Techniques
2.2. Roasting and Leaching Experiments
3. Results and Discussion
3.1. Characterization Results of Mineralogical Properties of Slag
3.2. Elemental Content in Tantalum-Niobium Slag
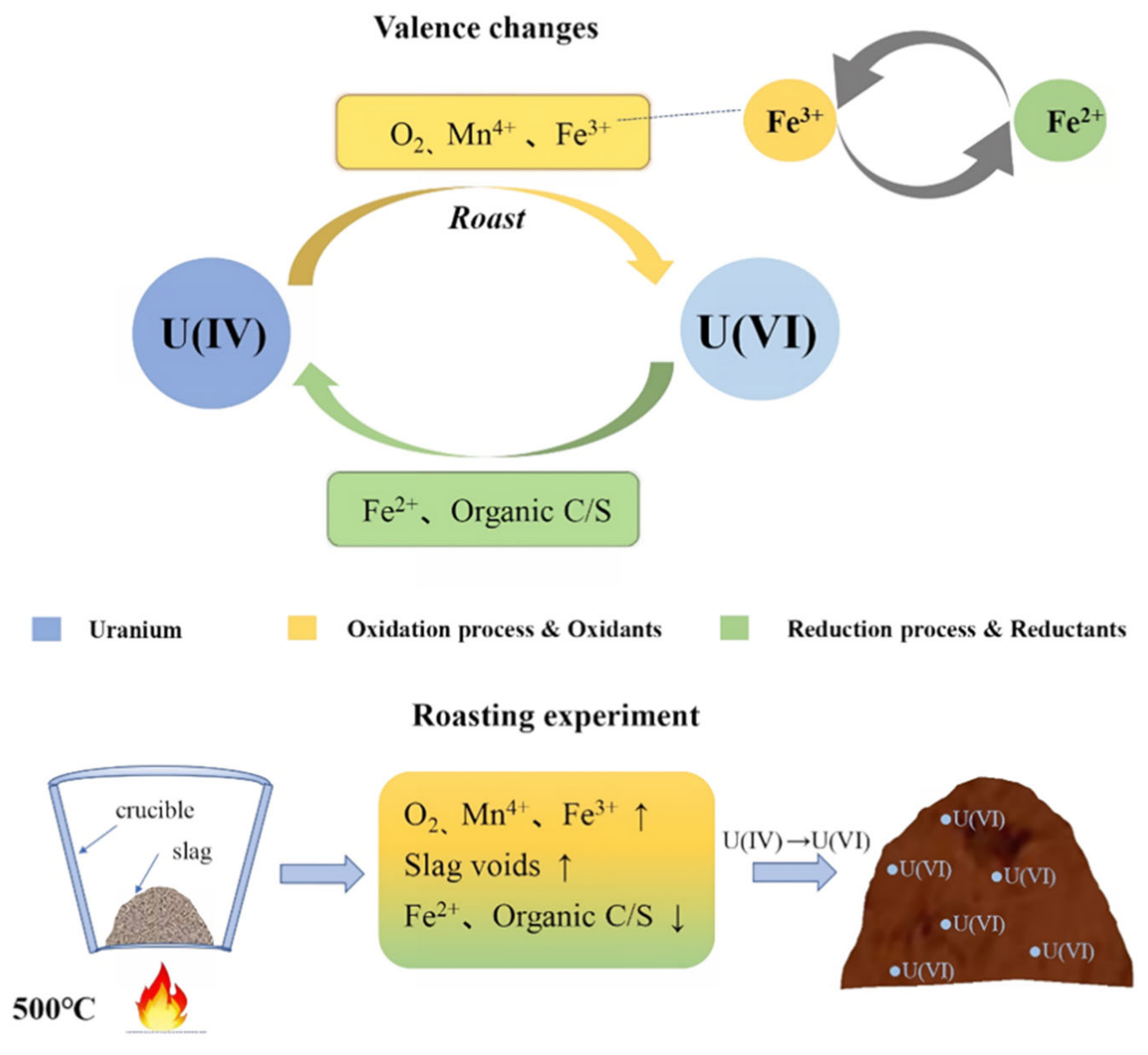
3.3. Elemental Valence Analysis in Tantalum-Niobium Slag
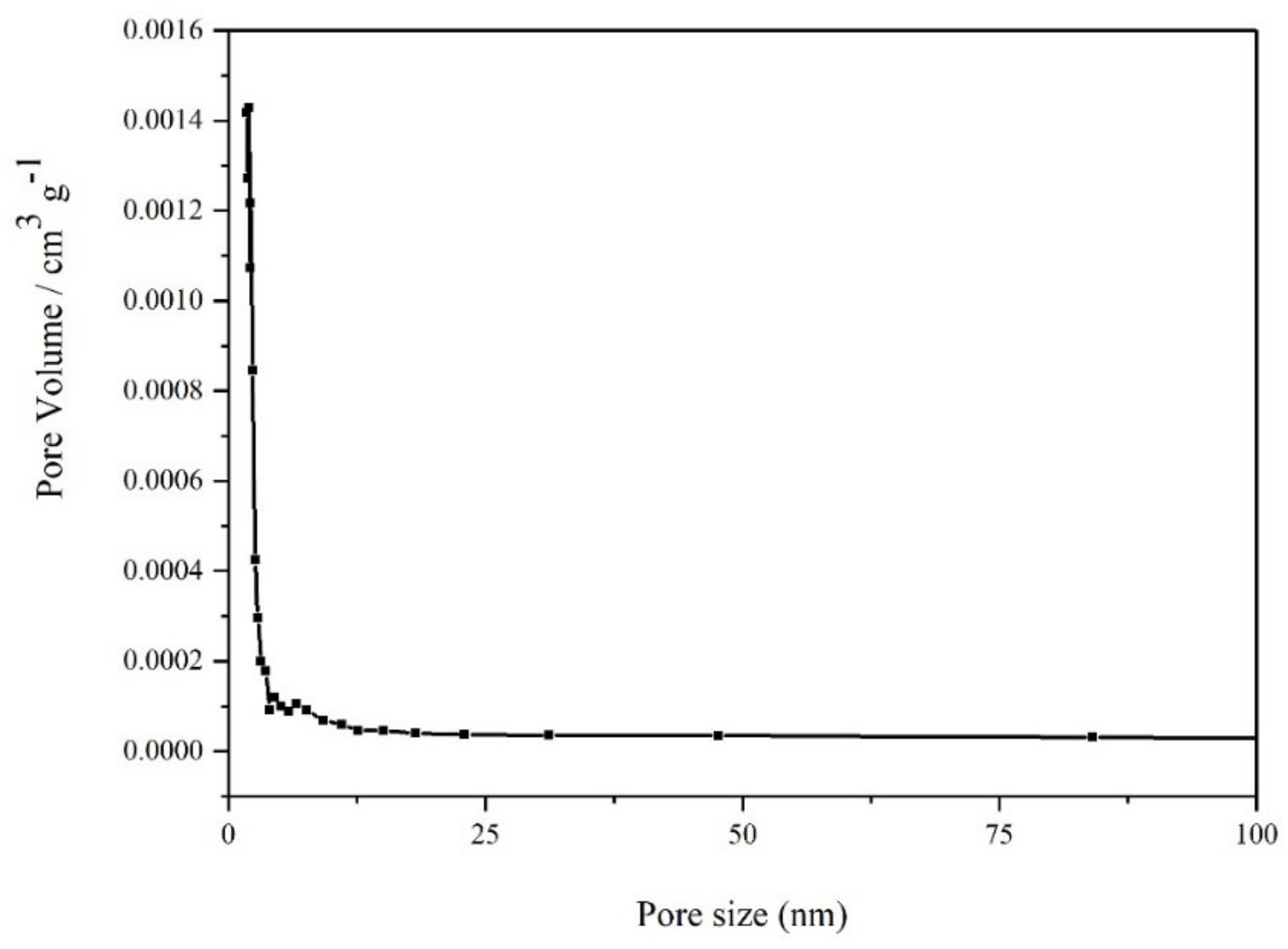
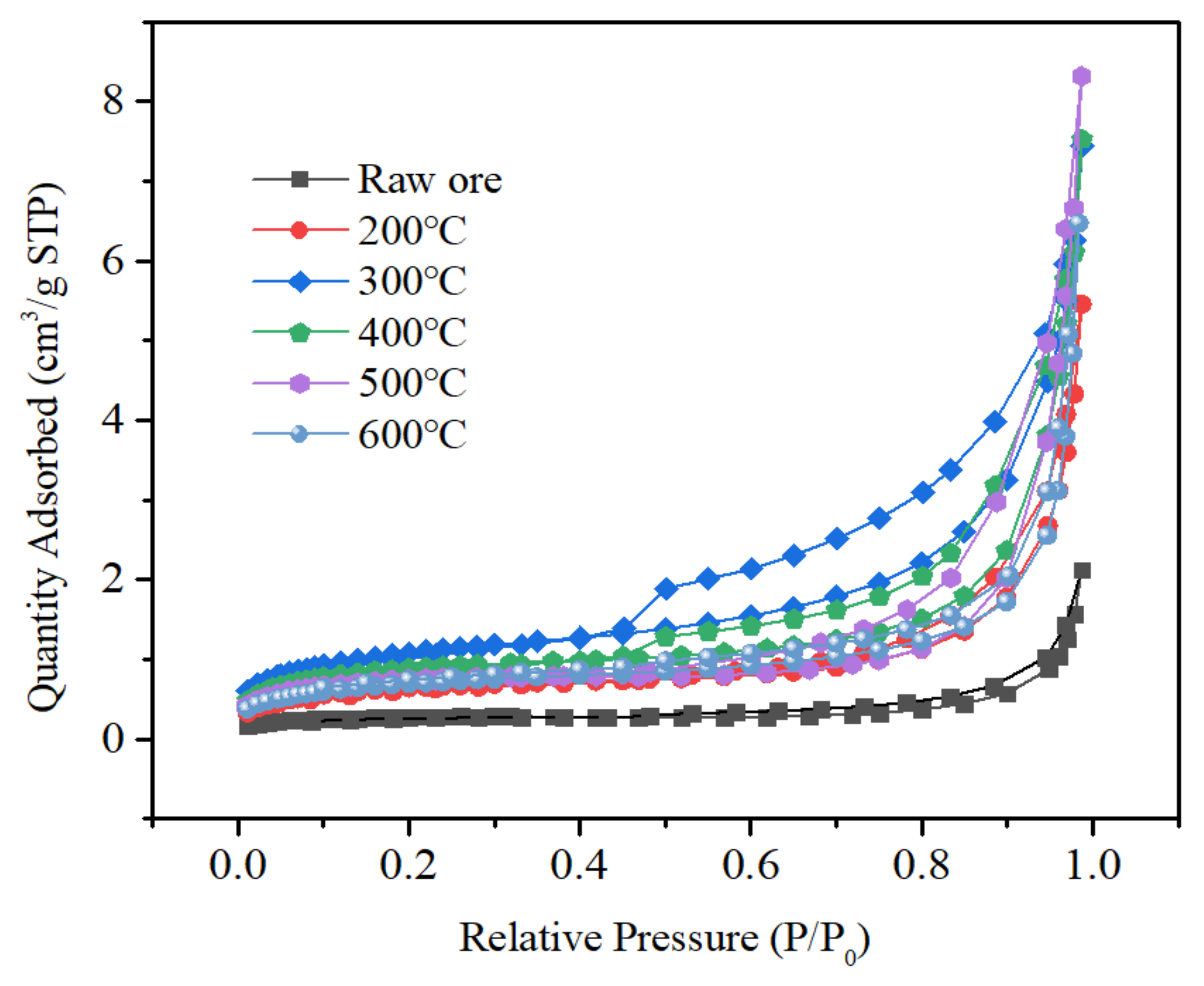
3.4. Specific Surface Area and Pore Size Analysis of Tantalum-Niobium Slag
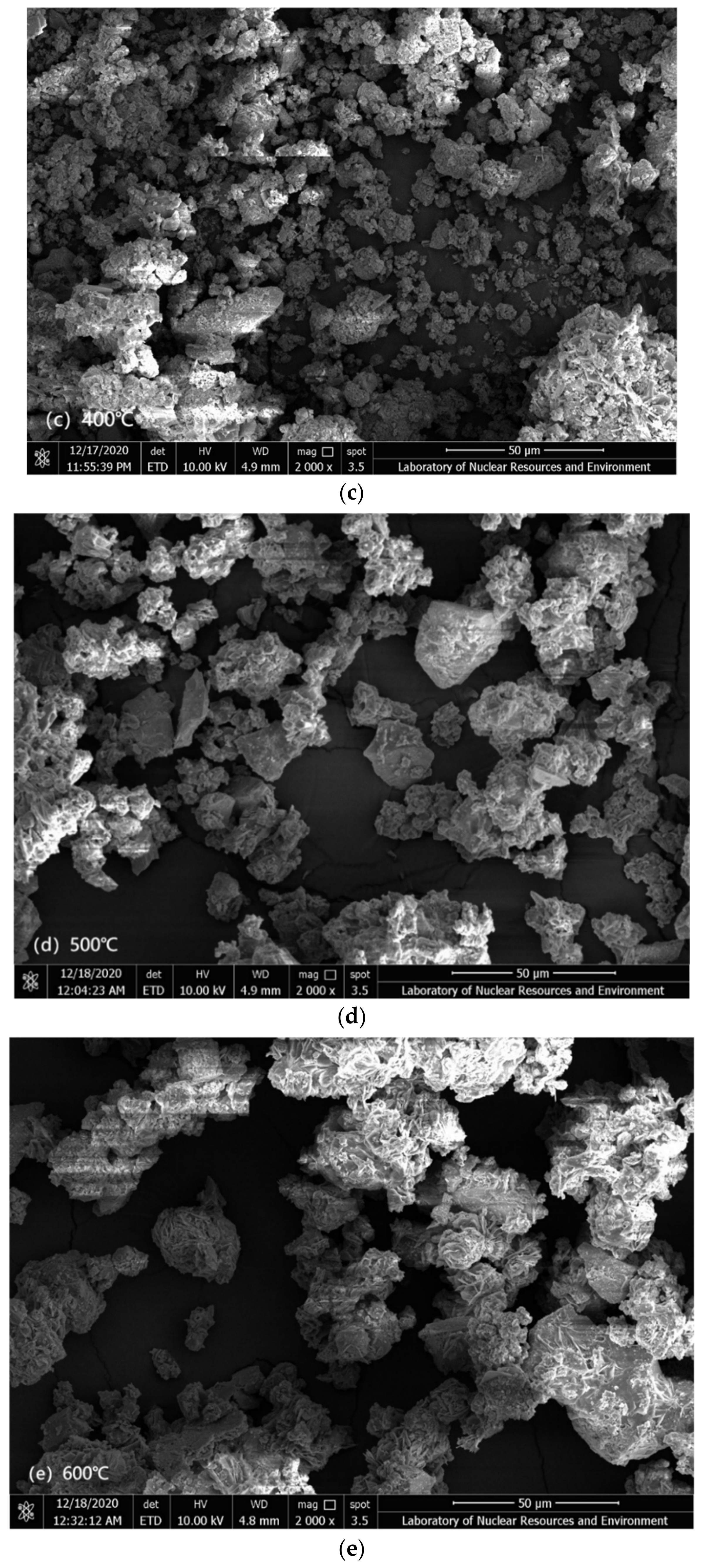
3.5. Mechanism of the Effect of Roasting on the Leaching of Uranium-Thorium
3.6. Morphological Mechanism Analysis of U
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, T.H.; Lee, M.S. A review on the separation of niobium and tantalum by solvent extraction. Miner. Process. Extr. Metall. Rev. 2018, 40, 265–277. [Google Scholar] [CrossRef]
- Shikika, A.; Sethurajan, M.; Muvundja, F.; Mugumaoderha, M.C.; Gaydardzhiev, S. A review on extractive metallurgy of tantalum and niobium. Hydrometallurgy 2020, 198, 105496. [Google Scholar] [CrossRef]
- Belay, A.N.; Venter, J.A.; Roodt, A. Coordination chemistry: Niobium(V) and tantalum(V) with O-donor ligands. Acta Crystallogr. Sect. A Found. Adv. 2017, 73, C1163. [Google Scholar] [CrossRef]
- Bălţatu, S.; Vizureanu, P.; Mareci, D.; Burtan, L.C.; Chiruţă, C.; Trincă, L.C. Effect of Ta on the electrochemical behavior of new TiMoZrTa alloys in artificial physiological solution simulating in vitro inflammatory conditions. Mater. Corros. 2016, 67, 1314–1320. [Google Scholar] [CrossRef]
- Ray, R.; Dutta, B.; Mandal, S.K.; González, A.G.; Pokrovsky, O.S.; Jana, T.K. Bioaccumulation of vanadium (V), niobium (Nb) and tantalum (Ta) in diverse mangroves of the Indian Sundarbans. Plant Soil 2020, 448, 553–564. [Google Scholar] [CrossRef]
- Ibrahim, M.E.; Orabi, A.H.; Falila, N.I.; Ismaiel, D.A.; Salem, H.M. Processing of the mineralized Black Mica for the recovery of uranium, rare earth elements, niobium, and tantalum. Hydrometallurgy 2020, 197, 105474. [Google Scholar] [CrossRef]
- Nete, M.; Purcell, W.; Nel, J.T. Tantalite beneficiation through sequential separation of radioactive elements, iron and titanium by magnetic separation and acid leaching. Adv. Mat. Res. 2014, 1019, 419–425. [Google Scholar] [CrossRef]
- Cui, C.Q.; Wang, B.; Zhao, Y.X.; Wang, Q.; Sun, Z.M. China’s regional sustainability assessment on mineral resources: Results from an improved analytic hierarchy process-based normal cloud model. J. Clean. Prod. 2019, 210, 105–120. [Google Scholar] [CrossRef]
- Wu, B.; Shang, H.; Wen, J.K. Sulfuric acid leaching of low-grade refractory tantalum–niobium and associated rare earths minerals in Panxi area of China. Rare Met. 2015, 34, 202–206. [Google Scholar] [CrossRef]
- Jacob-Tatapu, K.J.; Albert, S.; Grinham, A. Sediment arsenic hotspots in an abandoned tailings storage facility, Gold Ridge Mine, Solomon Islands. Chemosphere 2020, 269, 128756. [Google Scholar] [CrossRef]
- Pakhomova, S.; Yakushev, E.; Schaanning, M. Modeling nickel leaching from abandoned mine tailing deposits in Jøssingfjorden. Water 2021, 13, 967. [Google Scholar] [CrossRef]
- Kusin, F.M.; Sulong, N.A.; Affandi, F.N.A.; Molahid, V.L.M.; Jusop, S. Prospect of abandoned metal mining sites from a hydrogeochemical perspective. Environ. Sci. Pollut. Res. 2021, 28, 2678–2695. [Google Scholar] [CrossRef]
- Ding, Y.G.; Wang, J.S.; Wang, G.; Xue, Q.G. Innovative methodology for separating of rare earth and iron from Bayan Obo complex iron ore. ISIJ Int. 2012, 52, 1772–1777. [Google Scholar] [CrossRef]
- Melcher, F.; Graupner, T.; Gäbler, H.E.; Sitnikova, M.; Henjes-Kunst, F.; Oberthür, T.; Gerdes, A.; Dewaele, S. Tantalum–(niobium–tin) mineralisation in African pegmatites and rare metal granites: Constraints from Ta–Nb oxide mineralogy, geochemistry and U–Pb geochronology. Ore Geol. Rev. 2015, 64, 667–719. [Google Scholar] [CrossRef]
- Rodriguez, M.H.; Rosales, G.D.; Pinna, E.G.; Suarez, D.S. Extraction of niobium and tantalum from ferrocolumbite by hydrofluoric acid pressure leaching. Hydrometallurgy 2015, 156, 17–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Sun, N.; Liu, R.; Wang, Z.; Wang, L.; Sun, W. Systematic review of feldspar beneficiation and its comprehensive application. Miner. Eng. 2018, 128, 141–152. [Google Scholar] [CrossRef]
- Dai, Y.Y.; Zhong, Z.; Zhong, H.Y. Recovery leaching of niobium from metallurgical residues. Adv. Mat. Res. 2011, 236–238, 2554–2559. [Google Scholar] [CrossRef]
- Li, Z.; Hadioui, M.; Wilkinson, K.J. Conditions affecting the release of thorium and uranium from the tailings of a niobium mine. Environ. Pollut. 2019, 247, 206–215. [Google Scholar] [CrossRef]
- Lin, Y.; Jiao, Y.; Zhao, M.; Wang, G.; Wang, D.; Xiao, W.; Li, H.; Xu, Z.; Jiang, Y. Ecological restoration of wetland polluted by heavy metals in Xiangtan manganese mine area. Processes 2021, 9, 1702. [Google Scholar] [CrossRef]
- Xiang, M.; Li, Y.; Yang, J.; Lei, K.; Li, Y.; Li, F.; Zheng, D.; Fang, X.; Cao, Y. Heavy metal contamination risk assessment and correlation analysis of heavy metal contents in soil and crops. Environ. Pollut. 2021, 278, 116911. [Google Scholar] [CrossRef]
- Soesoo, A.; Vind, J.; Hade, S. Uranium and thorium resources of Estonia. Minerals 2020, 10, 798. [Google Scholar] [CrossRef]
- Nalivaiko, K.; Skripchenko, S.; Titova, S.; Rychkov, V. Characterization and processing of radioactive uranium containing waste sludge by sulfuric acid leaching. J. Environ. Chem. Eng. 2022, 10, 106972. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.A.; Dreisinger, D.; Lv, C.; Lv, G.; Zhang, W. Recovery of vanadium from calcification roasted-acid leaching tailing by enhanced acid leaching. J. Hazard. Mater. 2019, 369, 632–641. [Google Scholar] [CrossRef]
- Korehi, H.; Blöthe, M.; Sitnikova, M.A.; Dold, B.; Schippers, A. Metal mobilization by iron- and sulfur-oxidizing bacteria in a multiple extreme mine tailings in the Atacama desert, Chile. Environ. Sci. Technol. 2013, 47, 2189–2196. [Google Scholar] [CrossRef]
- Stasik, S.; Wick, L.Y.; Wendt-Potthoff, K. Anaerobic BTEX degradation in oil sands tailings ponds: Impact of labile organic carbon and sulfate-reducing bacteria. Chemosphere 2015, 138, 133–139. [Google Scholar] [CrossRef]
- Lv, S.Y.; Li, M.; Wu, X.Y.; Zhang, X.W.; Hua, Y.L.; Bi, L.; Fang, Q.; Cai, T. A non-polluting method for rapidly purifying uranium-containing wastewater and efficiently recovering uranium through electrochemical mineralization and oxidative roasting. J. Hazard. Mater. 2021, 416, 125885. [Google Scholar] [CrossRef]
- Sun, Y.W.; Zeng, B.Y.; Dai, Y.T.; Liang, X.J.; Zhang, L.J.; Ahmad, R.; Su, X.T. Modification of sludge-based biochar using air roasting-oxidation and its performance in adsorption of uranium(VI) from aqueous solutions. J. Colloid Interface Sci. 2022, 614, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Liu, Z.R.; Xiu, T.Y.; Zhou, L.M.; Wang, Y. Removal of thorium from aqueous solution by adsorption with Cu3(BTC)2. J. Radioanal. Nucl. Chem. 2020, 326, 185–192. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a theory of the van der Waals adsorption of gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Naik, B.; Ghosh, N. A review on chemical methodologies for preparation of mesoporous silica and alumina based materials. Recent Pat. Nanotechnol. 2009, 3, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Boreen, M.A.; Arnold, J. The synthesis and versatile reducing power of low-valent uranium complexes. Dalton Trans. 2020, 49, 15124–15138. [Google Scholar] [CrossRef]
- Puchkova, E.V.; Bogdanov, R.V.; Gieré, R. Redox states of uranium in samples of microlite and monazite. Am. Mineral. 2016, 101, 1884–1891. [Google Scholar] [CrossRef]
- Fu, H.Y.; Zhang, H.; Sui, Y.; Hu, N.; Ding, D.X.; Ye, Y.J.; Li, G.Y.; Wang, Y.D.; Dai, Z.R. Transformation of uranium species in soil during redox oscillations. Chemosphere 2018, 208, 846–853. [Google Scholar] [CrossRef]
- Wang, P.M.; Anderko, A.; Kosinski, J.J.; Springer, R.D.; Lencka, M.M. Modeling speciation and solubility in aqueous systems containing U(IV, VI), Np(IV, V, VI), Pu(III, IV, V, VI), Am(III), and Cm(III). J. Solut. Chem. 2017, 46, 521–588. [Google Scholar] [CrossRef]
- Leventhal, J.S.; Daws, T.A.; Frye, J.S. Organic geochemical analysis of sedimentary organic matter associated with uranium. Appl. Geochem. 1986, 1, 241–247. [Google Scholar] [CrossRef]
- Fuller, A.J.; Leary, P.; Gray, N.D.; Davies, H.S.; Mosselmans, J.F.W.; Cox, F.; Robinson, C.H.; Pittman, J.K.; McCann, C.M.; Muir, M.; et al. Organic complexation of U(VI) in reducing soils at a natural analogue site: Implications for uranium transport. Chemosphere 2020, 254, 126859. [Google Scholar] [CrossRef]
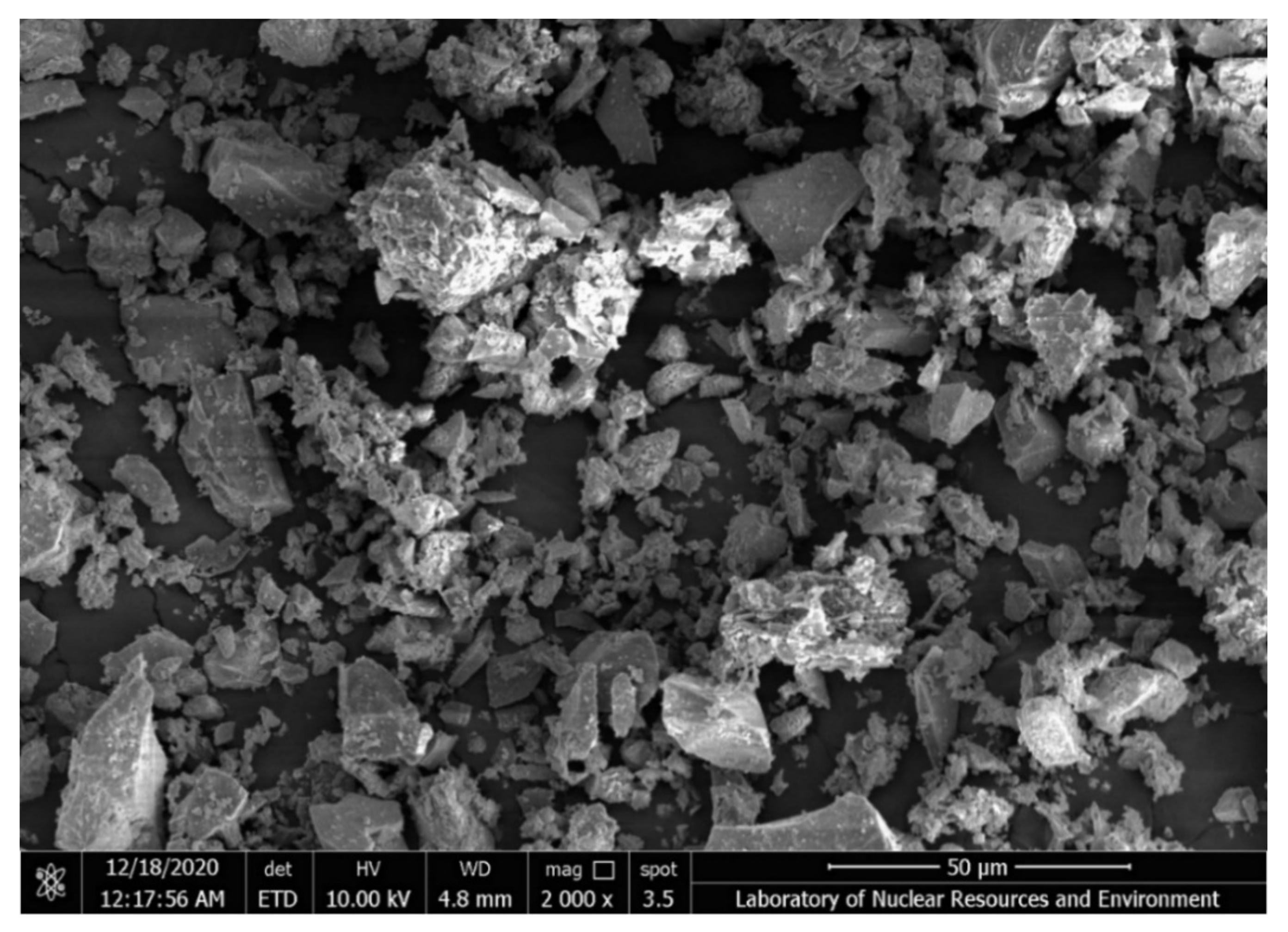
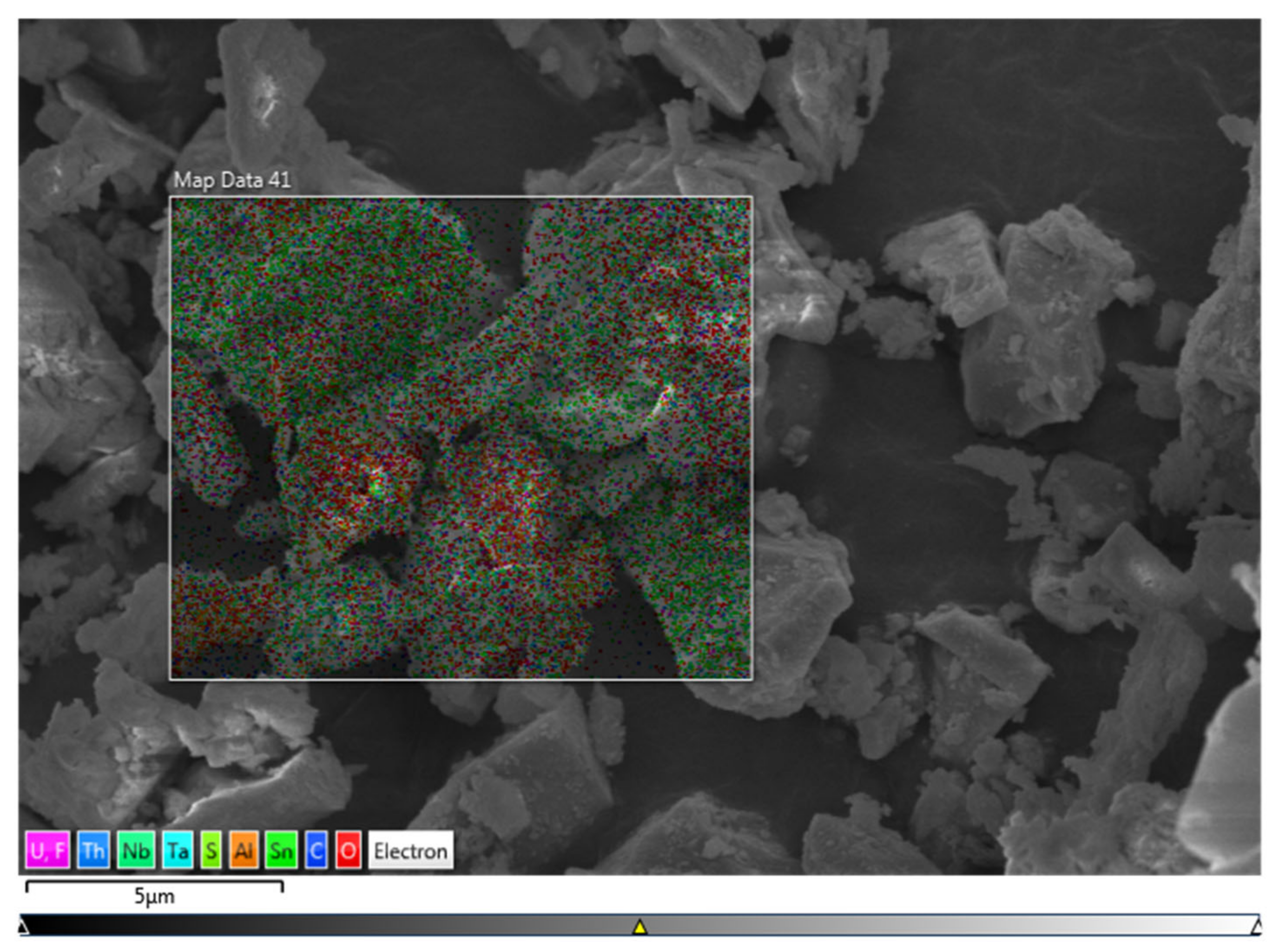


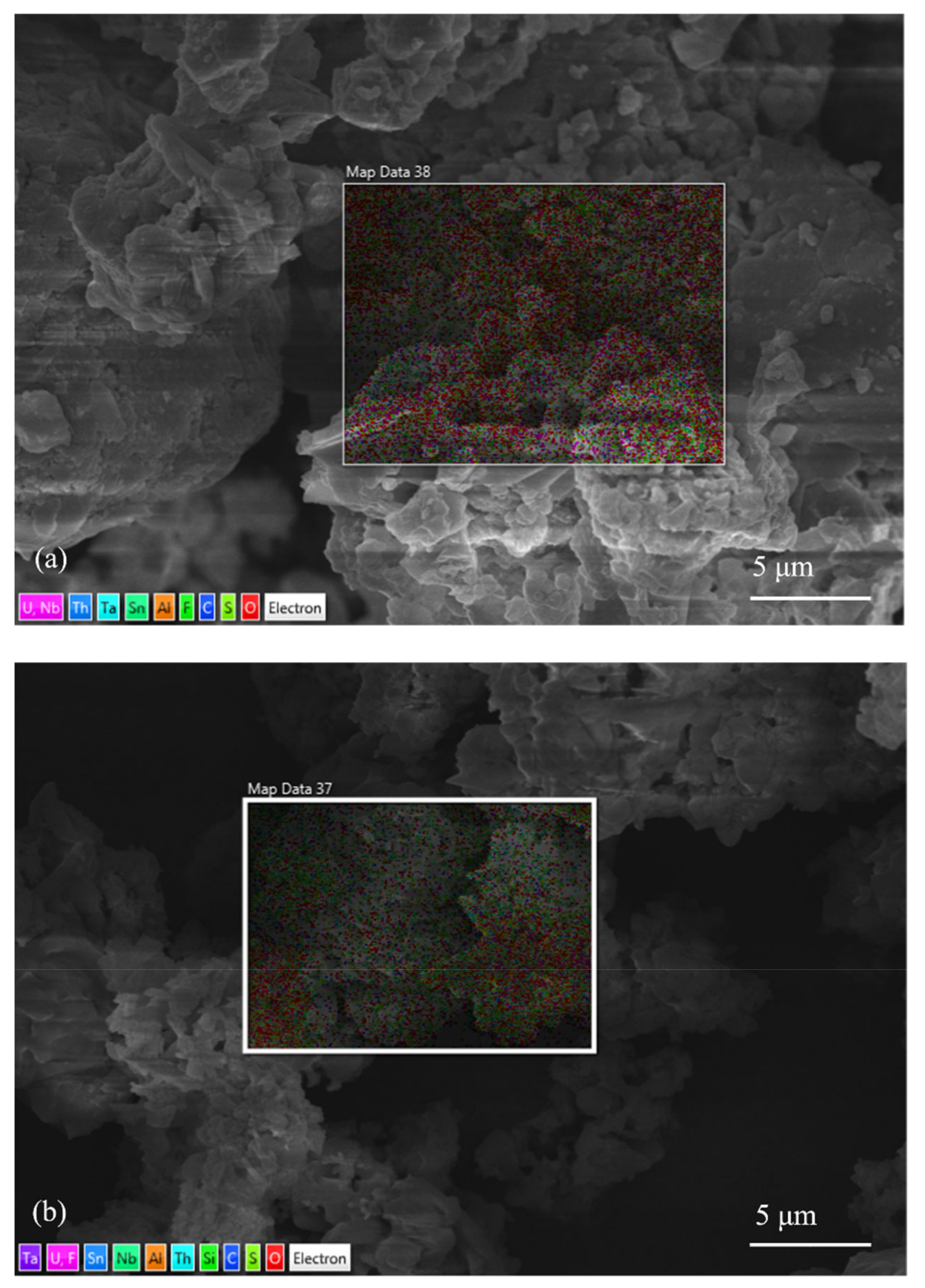
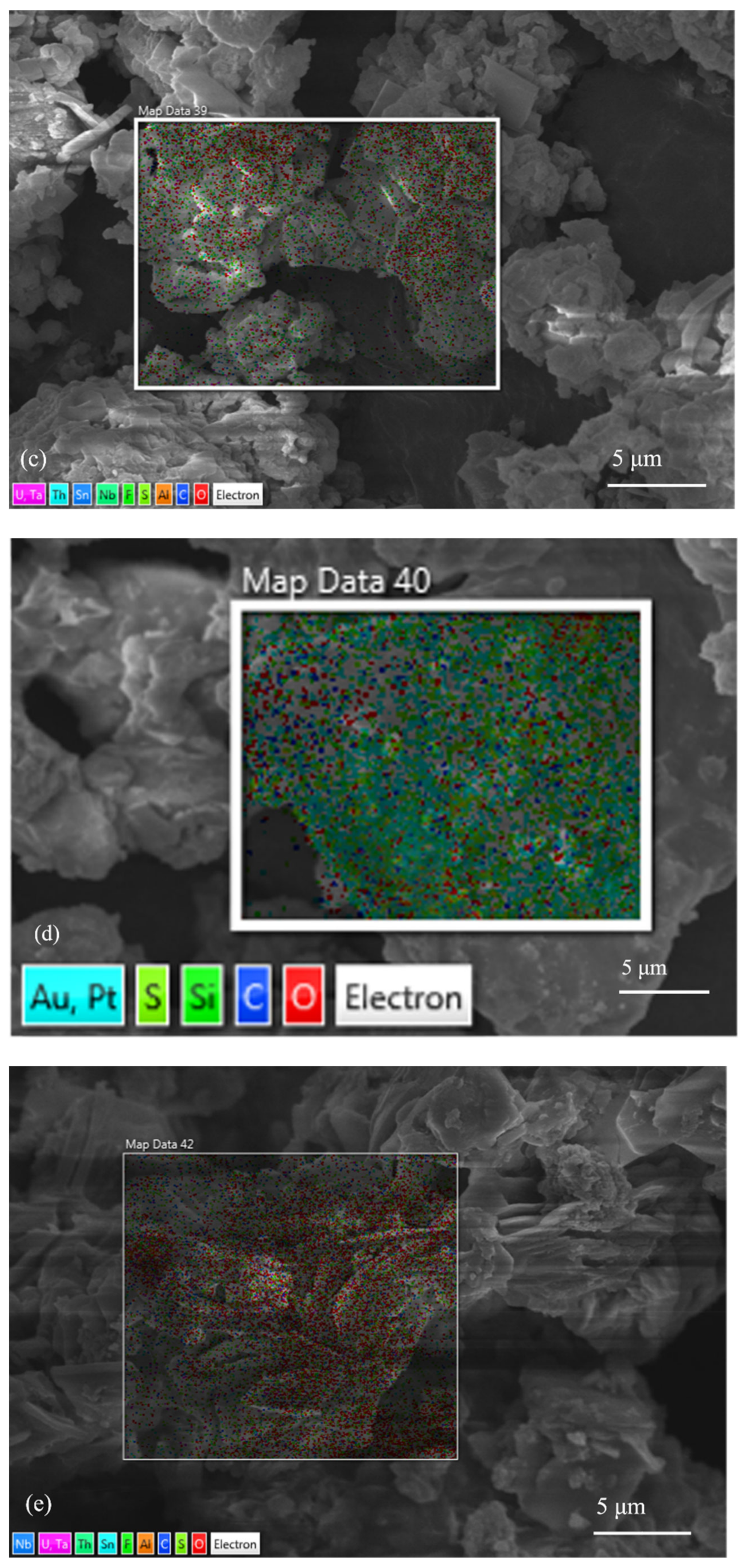


| Element | C | O | F | Al | Mn | Fe | Nb | Sn | Ta | Th | U | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wt % | 2.18 | 31.20 | 1.44 | 6.44 | 0.24 | 1.08 | 12.88 | 37.54 | 5.58 | 0.04 | 1.38 | 100 |
| Atomic % | 6.12 | 65.86 | 2.55 | 8.06 | 0.14 | 0.66 | 4.68 | 10.68 | 1.04 | 0.01 | 0.20 | 100 |
| Composition | Sn | Fe | U | Th | O | Al | S | Ta | Nb |
|---|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 8.38 | 28.5 | 0.38 | 1.22 | 28.5 | 3.36 | 16.8 | 0.08 | 0.799 |
| Composition | Nd | P | Sc | Si | Yb | Pb | Cr | Ca | Ti |
| Content (wt.%) | 0.55 | 2.73 | 0.041 | 2.58 | 0.59 | 0.099 | 1.27 | 0.71 | 0.79 |
| Ta-Nb Slag | U | Th | Ta | Nb | Fe |
|---|---|---|---|---|---|
| Content (mg/kg) | 2.26 × 103 | 7.84 × 103 | 0.88 × 103 | 2.01 × 103 | 1.66 × 105 |
| Valence States | Fe2+ (%) | Fe3+ (%) | U(IV) (%) | U(VI) (%) |
|---|---|---|---|---|
| Original slag | 62.61 | 37.39 | 63.83 | 36.17 |
| Materials | Pore Volume (cm3/g) | Average Pore Size (nm) | BET Surface Area (m2/g) |
|---|---|---|---|
| Ta-Nb slag | 3.12 × 10−3 | 5.5453 | 99.9 × 10−2 |
| Roasting Temperatures (°C) | U Content (mg/kg) | Th Content (mg/kg) |
|---|---|---|
| 0 | 2.26 × 103 | 7.84 × 103 |
| 200 | 2.31 × 103 | 7.88 × 103 |
| 300 | 2.42 × 103 | 7.96 × 103 |
| 400 | 2.46 × 103 | 8.04 × 103 |
| 500 | 2.54 × 103 | 8.10 × 103 |
| 600 | 2.59 × 103 | 8.12 × 103 |
| Elements (%) | Raw Slag | 200 °C | 300 °C | 400 °C | 500 °C | 600 °C |
|---|---|---|---|---|---|---|
| C | 0.1010 | 0.0924 | 0.0845 | 0.0698 | 0.0654 | 0.0762 |
| S | 0.0354 | 0.0321 | 0.0306 | 0.0241 | 0.0208 | 0.0256 |
| Roasting Temperature (°C) | Fe2+ (%) | Fe3+ (%) | U(IV) (%) | U(VI) (%) |
|---|---|---|---|---|
| 200 °C | 47.89 | 52.11 | 62.54 | 37.46 |
| 300 °C | 23.01 | 76.99 | 52.52 | 47.48 |
| 400 °C | 13.13 | 86.87 | 38.96 | 61.04 |
| 500 °C | 10.57 | 89.43 | 33.21 | 66.79 |
| 600 °C | 19.11 | 80.89 | 45.55 | 54.45 |
| Roasting Temperature (°C) | Pore Volume (cm3/g) | Average Pore Size (nm) | BET Surface Area (m2/g) |
|---|---|---|---|
| 200 °C | 8.233 × 10−3 | 7.4207 | 2.2868 |
| 300 °C | 11.619 × 10−3 | 7.3708 | 3.8298 |
| 400 °C | 11.489 × 10−3 | 7.8396 | 3.1209 |
| 500 °C | 12.720 × 10−3 | 9.2171 | 2.6696 |
| 600 °C | 9.553 × 10−3 | 6.7793 | 2.4359 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Hu, K.; Li, X.; Wang, Y.; Ouyang, J.; Zhou, L.; Liu, Z. Mineralogical Properties of a Refractory Tantalum-Niobium Slag and the Effect of Roasting on the Leaching of Uranium-Thorium. Toxics 2022, 10, 469. https://doi.org/10.3390/toxics10080469
Huang M, Hu K, Li X, Wang Y, Ouyang J, Zhou L, Liu Z. Mineralogical Properties of a Refractory Tantalum-Niobium Slag and the Effect of Roasting on the Leaching of Uranium-Thorium. Toxics. 2022; 10(8):469. https://doi.org/10.3390/toxics10080469
Chicago/Turabian StyleHuang, Min, Ke Hu, Xiang Li, Yun Wang, Jinbo Ouyang, Limin Zhou, and Zhirong Liu. 2022. "Mineralogical Properties of a Refractory Tantalum-Niobium Slag and the Effect of Roasting on the Leaching of Uranium-Thorium" Toxics 10, no. 8: 469. https://doi.org/10.3390/toxics10080469
APA StyleHuang, M., Hu, K., Li, X., Wang, Y., Ouyang, J., Zhou, L., & Liu, Z. (2022). Mineralogical Properties of a Refractory Tantalum-Niobium Slag and the Effect of Roasting on the Leaching of Uranium-Thorium. Toxics, 10(8), 469. https://doi.org/10.3390/toxics10080469








