The Response of Thiols to Cadmium Stress in Spinach (Spinacia Oleracea L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Condition and Treatment
2.2. Sample Collection
2.3. Measurement of Contents of Thiols
2.4. Cd Concentration Analysis
2.5. Statistical Analysis
3. Results
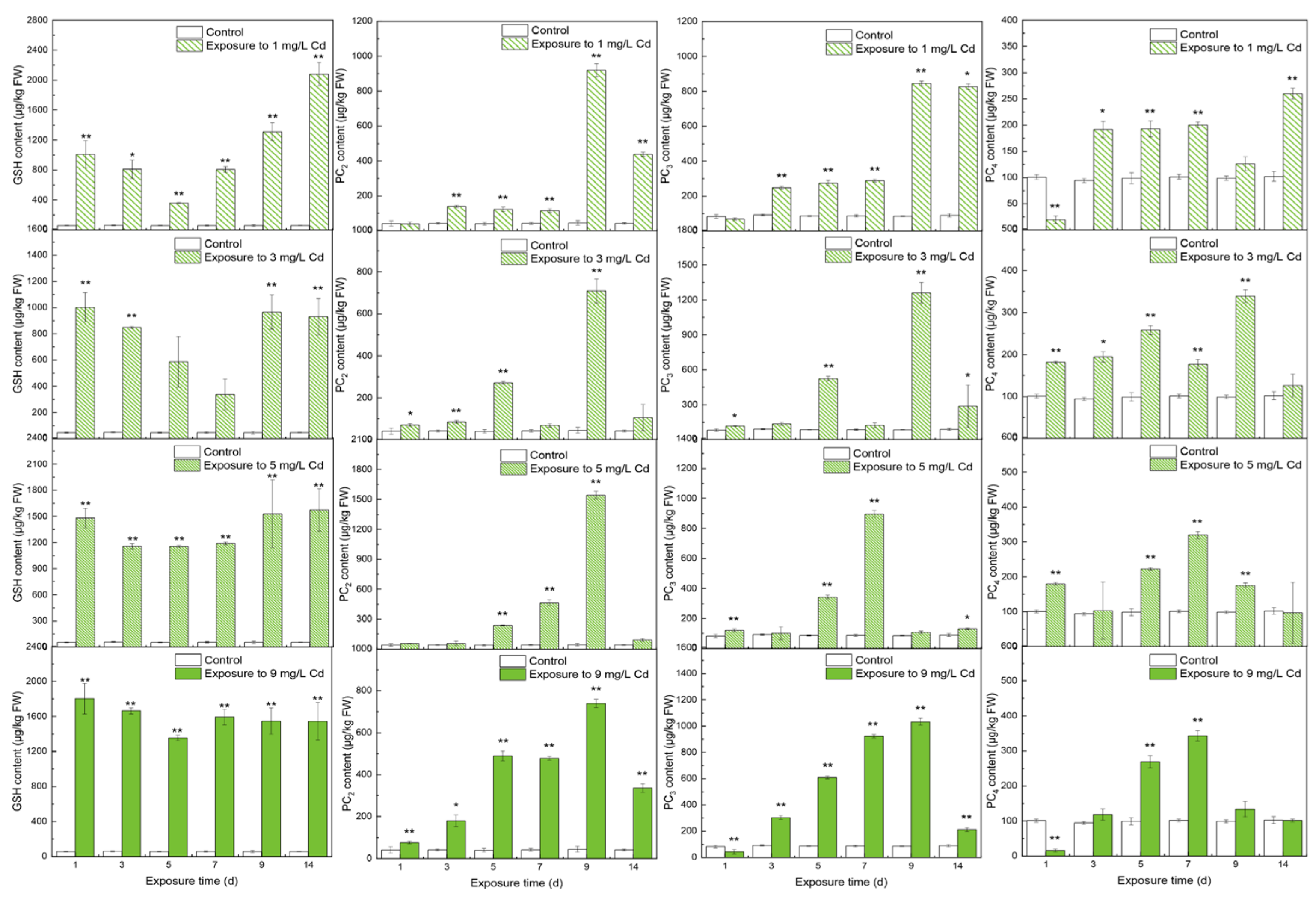
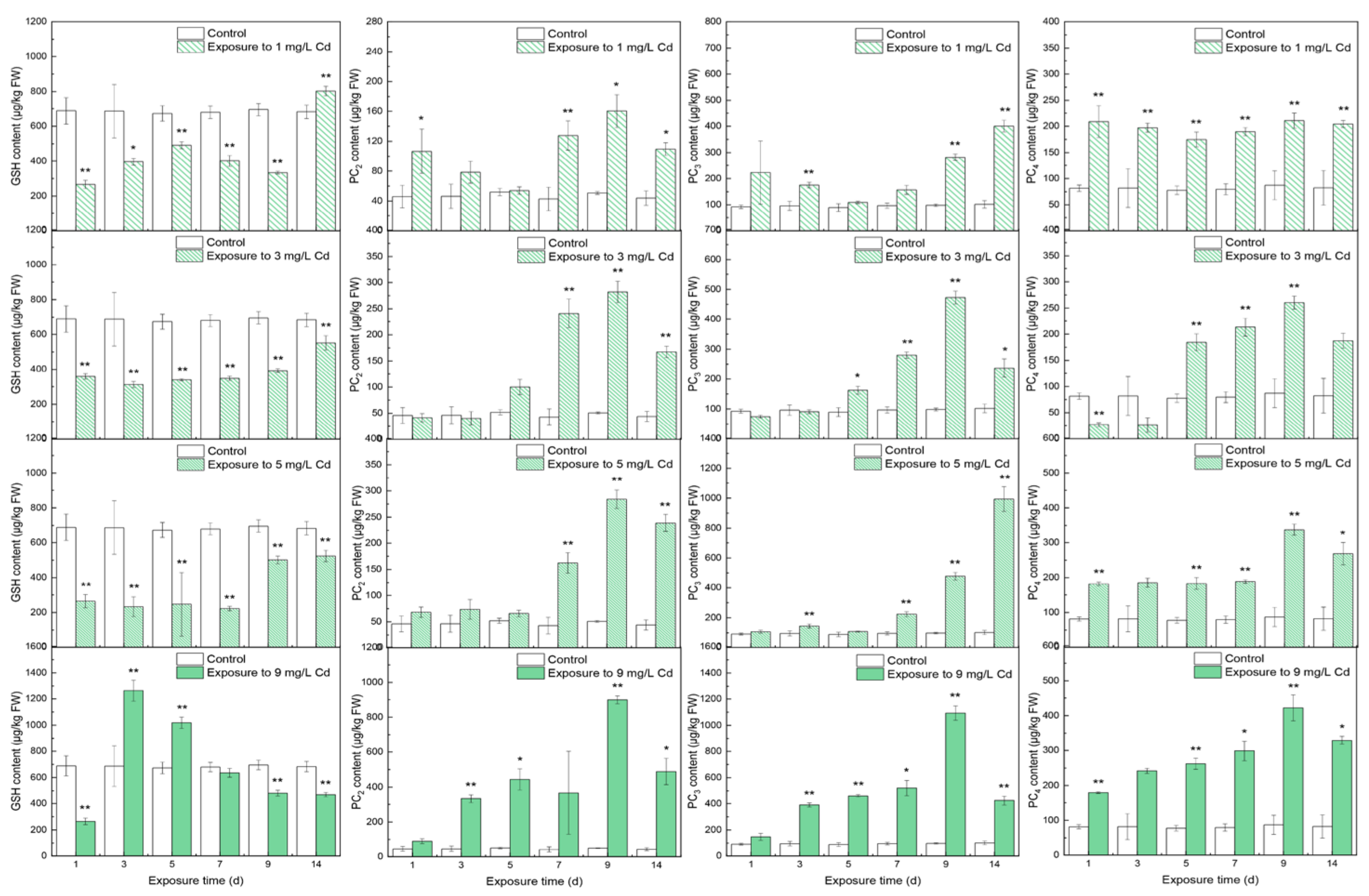
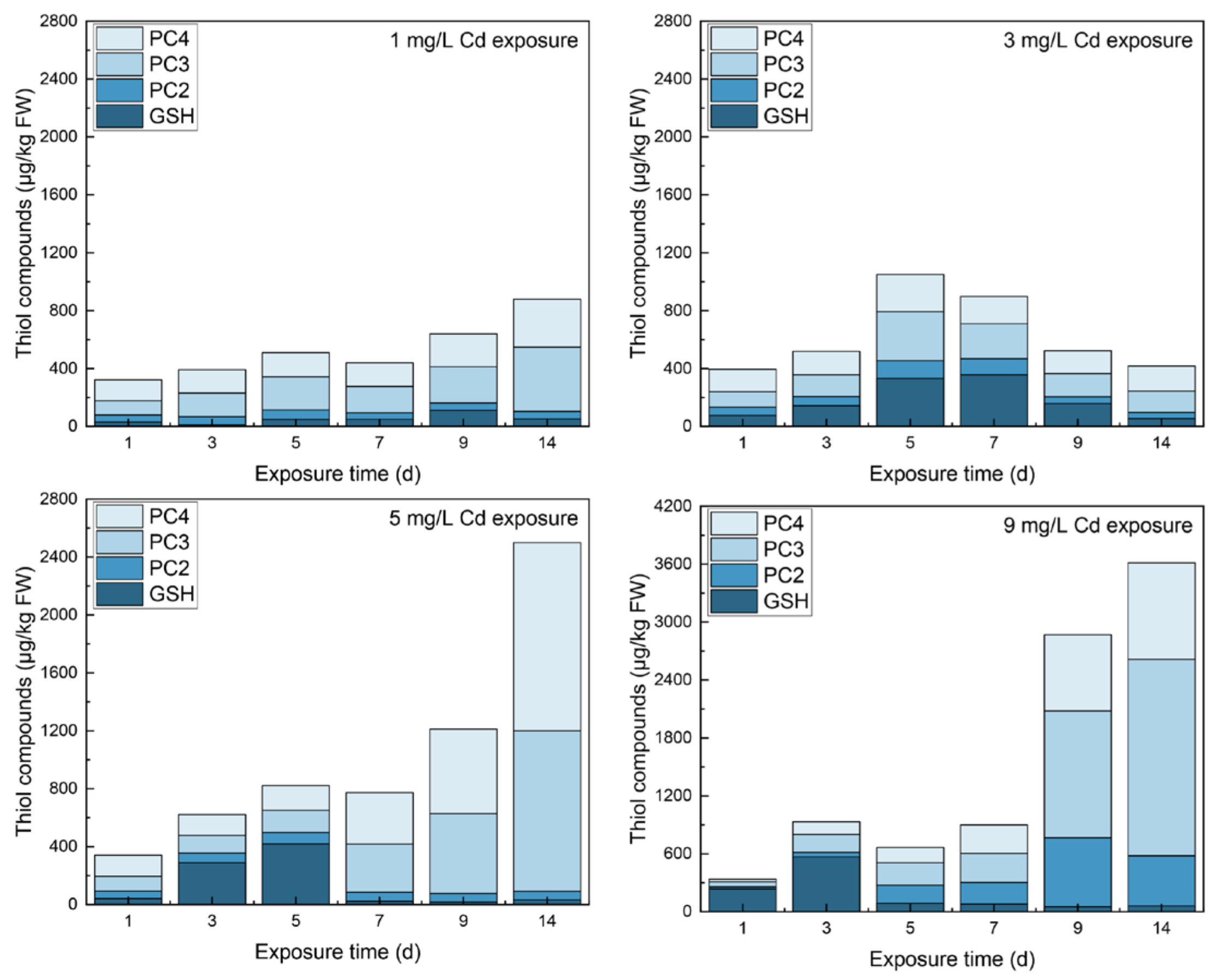
3.1. Contents of Thiols in Different Tissues
3.2. Cd Concentrations in Different Tissues
4. Discussion
4.1. The Responses of Thiols to Cd Concentrations
4.2. The Inner Conversions of Thiols
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younis, U.; Malik, S.A.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Shah, M.H.R.; Rehman, R.A.; Ahmad, N. Biochar enhances the cadmium tolerance in spinach (Spinacia oleracea) through modification of Cd uptake and physiological and biochemical attributes. Environ. Sci. Pollut. Res. 2016, 23, 21385–21394. [Google Scholar] [CrossRef] [PubMed]
- Chunhabundit, R. Cadmium Exposure and Potential Health Risk from Foods in Contaminated Area, Thailand. Toxicol. Res. 2016, 32, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.-J.; Ma, Y.; Zhu, Y.-G.; Tang, Z.; McGrath, S.P. Soil Contamination in China: Current Status and Mitigation Strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-B.; Wang, M.; Li, S.-S.; Zhao, Z.-Q.; Wen-di, E. Overview on current criteria for heavy metals and its hint for the revision of soil environmental quality standards in China. J. Integr. Agric. 2018, 17, 765–774. [Google Scholar] [CrossRef]
- Arao, T.; Ishikawa, S.; Murakami, M.; Abe, K.; Maejima, Y.; Makino, T. Heavy metal contamination of agricultural soil and countermeasures in Japan. Paddy Water Environ. 2010, 8, 247–257. [Google Scholar] [CrossRef]
- Jacob, D.L.; Yellick, A.H.; Kissoon, L.T.T.; Asgary, A.; Wijeyaratne, D.N.; Saini-Eidukat, B.; Otte, M.L. Cadmium and associated metals in soils and sediments of wetlands across the Northern Plains, USA. Environ. Pollut. 2013, 178, 211–219. [Google Scholar] [CrossRef]
- Global Mercury Assessment. United Nations Environment Programme Chemicals; United Nations: Geneva, Switzerland, 2002. [Google Scholar]
- Garrett, R.G. The distribution of cadmium in a horizon soils in the prairies of Canada and adjoining United States. Curr. Res. B Geol. Surv. Can. 1994, 1994, 73–82. [Google Scholar] [CrossRef]
- Csillag, J.; Lukacs, A.; Osztoics, E.; Csathó, P.; Baczó, G. Trace Metal Concentrations in the Liquid Phase of Phosphate Rock-Treated Soils. Agrokémia Talajt. 2006, 55, 203–212. [Google Scholar] [CrossRef][Green Version]
- Tóth, G.; Hermann, T.; Da Silva, M.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Pal, R.; Rai, J.P.N. Phytochelatins: Peptides Involved in Heavy Metal Detoxification. Appl. Biochem. Biotechnol. 2009, 160, 945–963. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 17859–17879. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, F.; Li, H.; Qiu, B.; Gao, Y.; Cui, D.; Yang, Z. Antioxidant defense system in lettuces tissues upon various As species exposure. J. Hazard. Mater. 2020, 399, 123003. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and Glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Jung, H.-I.; Lee, B.-R.; Chae, M.-J.; Lee, E.-J.; Lee, T.-G.; Jung, G.-B.; Kim, M.-S.; Lee, J. Ascorbate-Mediated Modulation of Cadmium Stress Responses: Reactive Oxygen Species and Redox Status in Brassica napus. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Cobbett, C.S. Phytochelatins and Their Roles in Heavy Metal Detoxification. Plant Physiol. 2000, 123, 825–832. [Google Scholar] [CrossRef]
- Weerakoon, S.R. Chapter 2—Genetic engineering for metal and metalloid detoxification. In Transgenic Plant Technology for Remediation of Toxic Metals and Metalloids; Prasad, M.N.V., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 23–41. [Google Scholar]
- Romano, R.L.; Liria, C.W.; Machini, M.T.; Colepicolo, P.; Zambotti-Villela, L. Cadmium decreases the levels of glutathione and enhances the phytochelatin concentration in the marine dinoflagellate Lingulodinium polyedrum. J. Appl. Phycol. 2017, 29, 811–820. [Google Scholar] [CrossRef]
- Adamis, P.D.; Gomes, D.S.; Pinto, M.L.C.; Panek, A.D.; Eleutherio, E.C. The role of glutathione transferases in cadmium stress. Toxicol. Lett. 2004, 154, 81–88. [Google Scholar] [CrossRef]
- Gallego, S.M.; Kogan, M.J.; Azpilicueta, C.E.; Peña, C.; Tomaro, M.L. Glutathione-mediated Antioxidative Mechanisms in Sunflower (Helianthus Annuus L.) Cells in Response to Cadmium Stress. Plant Growth Regul. 2005, 46, 267–276. [Google Scholar] [CrossRef]
- Raab, A.; Schat, H.; Meharg, A.A.; Feldmann, J. Uptake, translocation and transformation of arsenate and arsenite in sunflower (Helianthus annuus): Formation of arsenic–phytochelatin complexes during exposure to high arsenic concentrations. New Phytol. 2005, 168, 551–558. [Google Scholar] [CrossRef]
- Scarano, G.; Morelli, E. Characterization of cadmium- and lead-phytochelatin complexes formed in a marine microalga in response to metal exposure. BioMetals 2002, 15, 145–151. [Google Scholar] [CrossRef]
- Xie, M.; Chen, W.; Lai, X.; Dai, H.; Sun, H.; Zhou, X.; Chen, T. Metabolic responses and their correlations with phytochelatins in Amaranthus hypochondriacus under cadmium stress. Environ. Pollut. 2019, 252, 1791–1800. [Google Scholar] [CrossRef]
- Yang, L.; Li, N.; Kang, Y.; Liu, J.; Wang, Y.; Sun, H.; Ao, T.; Chen, W. Selenium alleviates toxicity in Amaranthus hypochondriacus by modulating the synthesis of thiol compounds and the subcellular distribution of cadmium. Chemosphere 2021, 291, 133108. [Google Scholar] [CrossRef]
- Głowacka, K.; Źróbek-Sokolnik, A.; Okorski, A.; Najdzion, J. The Effect of Cadmium on the Activity of Stress-Related Enzymes and the Ultrastructure of Pea Roots. Plants 2019, 8, 413. [Google Scholar] [CrossRef]
- Muradoglu, F.; Gundogdu, M.; Ercisli, S.; Encu, T.; Balta, F.; Jaafar, H.Z.; Zia-Ul-Haq, M. Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol. Res. 2015, 48, 11. [Google Scholar] [CrossRef]
- Yu, F.; Liu, K.; Li, M.; Zhou, Z.; Deng, H.; Chen, B. Effects of Cadmium on Enzymatic and Non-Enzymatic Antioxidative Defences of Rice (Oryza Sativa L.). Int. J. Phytoremediat. 2013, 15, 513–521. [Google Scholar] [CrossRef]
- Salaskar, D.; Shrivastava, M.; Kale, S. Bioremediation potential of spinach (Spinacia oleracea L.) for decontamination of cadmium in soil. Curr. Sci. 2011, 101, 1359–1363. [Google Scholar]
- Pinto, F.R.; Mourato, M.P.; Sales, J.R.; Fangueiro, D.; Martins, L.L. Effect of Cattle Slurry on the Growth of Spinach Plants in Cd-contaminated Soil. Commun. Soil Sci. Plant Anal. 2020, 51, 1370–1381. [Google Scholar] [CrossRef]
- Yu, S.; Bian, Y.; Zhou, R.; Mou, R.; Chen, M.; Cao, Z. Robust method for the analysis of phytochelatins in rice by high-performance liquid chromatography coupled with electrospray tandem mass spectrometry based on polymeric column materials. J. Sep. Sci. 2015, 38, 4146–4152. [Google Scholar] [CrossRef]
- Mishra, S.; Tripathi, R.; Srivastava, S.; Dwivedi, S.; Trivedi, P.K.; Dhankher, O.; Khare, A. Thiol metabolism play significant role during cadmium detoxification by Ceratophyllum demersum L. Bioresour. Technol. 2009, 100, 2155–2161. [Google Scholar] [CrossRef]
- Baba, S.P.; Bhatnagar, A. Role of thiols in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 133–139. [Google Scholar] [CrossRef]
- Rajput, V.; Harish; Singh, R.; Verma, K.; Sharma, L.; Quiroz-Figueroa, F.; Meena, M.; Gour, V.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Figueira, E.; Freitas, R.; Guasch, H.; Almeida, S.F.P. Efficiency of cadmium chelation by phytochelatins in Nitzschia palea (Kützing) W. Smith. Ecotoxicology 2014, 23, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, H.; Song, Y.; Zhang, F.; Lu, Y.; Peng, F.; Yang, Z. Decisive Enzymes and Prediction Models for the Glutathione Content in Spinach (Spinacia oleracea L.) Exposed to Cadmium. J. Agric. Food Chem. 2020, 68, 11855–11862. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, S.; Jozefczak, M.; Wójcik, M.; Deckers, J.; Vangronsveld, J.; Cuypers, A. Glutathione: A key player in metal chelation, nutrient homeostasis, cell cycle regulation and the DNA damage response in cadmium-exposed Arabidopsis thaliana. Plant Physiol. Biochem. 2020, 154, 498–507. [Google Scholar] [CrossRef]
- Morrell, C.N. Reactive Oxygen Species: Finding the right balance. Circ. Res. 2008, 103, 571–572. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Castagna, A.; Ranieri, A.; di Toppi, L.S. Cadmium tolerance in Brassica juncea roots and shoots is affected by antioxidant status and phytochelatin biosynthesis. Plant Physiol. Biochem. 2012, 57, 15–22. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, Y.; Cheng, S.; Osorio, S.; Sun, Y.; Fernie, A.R.; Cheung, C.Y.M.; Lim, B.L. Impacts of high ATP supply from chloroplasts and mitochondria on the leaf metabolism of Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 922. [Google Scholar] [CrossRef]
- Aghajanzadeh, T.A.; Prajapati, D.H.; Burow, M. Differential partitioning of thiols and glucosinolates between shoot and root in Chinese cabbage upon excess zinc exposure. J. Plant Physiol. 2020, 244, 153088. [Google Scholar] [CrossRef]
- Zhan, F.; Li, B.; Jiang, M.; Qin, L.; Wang, J.; He, Y.; Li, Y. Effects of a root-colonized dark septate endophyte on the glutathione metabolism in maize plants under cadmium stress. J. Plant Interact. 2017, 12, 421–428. [Google Scholar] [CrossRef]
- Loscos, J.; Naya, L.; Ramos, J.; Clemente, M.R.; A Matamoros, M.; Becana, M. A Reassessment of Substrate Specificity and Activation of Phytochelatin Synthases from Model Plants by Physiologically Relevant Metals. Plant Physiol. 2006, 140, 1213–1221. [Google Scholar] [CrossRef]
- Wood, B.A.; Feldmann, J. Quantification of phytochelatins and their metal(loid) complexes: Critical assessment of current analytical methodology. Anal. Bioanal. Chem. 2012, 402, 3299–3309. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, X.; Gao, K.; Ge, Y.; Cheng, W. Non-protein thiols and glutathione S-transferase alleviate Cd stress and reduce root-to-shoot translocation of Cd in rice. J. Plant Nutr. Soil Sci. 2013, 176, 626–633. [Google Scholar] [CrossRef]
- Rea, P.A.; Vatamaniuk, O.K.; Rigden, D. Weeds, Worms, and More. Papain’s Long-Lost Cousin, Phytochelatin Synthase. Plant Physiol. 2004, 136, 2463–2474. [Google Scholar] [CrossRef]
- Da Cruz, T.N.M.; Savassa, S.M.; Montanha, G.S.; Ishida, J.K.; De Almeida, E.; Tsai, S.M.; Junior, J.L.; De Carvalho, H.W.P. A new glance on root-to-shoot in vivo zinc transport and time-dependent physiological effects of ZnSO4 and ZnO nanoparticles on plants. Sci. Rep. 2019, 9, 10416. [Google Scholar] [CrossRef]



| Tissue | GSH-PC2 | GSH-PC3 | GSH-PC4 | PC2-PC3 | PC2-PC4 | PC3-PC4 |
|---|---|---|---|---|---|---|
| Roots | −0.374 ** | −0.485 ** | −0.601 ** | 0.699 ** | 0.691 ** | 0.933 ** |
| Shoots | 0.052 | 0.094 | 0.053 | 0.908 ** | 0.904 ** | 0.928 ** |
| Leaves | 0.607 ** | 0.439 ** | 0.230 ** | 0.842 ** | 0.626 ** | 0.763 ** |
| Tissue | GSH | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| Roots | −0.609 ** | 0.662 ** | 0.687 ** | 0.678 ** |
| Shoots | −0.105 | 0.832 ** | 0.816 ** | 0.790 ** |
| Leaves | 0.642 ** | 0.786 ** | 0.672 ** | 0.424 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Li, H.; Song, Y.; Zhang, F.; Yang, Z. The Response of Thiols to Cadmium Stress in Spinach (Spinacia Oleracea L.). Toxics 2022, 10, 429. https://doi.org/10.3390/toxics10080429
Gao Y, Li H, Song Y, Zhang F, Yang Z. The Response of Thiols to Cadmium Stress in Spinach (Spinacia Oleracea L.). Toxics. 2022; 10(8):429. https://doi.org/10.3390/toxics10080429
Chicago/Turabian StyleGao, Ya, Haipu Li, Yang Song, Fenglin Zhang, and Zhaoguang Yang. 2022. "The Response of Thiols to Cadmium Stress in Spinach (Spinacia Oleracea L.)" Toxics 10, no. 8: 429. https://doi.org/10.3390/toxics10080429
APA StyleGao, Y., Li, H., Song, Y., Zhang, F., & Yang, Z. (2022). The Response of Thiols to Cadmium Stress in Spinach (Spinacia Oleracea L.). Toxics, 10(8), 429. https://doi.org/10.3390/toxics10080429






