Boron Compounds Exhibit Protective Effects against Aluminum-Induced Neurotoxicity and Genotoxicity: In Vitro and In Vivo Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. In Vitro Applications
2.3. Cytotoxicity Testing
2.4. Biochemical Assays
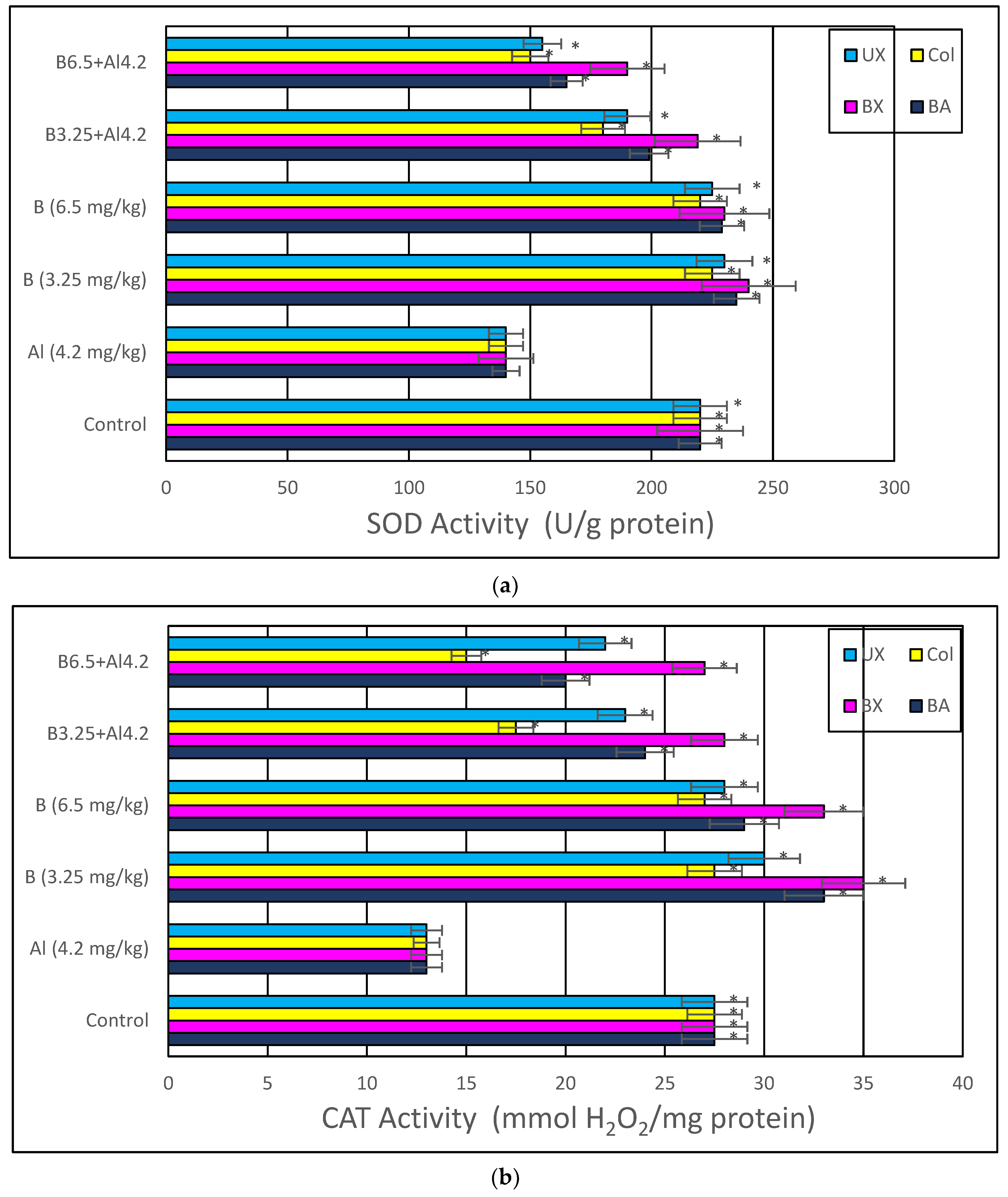
2.4.1. Superoxide Dismutase (SOD) Activity
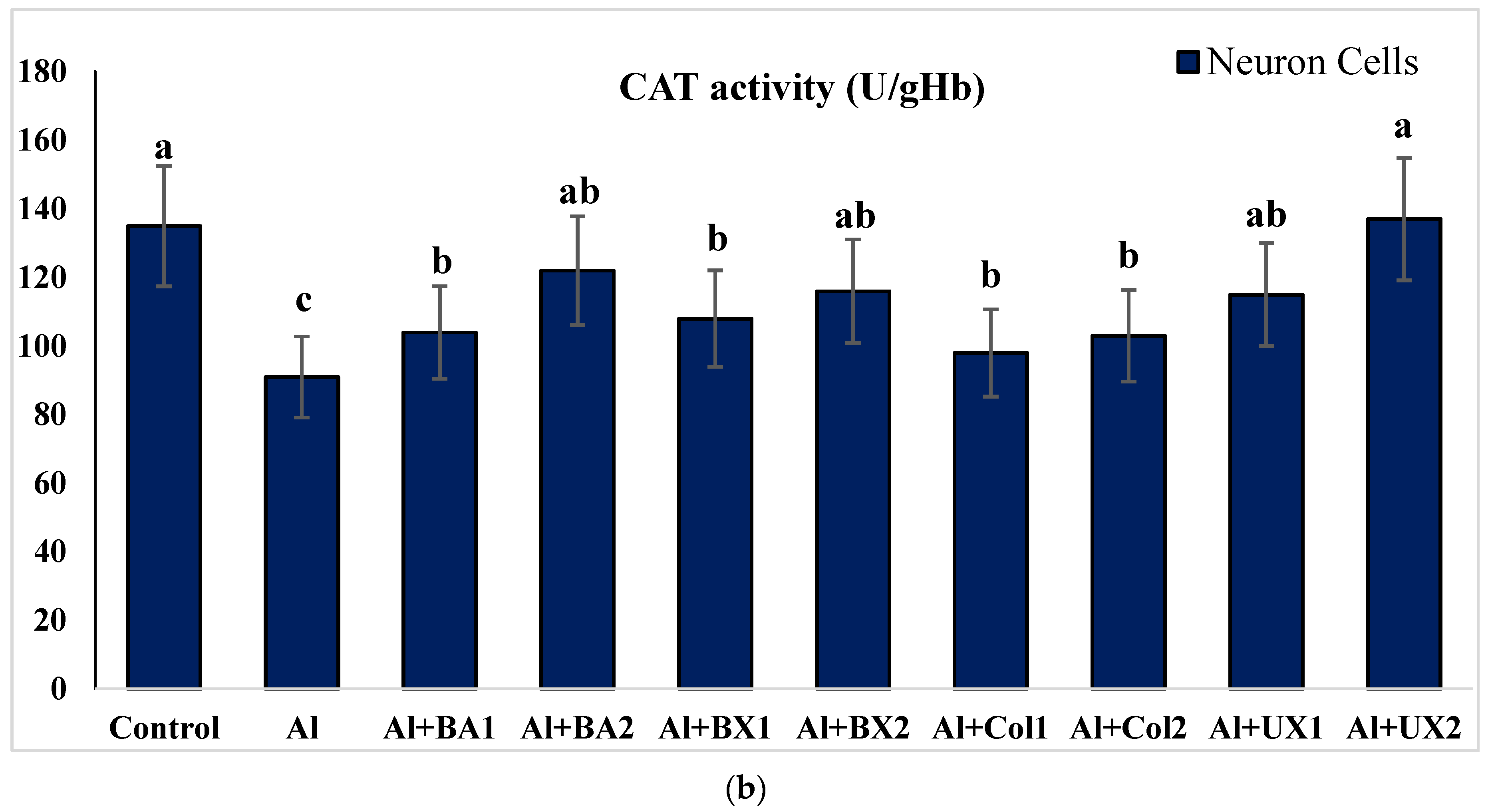
2.4.2. Catalase (CAT) Activity
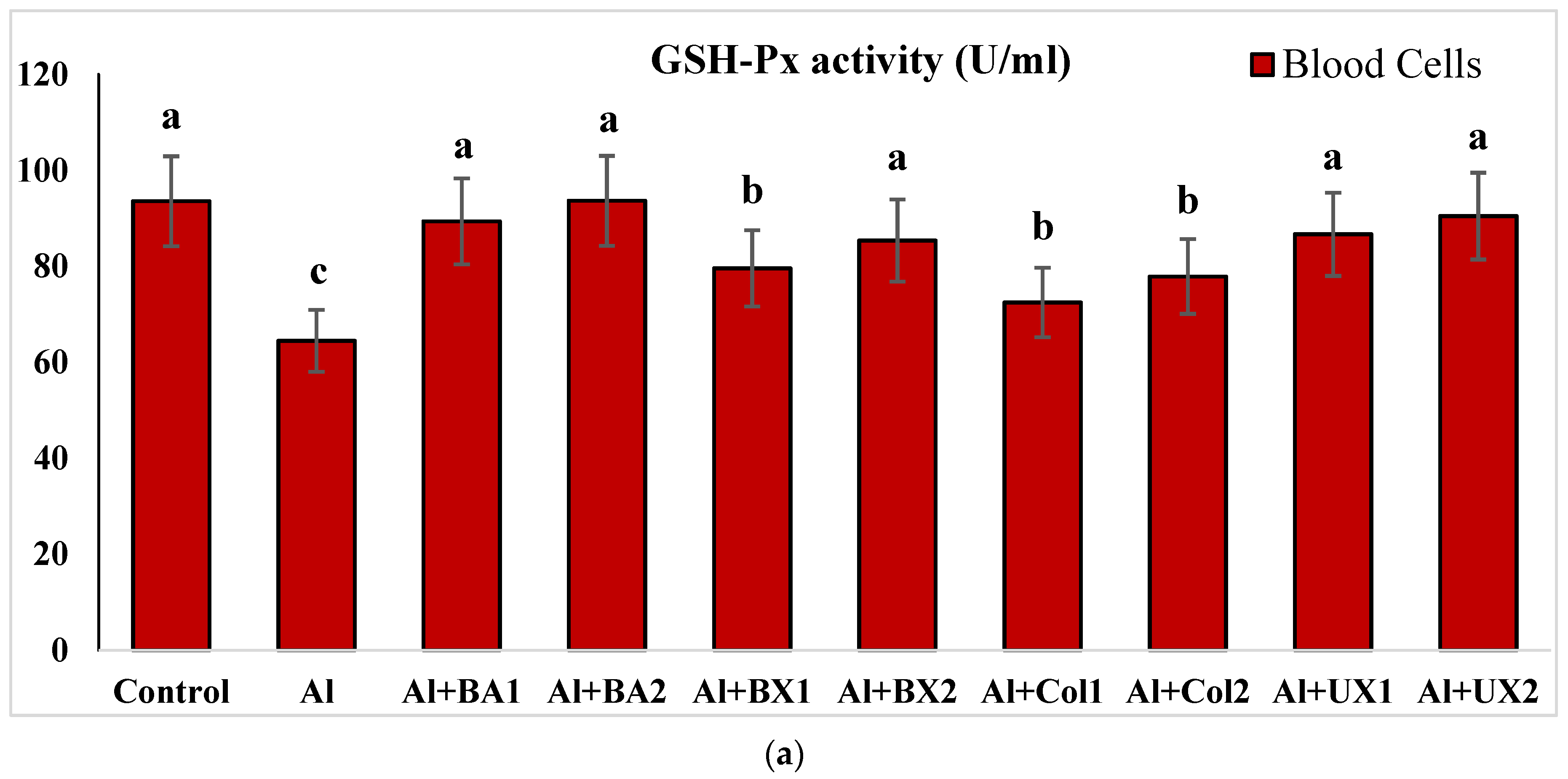
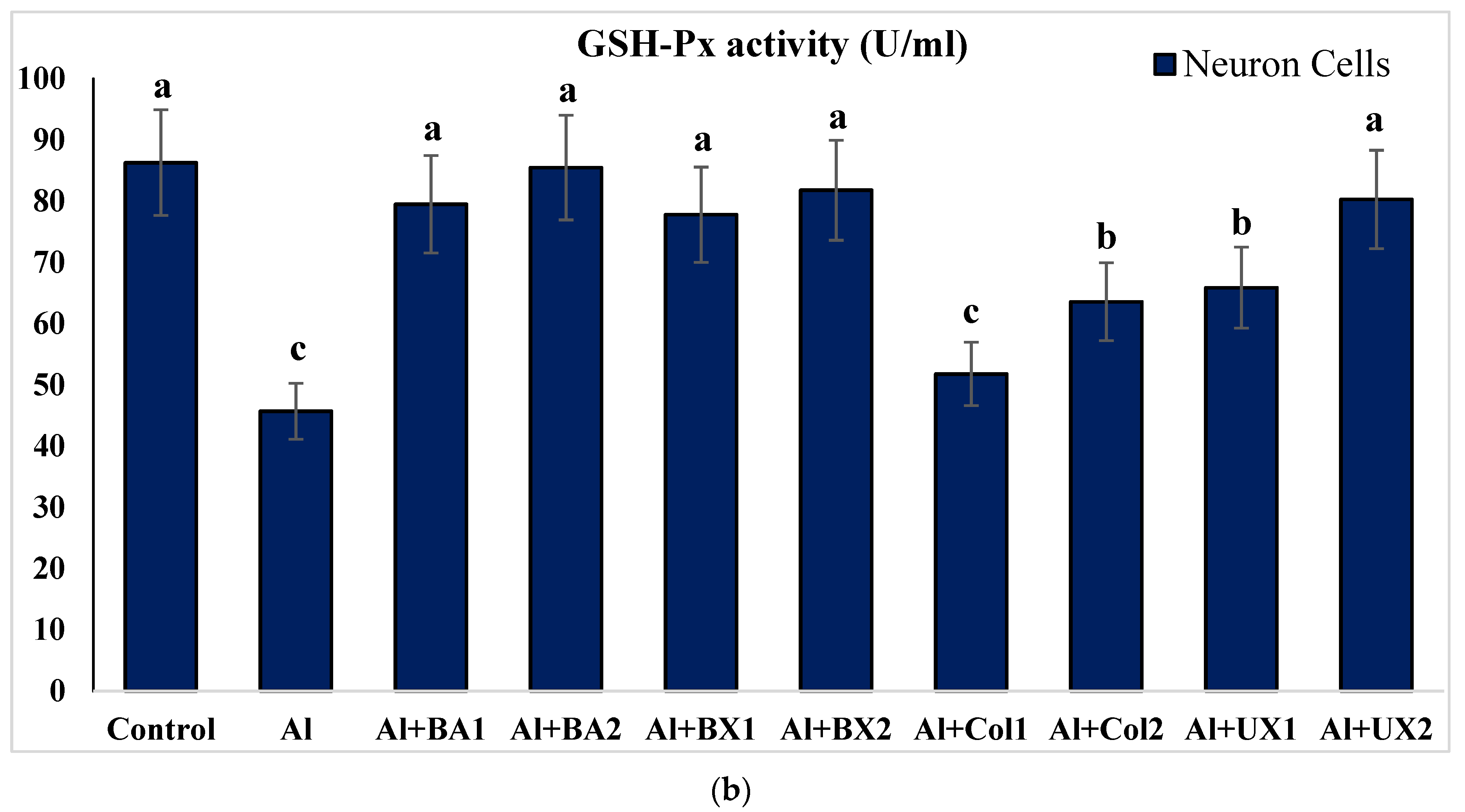
2.4.3. Glutathione Peroxidase (GSH-Px) Activity
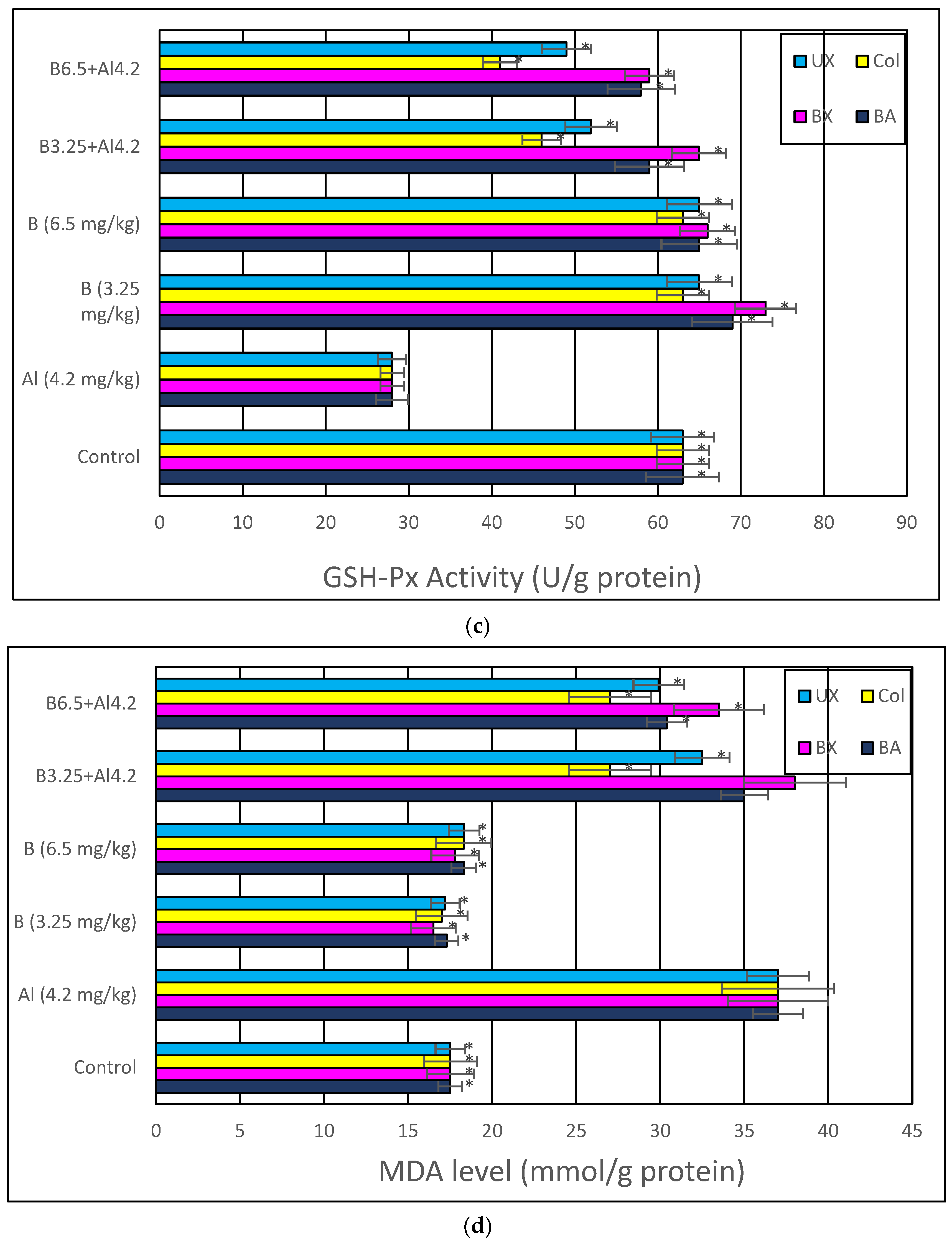
2.4.4. Malondialdehyde (MDA) Analysis
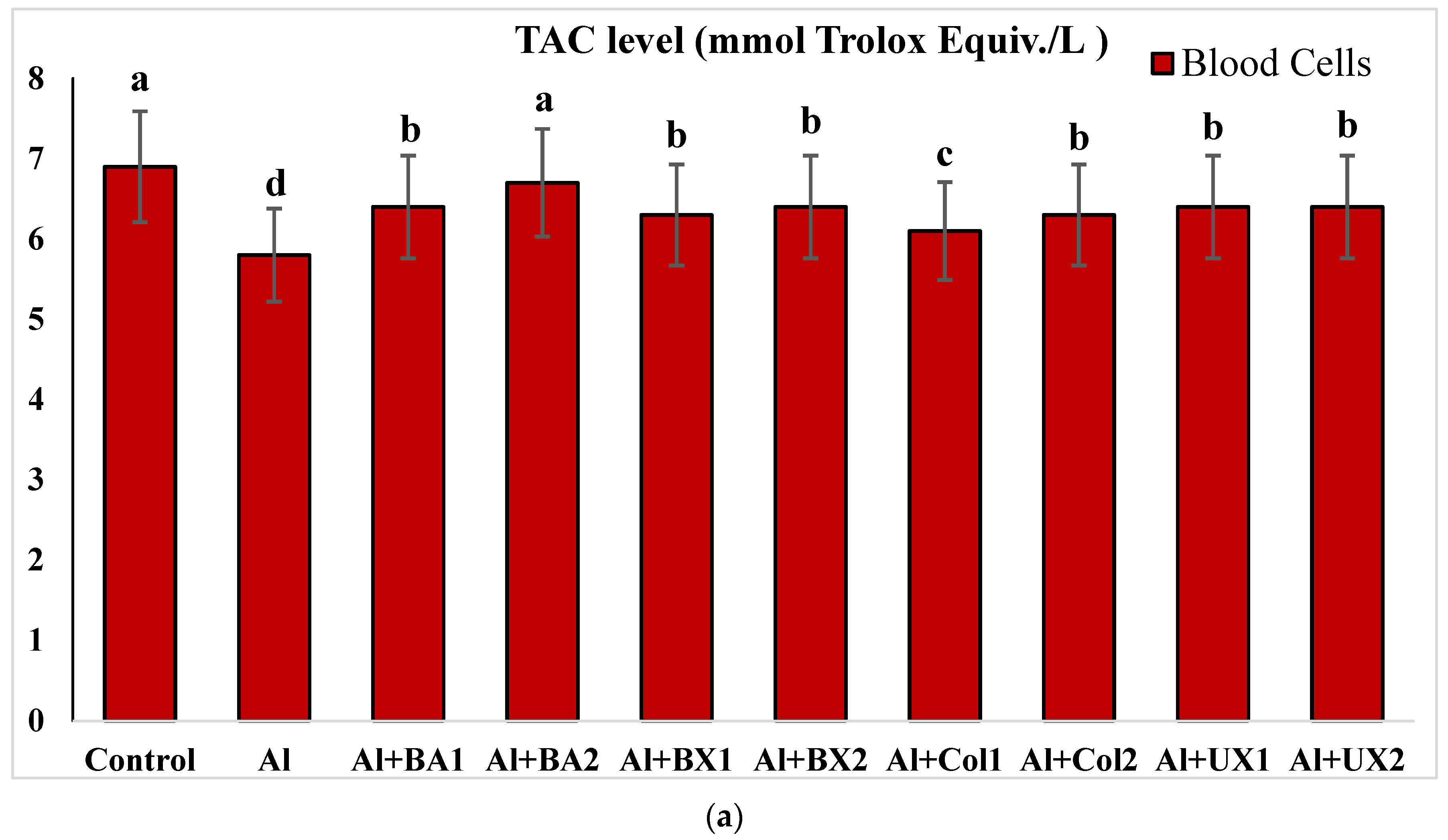
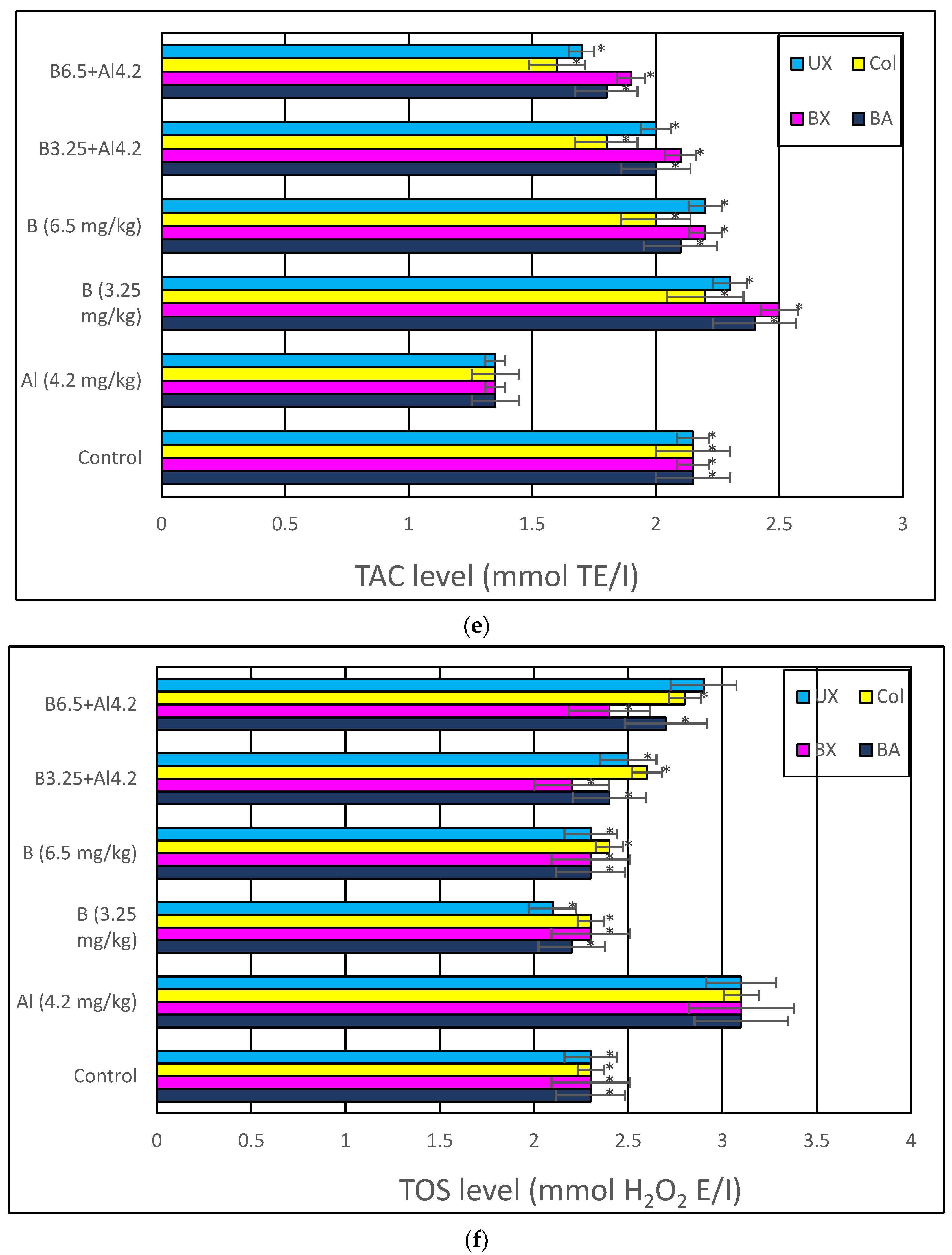
2.4.5. Total Antioxidant Capacity (TAC) and Total Oxidative Stress (TOS) Levels
2.5. Genotoxicity Testing on Human Blood Cultures
2.5.1. In Vitro CA Assay
2.5.2. In Vitro MN Assay
2.5.3. In Vitro SCGE Assay
2.6. Experimental Animals
2.7. Treatments with Al, BA, BX, Col and UX
2.8. In Vivo Biochemical Assays
2.9. In Vivo Genotoxicity Testing
2.9.1. In Vivo CA Assay
2.9.2. In Vivo MN Assay
2.9.3. In Vivo SCGE Assay
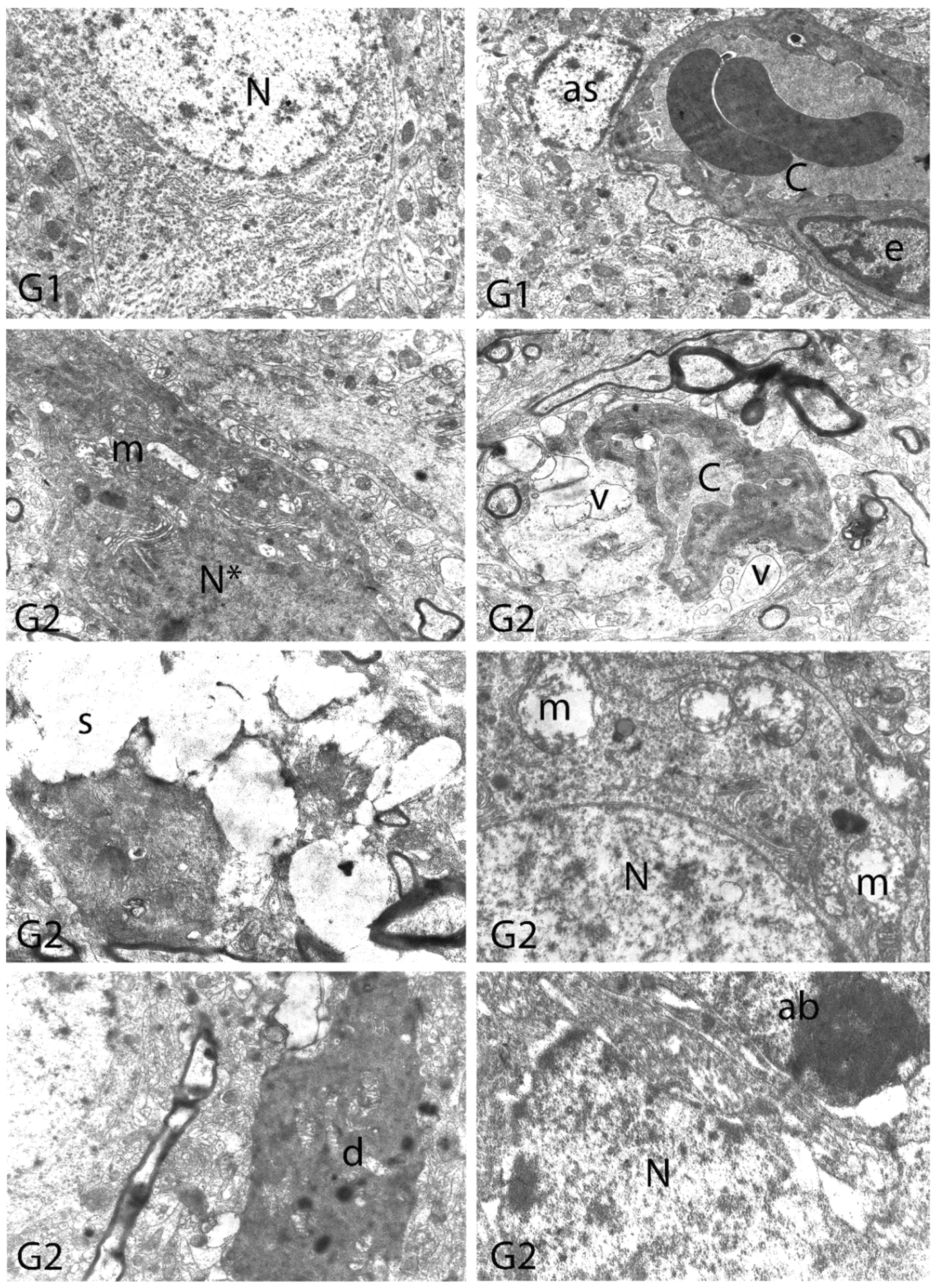
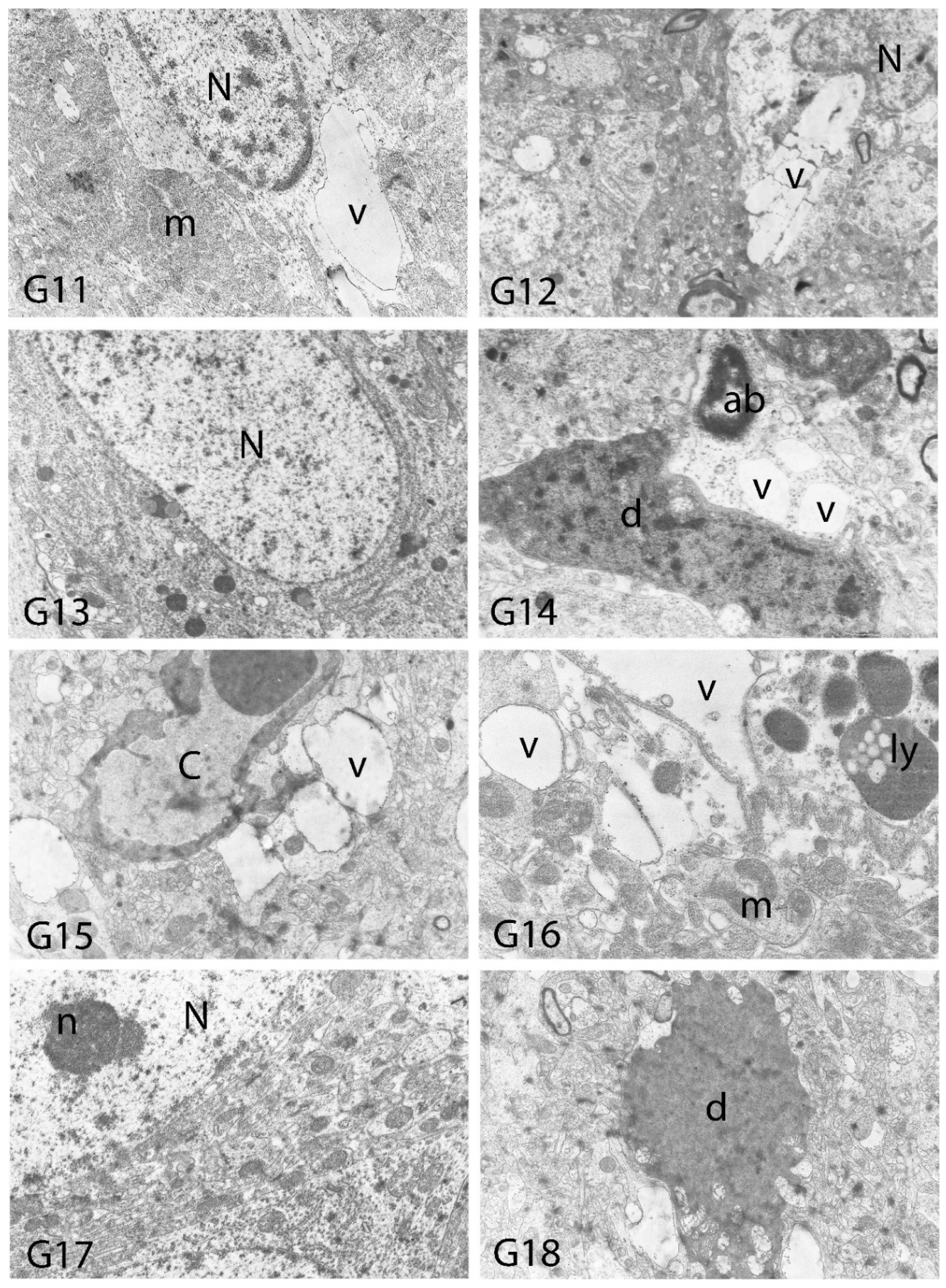
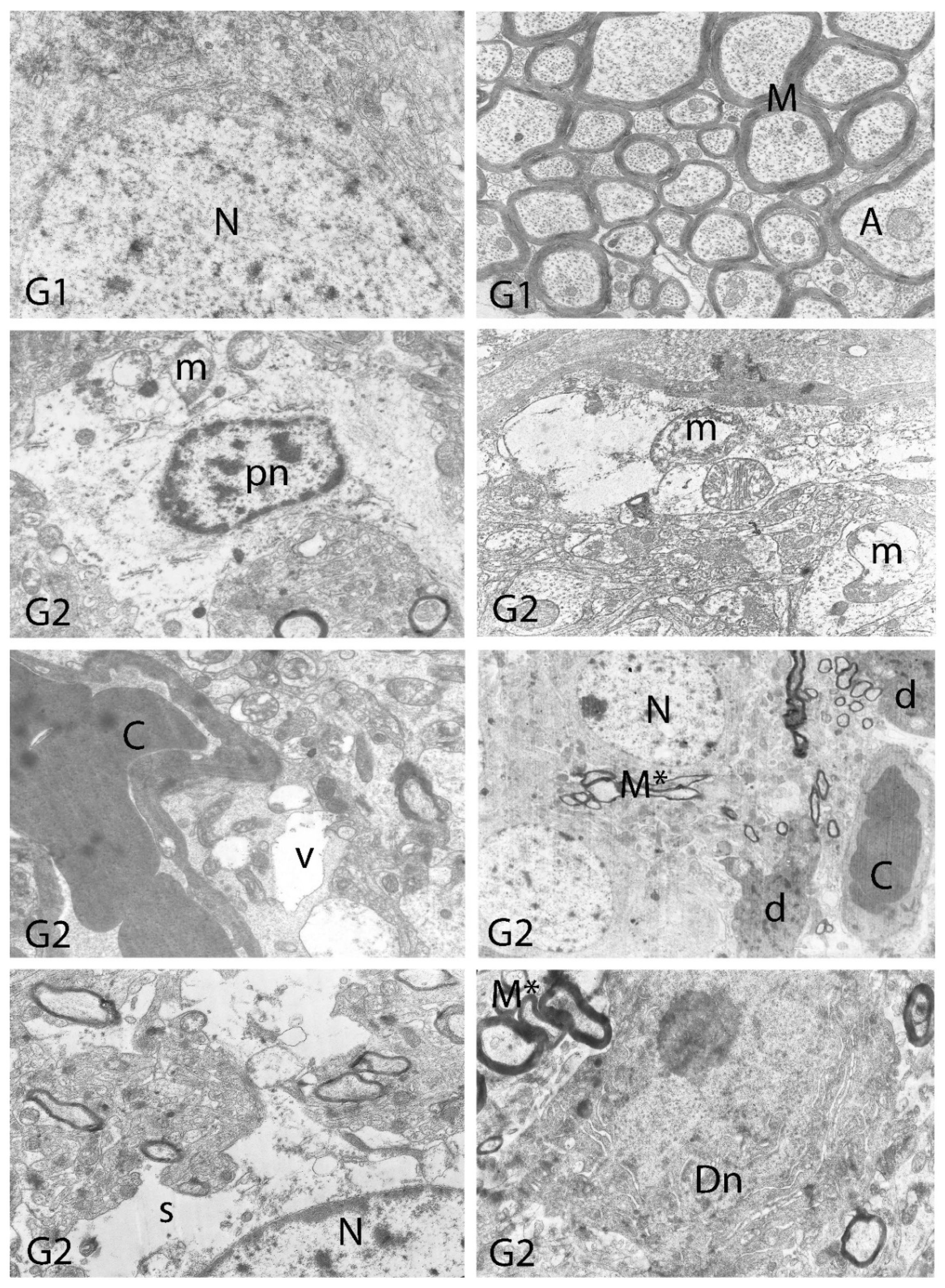
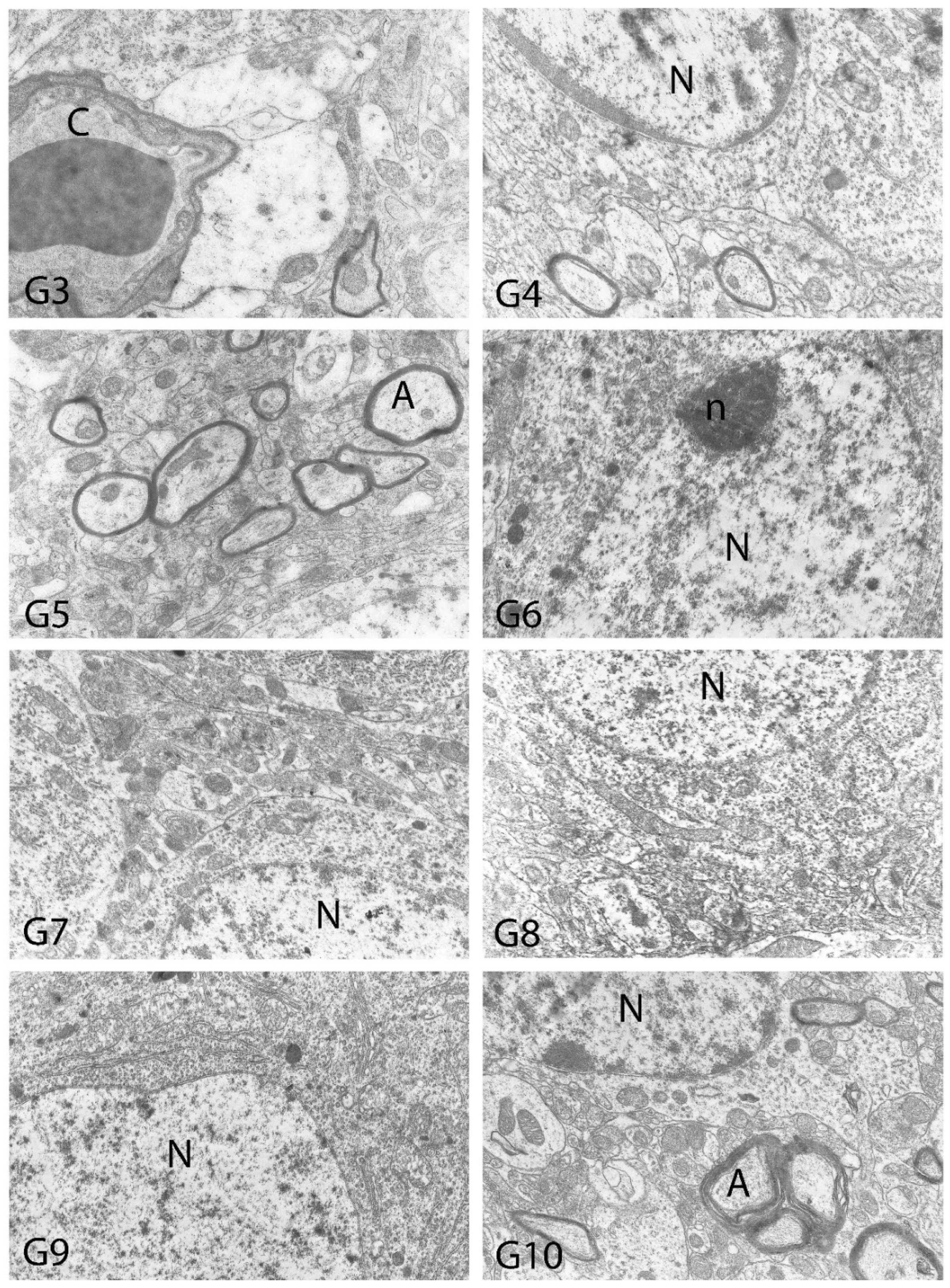
2.10. Histopathological Examination
2.11. Statistical Analysis
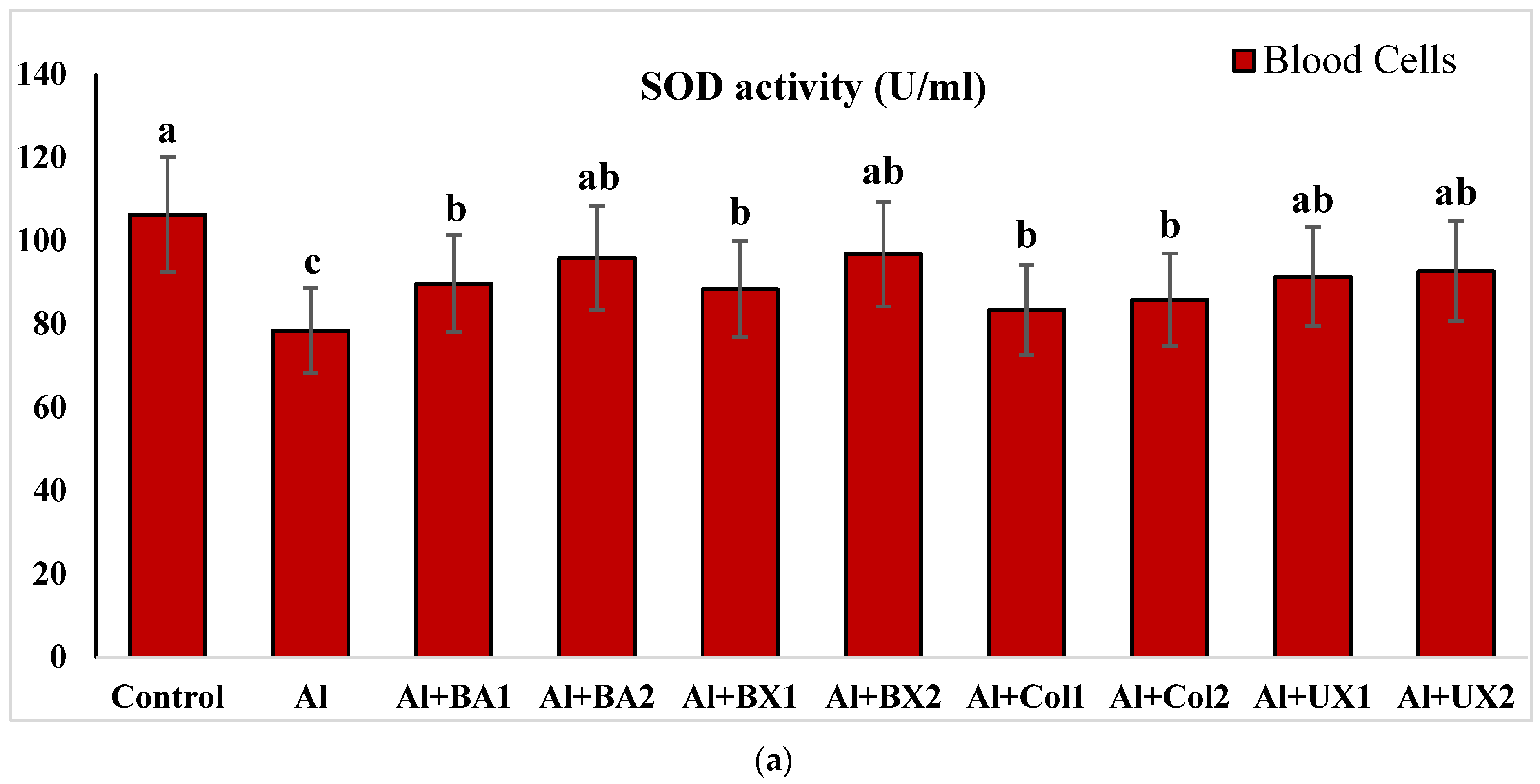
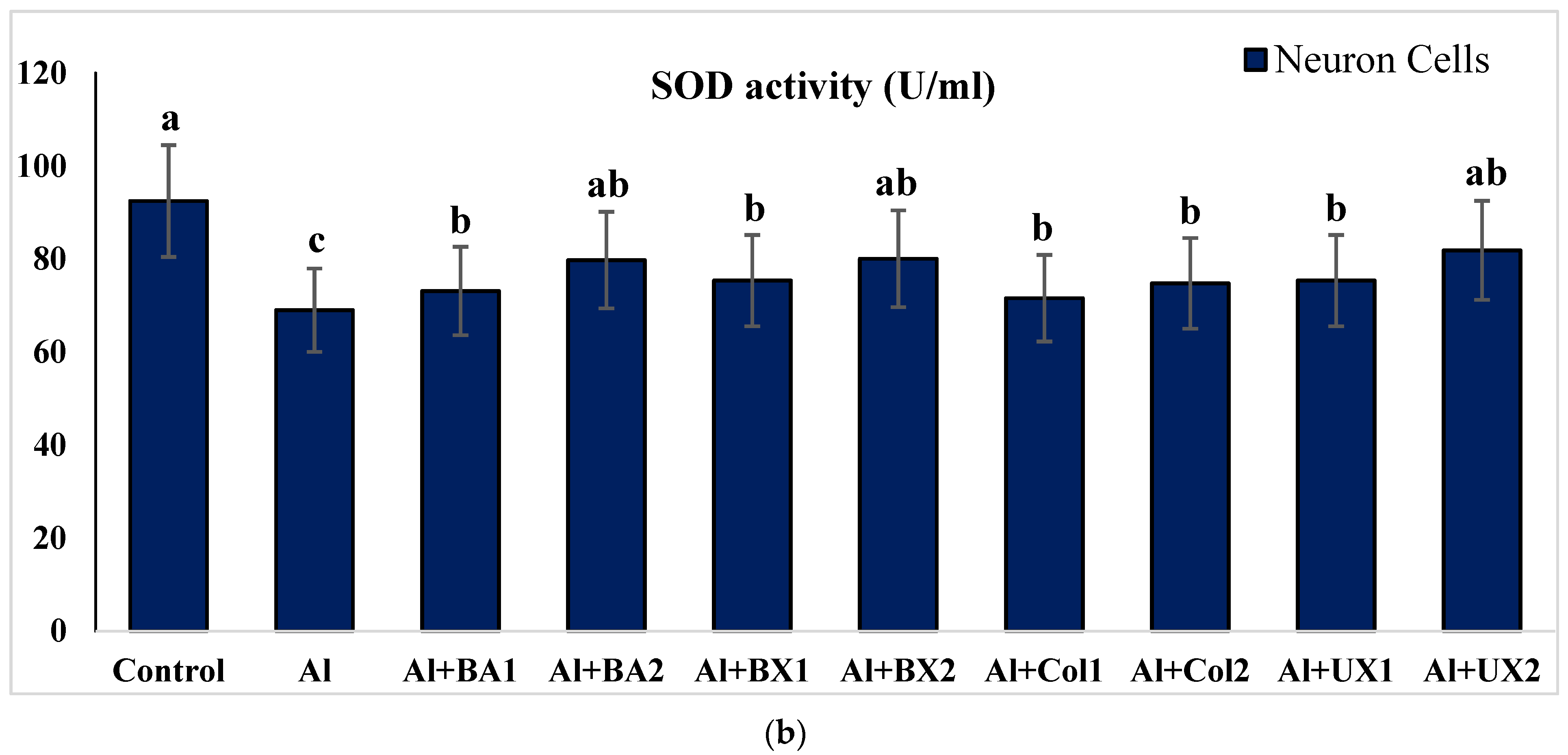
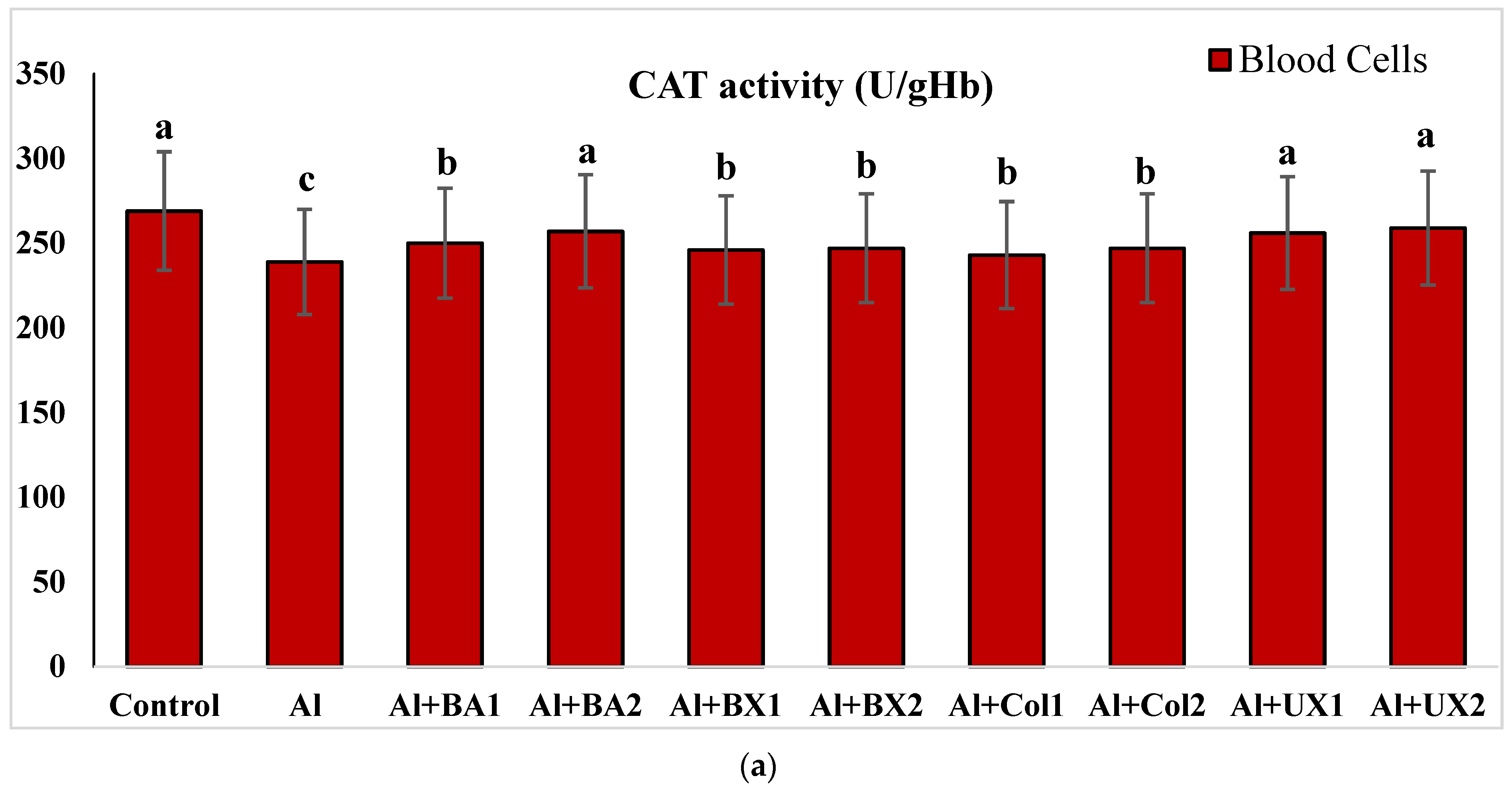
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stacchiotti, A.; Rodella, L.F.; Ricci, F.; Rezzani, R.; Lavazza, A.; Bianchi, R. Stress proteins expression in rat kidney and liver chronically exposed to aluminium sulphate. Histol. Histopathol. 2006, 21, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Dordevic, D.; Buchtova, H.; Jancikova, S.; Macharackova, B.; Jarosova, M.; Vítěz, T.; Kushkevych, I. Aluminum contamination of food during culinary preparation: Case study with aluminum foil and consumers’ preferences. Food Sci. Nutr. 2019, 7, 3349–3360. [Google Scholar] [CrossRef] [PubMed]
- Loughran, D.; Calello, D.; Nelson, L. Treatment of acute aluminum toxicity due to alum bladder irrigation in a hemodialysis patient: A case report. Toxicol. Commun. 2022, 6, 35–38. [Google Scholar] [CrossRef]
- Alasfar, R.H.; Isaifan, R.J. Aluminum environmental pollution: The silent killer. Environ. Sci. Pollut. Res. 2021, 28, 44587–44597. [Google Scholar] [CrossRef]
- Schetinger, M.R.C.; Bonan, C.D.; Morsch, V.M.; Bohrer, D.; Valentim, L.M.; Rodrigues, S.R. Effects of aluminum sulfate on delta-aminolevulinate dehydratase from kidney, brain, and liver of adult mice. Braz. J. Med. Biol. Res. 1999, 32, 761–766. [Google Scholar] [CrossRef]
- Bondy, S.C. The neurotoxicity of environmental aluminum is still an issue. NeuroToxicology 2010, 31, 575–581. [Google Scholar] [CrossRef]
- Promyo, K.; Iqbal, F.; Chaidee, N.; Chetsawang, B. Aluminum chloride-induced amyloid β accumulation and endoplasmic reticulum stress in rat brain are averted by melatonin. Food Chem. Toxicol. 2020, 146, 111829. [Google Scholar] [CrossRef]
- Mold, M.J.; O’Farrell, A.; Morris, B.; Exley, C. Aluminum and Neurofibrillary Tangle Co-Localization in Familial Alzheimer’s Disease and Related Neurological Disorders. J. Alzheimer’s Dis. 2020, 78, 139–149. [Google Scholar] [CrossRef]
- Osińska, E.; Kanoniuk, D.; Kusiak, A. Aluminum hemotoxicity mechanisms. Ann. Univ. Mariae Curie. Sklodowska. Med. 2004, 59, 411–416. [Google Scholar]
- Murray, F.J. A comparative review of the pharmacokinetics of boric acid in rodents and humans. Biol. Trace Element Res. 1998, 66, 331–341. [Google Scholar] [CrossRef]
- Dinca, L.; Scorei, R. Boron in Human Nutrition and its Regulations Use. J. Nutr. Ther. 2013, 2, 22–29. [Google Scholar]
- Białek, M.; Czauderna, M.; Krajewska, K.A.; Przybylski, W. Selected physiological effects of boron compounds for animals and humans. A review. J. Anim. Feed Sci. 2019, 28, 307–320. [Google Scholar] [CrossRef]
- Nielsen, F.H. Update on human health effects of boron. J. Trace Elem. Med. Biol. 2014, 28, 383–387. [Google Scholar] [CrossRef]
- Pizzorno, L. Nothing Boring About Boron. Integr. Med. 2015, 14, 35–48. [Google Scholar]
- Çelikezen, F.; Turkez, H.; Togar, B.; Izgi, M.S. DNA damaging and biochemical effects of potassium tetraborate. EXCLI J. 2014, 13, 446. [Google Scholar] [CrossRef]
- Turkez, H.; Geyikoglu, F.; Tatar, A.; Keles, M.S.; Kaplan, I. The effects of some boron compounds against heavy metal toxicity in human blood. Exp. Toxicol. Pathol. 2012, 64, 93–101. [Google Scholar] [CrossRef]
- Gündüz, M.K.; Bolat, M.; Kaymak, G.; Berikten, D.; Köse, D.A. Therapeutic Effects of Newly Synthesized Boron Compounds (BGM and BGD) on Hepatocellular Carcinoma. Biol. Trace Elem. Res. 2021, 200, 134–146. [Google Scholar] [CrossRef]
- Alak, G.; Ucar, A.; Parlak, V.; Yeltekin, A.Ç.; Özgeriş, F.B.; Atamanalp, M.; Türkez, H. Antioxidant Potential of Ulexite in Zebrafish Brain: Assessment of Oxidative DNA Damage, Apoptosis, and Response of Antioxidant Defense System. Biol. Trace Elem. Res. 2021, 199, 1092–1099. [Google Scholar] [CrossRef]
- Küçükdoğru, R.; Türkez, H.; Arslan, M.E.; Tozlu, Ö.Ö.; Sönmez, E.; Mardinoğlu, A.; Cacciatore, I.; Di Stefano, A. Neuroprotective effects of boron nitride nanoparticles in the experimental Parkinson’s disease model against MPP+ induced apoptosis. Metab. Brain Dis. 2020, 35, 947–957. [Google Scholar] [CrossRef]
- Laabbar, W.; Abbaoui, A.; Elgot, A.; Mokni, M.; Amri, M.; Masmoudi-Kouki, O.; Gamrani, H. Aluminum induced oxidative stress, astrogliosis and cell death in rat astrocytes, is prevented by curcumin. J. Chem. Neuroanat. 2021, 112, 101915. [Google Scholar] [CrossRef]
- Swain, C.; Chainy, G.B.N. Aluminum Effect on Lipid Peroxidation and on the Activities of Superoxide Dismutase and Catalase in the Cerebral Hemisphere and Liver of Young Chicks. J. Trace Elem. Med. Biol. 1997, 11, 77–82. [Google Scholar] [CrossRef]
- Nayak, P.; Sharma, S.B.; Chowdary, N.V.S. Alpha-Tocopherol Supplementation Restricts Aluminium- and Ethanol-Induced Oxidative Damage in Rat Brain but Fails to Protect Against Neurobehavioral Damage. J. Diet. Suppl. 2019, 16, 257–268. [Google Scholar] [CrossRef]
- Cao, Z.; Geng, X.; Jiang, X.; Gao, X.; Liu, K.; Li, Y. Melatonin Attenuates AlCl3-Induced Apoptosis and Osteoblastic Differentiation Suppression by Inhibiting Oxidative Stress in MC3T3-E1 Cells. Biol. Trace Elem. Res. 2020, 196, 214–222. [Google Scholar] [CrossRef]
- Ghorbel, I.; Elwej, A.; Chaabane, M.; Jamoussi, K.; Mnif, H.; Boudawara, T.; Zeghal, N. Selenium Alleviates Oxidative Stress and Lung Damage Induced by Aluminum Chloride in Adult Rats: Biochemical and Histological Approach. Biol. Trace Elem. Res. 2016, 176, 181–191. [Google Scholar] [CrossRef]
- Esparza, J.L.; Gómez, M.; Domingo, J.L. Role of Melatonin in Aluminum-Related Neurodegenerative Disorders: A Review. Biol. Trace Elem. Res. 2019, 188, 60–67. [Google Scholar] [CrossRef]
- Türkez, H.; Yousef, M.I.; Geyikoglu, F. Propolis prevents aluminium-induced genetic and hepatic damages in rat liver. Food Chem. Toxicol. 2010, 48, 2741–2746. [Google Scholar] [CrossRef]
- Evans, H.J.; O’Riordan, M.L. Human peripheral blood lymphocytes for the analysis of chromosome aberrations in mutagen tests. Mutat. Res. Mutagen. Relat. Subj. 1975, 31, 135–148. [Google Scholar] [CrossRef]
- Türkez, H.; Çelik, K.; Toğar, B. Effects of copaene, a tricyclic sesquiterpene, on human lymphocytes cells in vitro. Cytotechnology 2014, 66, 597–603. [Google Scholar] [CrossRef]
- Ban, J.Y.; Jeon, S.Y.; Nguyen, T.T.H.; Bae, K.H.; Song, K.S.; Seong, Y.H. Neuroprotective effect of oxyresveratrol from Smilacis chinae rhizome on amyloid β protein (25-35)-induced neurotoxicity in cultured rat cortical neurons. Biol. Pharm. Bull. 2006, 29, 2419–2424. [Google Scholar] [CrossRef]
- Türkez, H.; Togar, B.; Di Stefano, A.; Taspınar, N.; Sozio, P. Protective effects of cyclosativene on H2O2-induced injury in cultured rat primary cerebral cortex cells. Cytotechnology 2014, 67, 299–309. [Google Scholar] [CrossRef]
- Hacimuftuoglu, A.; Tatar, A.; Çetin, D.; Taşpinar, N.; Saruhan, F.; Okkay, U.; Turkez, H.; Unal, D.; Stephens, R.L.; Süleyman, H. Astrocyte/neuron ratio and its importance on glutamate toxicity: An in vitro voltammetric study. Cytotechnology 2016, 68, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Togar, B.; Türkez, H.; Stefano, A.D.; Tatar, A.; Cetin, D. Zingiberene attenuates hydrogen peroxide-induced toxicity in neuronal cells. Hum. Exp. Toxicol. 2015, 34, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Türkez, H.; Geyikoǧlu, F.; Tatar, A.; Keleş, S.; Özkan, A. Effects of Some Boron Compounds on Peripheral Human Blood. Z. Naturforsch. C 2007, 62, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, I.; Mannervik, B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975, 250, 5475–5480. [Google Scholar] [CrossRef]
- Türkez, H.; Geyikoǧlu, F.; Yousef, M.I. Modulatory effect of l-glutamine on 2,3,7,8 tetrachlorodibenzo-p-dioxin- induced liver injury in rats. Toxicol. Ind. Health 2012, 28, 663–672. [Google Scholar] [CrossRef]
- Stocks, J.; Dormandy, T.L. The Autoxidation of Human Red Cell Lipids Induced by Hydrogen Peroxide. Br. J. Haematol. 1971, 20, 95–111. [Google Scholar] [CrossRef]
- Kılıç, Y.; Geyikoglu, F.; Çolak, S.; Turkez, H.; Bakır, M.; Hsseinigouzdagani, M. Carvacrol modulates oxidative stress and decreases cell injury in pancreas of rats with acute pancreatitis. Cytotechnology 2016, 68, 1243–1256. [Google Scholar] [CrossRef][Green Version]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Çadirci, K.; Türkez, H.; Özdemir, Ö. The In Vitro Cytotoxicity, Genotoxicity and Oxidative Damage Potential of the Oral Dipeptidyl Peptidase-4 Inhibitor, Linagliptin, on Cultured Human Mononuclear Blood Cells. Acta Endocrinol. 2019, 15, 9–15. [Google Scholar] [CrossRef]
- Türkez, H.; Gürbüz, H.; Aydin, E.; Aslan, A.; Dirican, E. The evaluation of the genotoxic and oxidative damage potentials of Ulothrix tenuissima (Kütz.) in vitro. Toxicol. Ind. Health 2012, 28, 147–151. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Turkez, H.; Togar, B.; Tatar, A.; Geyıkoglu, F.; Hacımuftuoglu, A. Cytotoxic and cytogenetic effects of α-copaene on rat neuron and N2a neuroblastoma cell lines. Biologia 2014, 69, 936–942. [Google Scholar] [CrossRef]
- Çolak, S.; Geyikoğlu, F.; Keles, O.N.; Türkez, H.; Topal, A.; Unal, B. The neuroprotective role of boric acid on aluminum chloride-induced neurotoxicity. Toxicol. Ind. Health. 2011, 27, 700–710. [Google Scholar] [CrossRef]
- Chakrabarty, M.; Bhat, P.; Kumari, S.; D’Souza, A.; Bairy, K.L.; Chaturvedi, A.; Natarajan, A.; Mohandas Rao, K.G.; Kamath, S. Cortico-hippocampal salvage in chronic aluminium induced neurodegeneration by Celastruspaniculatus seed oil: Neurobehavioural, biochemical, histological study. J. Pharmacol. Pharmacother. 2012, 3, 161–171. [Google Scholar]
- Türkez, H.; Toğar, B. Aluminum phosphide-induced genetic and oxidative damages in rats: Attenuation by Laurus nobilis leaf extract. Toxicol. Ind. Health. 2013, 29, 579–583. [Google Scholar] [CrossRef]
- Türkez, H.; Togar, B.; Polat, E. Olive leaf extract modulates permethrin induced genetic and oxidative damage in rats. Cytotechnology 2012, 64, 459–464. [Google Scholar] [CrossRef]
- Speit, G.; Hartmann, A. The Comet Assay (Single-Cell Gel Test): A Sensitive Genotoxicity Test for the Detection of DNA Damage and Repair. Methods Mol. Biol. 1999, 113, 203–212. [Google Scholar] [CrossRef]
- Downie, T. Theory and Practice of Histological Techniques Edited by J.D. Bancroft & A. Stevens, Churchill Livingstone, Edinburgh, 740 pages, £55.00. Histopathology 1990, 17, 386. [Google Scholar]
- Bancroft, J.D.; Layton, C. The hematoxylins and eosin. In Bancroft’s Theory and Practice of Histological Techniques; Elsevier: Amsterdam, The Netherlands, 2013; pp. 173–186. [Google Scholar]
- Yulug, B.; Altay, O.; Li, X.; Hanoglu, L.; Cankaya, S.; Lam, S.; Yang, H.; Coskun, E.; Idil, E.; Nogaylar, R.; et al. Combined Metabolic Activators Improves Cognitive Functions in Alzheimer’s Disease. MedRxiv 2021. [Google Scholar] [CrossRef]
- Altunkaynak, B.Z.; Özbek, E.; Unal, B.; Aydin, N.; Aydin, M.D.; Vuraler, O. Chronic treatment of haloperidol induces pathological changes in striatal neurons of guinea pigs: A light and electron microscopical study. Drug Chem. Toxicol. 2012, 35, 406–411. [Google Scholar] [CrossRef]
- Gómez, M.; Esparza, J.L.; Cabré, M.; García, T.; Domingo, J.L. Aluminum exposure through the diet: Metal levels in AβPP transgenic mice, a model for Alzheimer’s disease. Toxicology 2008, 249, 214–219. [Google Scholar] [CrossRef]
- Aly, H.F.; Metwally, F.M.; Ahmed, H.H. Neuroprotective effects of dehydroepiandrosterone (DHEA) in rat model of Alzheimer’s disease. Acta Biochim. Pol. 2011, 58, 513–520. [Google Scholar] [CrossRef]
- Garcia, T.; Esparza, J.L.; Nogués, M.R.; Romeu, M.; Domingo, J.L.; Gómez, M. Oxidative stress status and RNA expression in hippocampus of an animal model of Alzheimer’s disease after chronic exposure to aluminum. Hippocampus 2010, 20, 218–225. [Google Scholar] [CrossRef]
- Xiao, F.; Li, X.-G.; Zhang, X.-Y.; Hou, J.-D.; Lin, L.-F.; Gao, Q.; Luo, H.-M. Combined administration of D-galactose and aluminium induces Alzheimerlike lesions in brain. Neurosci. Bull. 2011, 27, 143–155. [Google Scholar] [CrossRef]
- Sood, P.K.; Nahar, U.; Nehru, B. Stress Proteins and Glial Cell Functions During Chronic Aluminium Exposures: Protective Role of Curcumin. Neurochem. Res. 2012, 37, 639–646. [Google Scholar] [CrossRef]
- Khaliq, H.; Juming, Z.; Ke-Mei, P. The Physiological Role of Boron on Health. Biol. Trace Elem. Res. 2018, 186, 31–51. [Google Scholar] [CrossRef]
- Routray, I.; Ali, S. Boron inhibits apoptosis in hyperapoptosis condition: Acts by stabilizing the mitochondrial membrane and inhibiting matrix remodeling. Biochim. Biophys. Acta BBA-Gen. Subj. 2019, 1863, 144–152. [Google Scholar] [CrossRef]
- Hacioglu, C.; Kar, F.; Kar, E.; Kara, Y.; Kanbak, G. Effects of Curcumin and Boric Acid Against Neurodegenerative Damage Induced by Amyloid Beta (1-42). Biol. Trace Elem. Res. 2021, 199, 3793–3800. [Google Scholar] [CrossRef]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Jiang, C. Mechanisms of organic acids and boron induced tolerance of aluminum toxicity: A review. Ecotoxicol. Environ. Saf. 2018, 165, 25–35. [Google Scholar] [CrossRef]
- Türkez, H.; Arslan, M.E.; Özdemir, Ö.; Chikha, O. Ameliorative effect of boric acid against nicotine-induced cytotoxicity on cultured human primary alveolar epithelial cells. J. Boron 2016, 1, 104–109. [Google Scholar]
- Pawa, S.; Ali, S. Boron ameliorates fulminant hepatic failure by counteracting the changes associated with the oxidative stress. Chem.-Biol. Interact. 2006, 160, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Nehru, B. Antioxidant Enzymatic System in Neuronal and Glial Cells Enriched Fractions of Rat Brain After Aluminum Exposure. Cell. Mol. Neurobiol. 2007, 27, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Seif-El-Nasr, M.; Atia, A.S.; Abdelsalam, R.M. Effect of MAO-B Inhibition against Ischemia-induced Oxidative Stress in the Rat Brain. Arzneimittelforschung 2008, 58, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Wang, Y.; Imran, M.; Jiang, C. Boron alleviates the aluminum toxicity in trifoliate orange by regulating antioxidant defense system and reducing root cell injury. J. Environ. Manage 2018, 208, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.G.; Cardwell, L.A.; Oussedik, E.; Feldman, S.R. Utility of boron in dermatology. J. Dermatol. Treat. 2020, 31, 2–12. [Google Scholar] [CrossRef]
- Sensi, S.L.; Granzotto, A.; Siotto, M.; Squitti, R. Copper and Zinc Dysregulation in Alzheimer’s Disease. Trends Pharmacol. Sci. 2018, 39, 1049–1063. [Google Scholar] [CrossRef]
- Yadav, E.; Yadav, P. Biofabricated zinc oxide nanoparticles impair cognitive function via modulating oxidative stress and acetylcholinesterase level in mice. Environ. Toxicol. 2021, 36, 572–585. [Google Scholar] [CrossRef]
- Narayanan, S.E.; Rehuman, N.A.; Harilal, S.; Vincent, A.; Rajamma, R.G.; Behl, T.; Uddin, M.S.; Ashraf, G.M.; Mathew, B. Molecular mechanism of zinc neurotoxicity in Alzheimer’s disease. Environ. Sci. Pollut. Res. 2020, 27, 43542–43552. [Google Scholar] [CrossRef]
- Feng, P.; Li, T.; Guan, Z.; Franklin, R.B.; Costello, L.C. The Involvement of Bax in Zinc-Induced Mitochondrial Apoptogenesis in Malignant Prostate Cells. Mol. Cancer 2008, 7, 25. [Google Scholar] [CrossRef]
- Pandya, J.D.; Dave, K.R.; Katyare, S.S. Effect of long-term aluminum feeding on lipid/phospholipid profiles of rat brain myelin. Lipids Health Dis. 2004, 3, 13. [Google Scholar] [CrossRef][Green Version]
- Princ, F.G.; Juknat, A.A.; Amitrano, A.A.; Batlle, A. Effect of Reactive Oxygen Species Promoted by δ-Aminolevulinic Acid on Porphyrin Biosynthesis and Glucose Uptake in Rat Cerebellum. Gen. Pharmacol. Vasc. Syst. 1998, 31, 143–148. [Google Scholar] [CrossRef]
- Ozdemir, H.; Yaren, B.; Oto, G. Effect of dietary boron on learning and behavior in rats administered with boric acid. Cell. Mol. Biol. 2019, 65, 65–72. [Google Scholar] [CrossRef]
- Kot, F.S. Boron sources, speciation and its potential impact on health. Rev. Environ. Sci. Bio/Technol. 2009, 8, 3–28. [Google Scholar] [CrossRef]
- De Pasquale, D.; Marino, A.; Tapeinos, C.; Pucci, C.; Rocchiccioli, S.; Michelucci, E.; Finamore, F.; McDonnell, L.; Scarpellini, A.; Lauciello, S.; et al. Homotypic targeting and drug delivery in glioblastoma cells through cell membrane-coated boron nitride nanotubes. Mater. Des. 2020, 192, 108742. [Google Scholar] [CrossRef]
- WHO. Boron in Drinking-water Background. Health Criteria Other Support. Inf. 2003, 2, 1–17. [Google Scholar]
- Arslan, M.; Topaktas, M.; Rencuzogullari, E. The effects of boric acid on sister chromatid exchanges and chromosome aberrations in cultured human lymphocytes. Cytotechnology 2008, 56, 91–96. [Google Scholar] [CrossRef][Green Version]
- Turkez, H.; Geyikoğlu, F.; Dirican, E.; Tatar, A. In vitro studies on chemoprotective effect of borax against aflatoxin B1-induced genetic damage in human lymphocytes. Cytotechnology 2012, 64, 607–612. [Google Scholar] [CrossRef][Green Version]
- Elikezen, F.Ç.; Toğar, B.; Özgeriş, F.B.; İzgi, M.S.; Türkez, H. Cytogenetic and oxidative alterations after exposure of cultured human whole blood cells to lithium metaborate dehydrate. Cytotechnology 2016, 68, 821–827. [Google Scholar] [CrossRef][Green Version]
- Martínez-Rodríguez, N.L.; Tavárez, S.; González-Sánchez, Z.I. In vitro toxicity assessment of zinc and nickel ferrite nanoparticles in human erythrocytes and peripheral blood mononuclear cell. Toxicol. Vitr. 2019, 57, 54–61. [Google Scholar] [CrossRef]
- Suljević, D.; Hodžić-Klapuh, L.; Handžić, N.; Fočak, M. Morpho-functional alterations in lymphocytes and erythrocytes of Japanese quail due to prolonged in vivo exposure to heavy metal complexes. J. Trace Elem. Med. Biol. 2020, 59, 126472. [Google Scholar] [CrossRef]
- Bai, Y.; Guan, X.; Wei, W.; Feng, Y.; Meng, H.; Li, G.; Li, H.; Li, M.; Wang, C.; Fu, M.; et al. Effects of polycyclic aromatic hydrocarbons and multiple metals co-exposure on the mosaic loss of chromosome Y in peripheral blood. J. Hazard. Mater. 2021, 414, 125519. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione Is a Key Player in Metal-Induced Oxidative Stress Defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef]
- Viezeliene, D.; Jansen, E.; Rodovicius, H.; Kasauskas, A.; Ivanov, L. Protective effect of selenium on aluminium-induced oxidative stress in mouse liver in vivo. Environ. Toxicol. Pharmacol. 2011, 31, 302–306. [Google Scholar] [CrossRef]
- Shen, X.; Li, R.; Chai, M.; Cheng, S.; Niu, Z.; Qiu, G.Y. Interactive effects of single, binary and trinary trace metals (lead, zinc and copper) on the physiological responses of Kandelia obovata seedlings. Environ. Geochem. Health 2019, 41, 135–148. [Google Scholar] [CrossRef]
- Kasperczyk, S.; Kasperczyk, J.; Ostałowska, A.; Zalejska-Fiolka, J.; Wielkoszyński, T.; Świętochowska, E.; Birkner, E. The Role of the Antioxidant Enzymes in Erythrocytes in the Development of Arterial Hypertension among Humans Exposed to Lead. Biol. Trace Element Res. 2009, 130, 95–106. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Mori, C.; Tokumoto, M.; Satoh, M. Time-Dependent Changes in the Gene Expression Levels in the Mouse Kidney by Long-Term Exposure to Cadmium. BPB Rep. 2021, 4, 69–73. [Google Scholar] [CrossRef]
- Agnihotri, S.K.; Kesari, K.K. Mechanistic Effect of Heavy Metals in Neurological Disorder and Brain Cancer. In Networking of Mutagens in Environmental Toxicology; Springer: Cham, Switzerland, 2019; pp. 25–47. [Google Scholar] [CrossRef]
- Garza-Lombó, C.; Posadas, Y.; Quintanar, L.; Gonsebatt, M.E.; Franco, R. Neurotoxicity Linked to Dysfunctional Metal Ion Homeostasis and Xenobiotic Metal Exposure: Redox Signaling and Oxidative Stress. Antioxid. Redox Signal. 2018, 28, 1669–1703. [Google Scholar] [CrossRef]
- Ahmad, W.; Ijaz, B.; Shabbiri, K.; Ahmed, F.; Rehman, S. Oxidative toxicity in diabetes and Alzheimer’s disease: Mechanisms behind ROS/ RNS generation. J. Biomed. Sci. 2017, 24, 76. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Aboul-Enein, H.Y. Reactive oxygen and nitrogen species in carcinogenesis: Implications of oxidative stress on the progression and development of several cancer types. Mini-Rev. Med. Chem. 2017, 17, 904–919. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M. The in vitro micronucleus technique. Mutat. Res. Mol. Mech. Mutagen. 2000, 455, 81–95. [Google Scholar] [CrossRef]
- Mosesso, P.; Cinelli, S.; Natarajan, A.T.; Palitti, F. In Vitro Cytogenetic Assays: Chromosomal Aberrations and Micronucleus Tests. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; pp. 123–146. ISBN 9781627035286. [Google Scholar]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.-C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Kiernan, J. Does progressive nuclear staining with hemalum (alum hematoxylin) involve DNA, and what is the nature of the dye-chromatin complex? Biotech. Histochem. 2018, 93, 133–148. [Google Scholar] [CrossRef]
- Lozano, H.J.; Busto, N.; Lari, M.; Leal, J.M.; García, B. Binding of aluminium/cacodylate complexes with DNA and RNA. Experimental and “in silico”study. New J. Chem. 2018, 42, 8137–8144. [Google Scholar] [CrossRef]
- Varella, S.D.; Pozetti, G.L.; Vilegas, W.; Varanda, E.A. Mutagenic activity in waste from an aluminum products factory in Salmonella/microsome assay. Toxicol. Vitr. 2004, 18, 895–900. [Google Scholar] [CrossRef]
- Banasik, A.; Lankoff, A.; Piskulak, A.; Adamowska, K.; Lisowska, H.; Wojcik, A. Aluminum-induced micronuclei and apoptosis in human peripheral-blood lymphocytes treated during different phases of the cell cycle. Environ. Toxicol. 2005, 20, 402–406. [Google Scholar] [CrossRef]
- Lima, P.D.L.; Leite, D.S.; Vasconcellos, M.C.; Cavalcanti, B.C.; Santos, R.A.; Costa-Lotufo, L.V.; Pessoa, C.; Moraes, M.O.; Burbano, R.R. Genotoxic effects of aluminum chloride in cultured human lymphocytes treated in different phases of cell cycle. Food Chem. Toxicol. 2007, 45, 1154–1159. [Google Scholar] [CrossRef]
- Abu-Taweel, G.M.; Ajarem, J.S.; Ahmad, M. Neurobehavioral toxic effects of perinatal oral exposure to aluminum on the developmental motor reflexes, learning, memory and brain neurotransmitters of mice offspring. Pharmacol. Biochem. Behav. 2012, 101, 49–56. [Google Scholar] [CrossRef]
- Jiang, T.; Zhi, X.-L.; Zhang, Y.-H.; Pan, L.-F.; Zhou, P. Inhibitory effect of curcumin on the Al(III)-induced Aβ42 aggregation and neurotoxicity in vitro. Biochim. et Biophys. Acta BBA-Mol. Basis Dis. 2012, 1822, 1207–1215. [Google Scholar] [CrossRef]
- Kawahara, M. Effects of aluminum on the nervous system and its possible link with neurodegenerative diseases. J. Alzheimer’s Dis. 2005, 8, 171–182. [Google Scholar] [CrossRef]
- Candan, N.; Tuzmen, N. Very rapid quantification of malondialdehyde (MDA) in rat brain exposed to lead, aluminium and phenolic antioxidants by high-performance liquid chromatography-fluorescence detection. NeuroToxicology 2008, 29, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Niu, Q.; Niu, P.Y.; Ji, X.L.; Zhang, C.; Wang, L. Novel interventions targeting on apoptosis and necrosis induced by aluminum chloride in neuroblastoma cells. J. Biol. Regul. Homeost. Agents 2010, 24, 137–148. [Google Scholar] [PubMed]
- Sumathi, T.; Shobana, C.; Kumari, B.R.; Nandhini, D.N. Protective Role of Cynodon dactylon in Ameliorating the Aluminium-Induced Neurotoxicity in Rat Brain Regions. Biol. Trace Elem. Res. 2011, 144, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Kwakowsky, A.; Calvo-Flores Guzmán, B.; Pandya, M.; Turner, C.; Waldvogel, H.J.; Faull, R.L. GABA A receptor subunit expression changes in the human Alzheimer’s disease hippocampus, subiculum, entorhinal cortex and superior temporal gyrus. J. Neurochem. 2018, 145, 374–392. [Google Scholar] [CrossRef]
- Roeske, M.J.; McHugo, M.; Vandekar, S.; Blackford, J.U.; Woodward, N.D.; Heckers, S. Incomplete hippocampal inversion in schizophrenia: Prevalence, severity, and impact on hippocampal structure. Mol. Psychiatry 2021, 26, 5407–5416. [Google Scholar] [CrossRef]
- Mohammadipour, A.; Abudayyak, M. Hippocampal toxicity of metal base nanoparticles. Is there a relationship between nanoparticles and psychiatric disorders? Rev. Environ. Health 2022, 37, 35–44. [Google Scholar] [CrossRef]
- Ekong, M.B.; Ekpo, M.M.; Akpanyung, E.O.; Nwaokonko, D.U. Neuroprotective effect of Moringa oleifera leaf extract on aluminium-induced temporal cortical degeneration. Metab. Brain Dis. 2017, 32, 1437–1447. [Google Scholar] [CrossRef]
- Mesole, S.B.; Alfred, O.O.; Yusuf, U.A.; Lukubi, L.; Ndhlovu, D. Apoptotic Inducement of Neuronal Cells by Aluminium Chloride and the Neuroprotective Effect of Eugenol in Wistar Rats. Oxid. Med. Cell. Longev. 2020, 2020, 8425643. [Google Scholar] [CrossRef]
- Zhao, Y.; Dang, M.; Zhang, W.; Lei, Y.; Ramesh, T.; Veeraraghavan, V.P.; Hou, X. Neuroprotective effects of Syringic acid against aluminium chloride induced oxidative stress mediated neuroinflammation in rat model of Alzheimer’s disease. J. Funct. Foods 2020, 71, 104009. [Google Scholar] [CrossRef]
- Wang, X.; Fan, X.; Yuan, S.; Jiao, W.; Liu, B.; Cao, J.; Jiang, W. Chlorogenic acid protects against aluminium-induced cytotoxicity through chelation and antioxidant actions in primary hippocampal neuronal cells. Food Funct. 2017, 8, 2924–2934. [Google Scholar] [CrossRef]
- Cheng, L.; Liang, R.; Li, Z.; Ren, J.; Yang, S.; Bai, J.; Niu, Q.; Yu, H.; Zhang, H.; Xia, N.; et al. Aluminum maltolate triggers ferroptosis in neurons: Mechanism of action. Toxicol. Mech. Methods 2021, 31, 33–42. [Google Scholar] [CrossRef]
- Yen, C.-M.; Shen, C.-C.; Yang, Y.-C.; Liu, B.-S.; Lee, H.-T.; Sheu, M.-L.; Tsai, M.-H.; Cheng, W.-Y. Novel electrospun poly(ε-caprolactone)/type I collagen nanofiber conduits for repair of peripheral nerve injury. Neural Regen. Res. 2019, 14, 1617. [Google Scholar]
- Aboelwafa, H.R.; El-kott, A.F.; Abd-Ella, E.M.; Yousef, H.N. The Possible Neuroprotective Effect of Silymarin against Aluminum Chloride-Prompted Alzheimer’s-Like Disease in Rats. Brain Sci. 2020, 10, 628. [Google Scholar] [CrossRef]
- Türk, E.; Ozan Tekeli, I.; Özkan, H.; Uyar, A.; Cellat, M.; Kuzu, M.; Yavas, I.; Alizadeh Yegani, A.; Yaman, T.; Güvenç, M. The protective effect of esculetin against aluminium chloride-induced reproductive toxicity in rats. Andrologia 2021, 53, e13930. [Google Scholar] [CrossRef]
- Bhadauria, M. Combined treatment of HEDTA and propolis prevents aluminum induced toxicity in rats. Food Chem. Toxicol. 2012, 50, 2487–2495. [Google Scholar]
- Yang, X.; Yu, K.; Wang, H.; Zhang, H.; Bai, C.; Song, M.; Han, Y.; Shao, B.; Li, Y.; Li, X. Bone impairment caused by AlCl3 is associated with activation of the JNK apoptotic pathway mediated by oxidative stress. Food Chem. Toxicol. 2018, 116, 307–314. [Google Scholar] [CrossRef]
- Al-Hazmi, M.A.; Rawi, S.M.; Hamza, R.Z. Biochemical, histological, and neuro-physiological effects of long-term aluminum chloride exposure in rats. Metab. Brain Dis. 2021, 36, 429–436. [Google Scholar] [CrossRef]
- Hu, C.; Li, J.; Zhu, Y.; Bai, C.; Zhang, J.; Xia, S.; Li, Y. Effects of Al on the splenic immune function and NE in rats. Food Chem. Toxicol. 2013, 62, 194–198. [Google Scholar] [CrossRef]
- Kushkuley, J.; Metkar, S.; Chan, W.K.-H.; Lee, S.; Shea, T.B. Aluminum induces neurofilament aggregation by stabilizing cross-bridging of phosphorylated c-terminal sidearms. Brain Res. 2010, 1322, 118–123. [Google Scholar] [CrossRef]
- Jendrach, M.; Mai, S.; Pohl, S.; Vöth, M.; Bereiter-Hahn, J. Short- and long-term alterations of mitochondrial morphology, dynamics and mtDNA after transient oxidative stress. Mitochondrion 2008, 8, 293–304. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, W.; Xu, F.; Cao, Z.; Jia, F.; Li, Y. Iron Dyshomeostasis Participated in Rat Hippocampus Toxicity Caused by Aluminum Chloride. Biol. Trace Elem. Res. 2020, 197, 580–590. [Google Scholar] [CrossRef]
- Bartesaghi, S.; Radi, R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018, 14, 618–625. [Google Scholar] [CrossRef]
- Ahmad, R.; Hussain, A.; Ahsan, H. Peroxynitrite: Cellular pathology and implications in autoimmunity. J. Immunoass. Immunochem. 2019, 40, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Kameritsch, P.; Singer, M.; Nuernbergk, C.; Rios, N.; Reyes, A.M.; Schmidt, K.; Kirsch, J.; Schneider, H.; Müller, S.; Pogoda, K.; et al. The mitochondrial thioredoxin reductase system (TrxR2) in vascular endothelium controls peroxynitrite levels and tissue integrity. Proc. Natl. Acad. Sci. USA 2021, 118, e1921828118. [Google Scholar] [CrossRef] [PubMed]
- Mccarty, M.F.; Lerner, A. Nutraceuticals Targeting Generation and Oxidant Activity of Peroxynitrite May Aid Prevention and Control of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 3624. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, W.; Fang, Y.; Yang, H.; Tian, L.; Li, K.; Lai, W.; Bian, L.; Lin, B.; Liu, X.; et al. Neurotoxicity of aluminum oxide nanoparticles and their mechanistic role in dopaminergic neuron injury involving p53-related pathways. J. Hazard. Mater. 2020, 392, 122312. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth, M.A.; Samson, P.; Itohan, O.R. Histomorphological evaluations on the frontal cortex extrapyramidal cell layer following administration of N-Acetyl cysteine in aluminum induced neurodegeneration rat model. Metab. Brain Dis. 2020, 35, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, M.; Lu, X.; Sun, Q.; Wang, C.; Zhang, J.; Fan, H. Aluminum trichloride caused hippocampal neural cells death and subsequent depression-like behavior in rats via the activation of IL-1β/JNK signaling pathway. Sci. Total Environ. 2020, 715, 136942. [Google Scholar] [CrossRef] [PubMed]
- Türkez, H.; Arslan, M.E.; Di Stefano, A.; Cacciatore, I.; Mardinoğlu, A. Nonpharmacological treatment options for Alzheimer’s disease: From animal testing to clinical studies. Turk. J. Zool. 2020, 44, 81–89. [Google Scholar] [CrossRef]
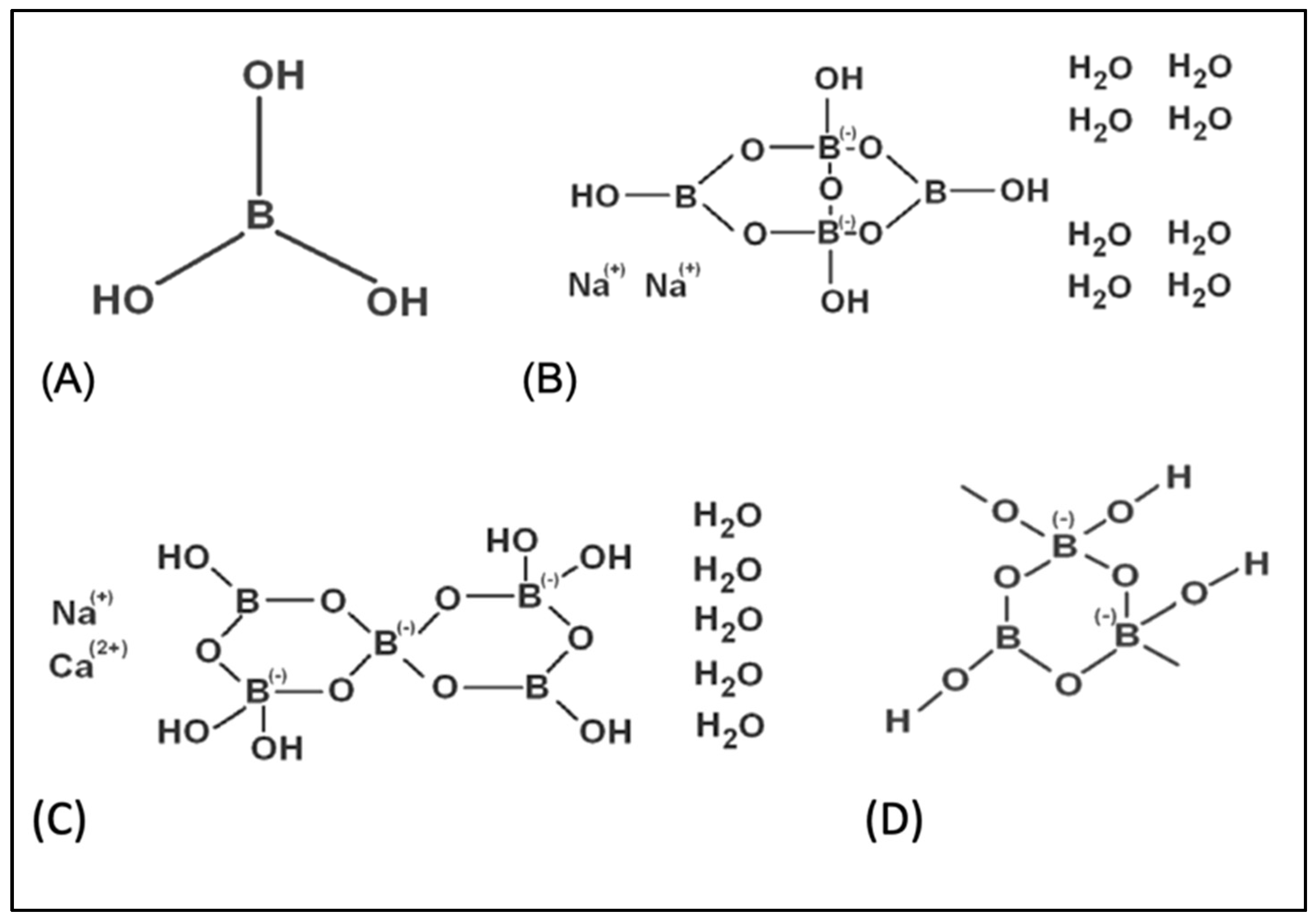

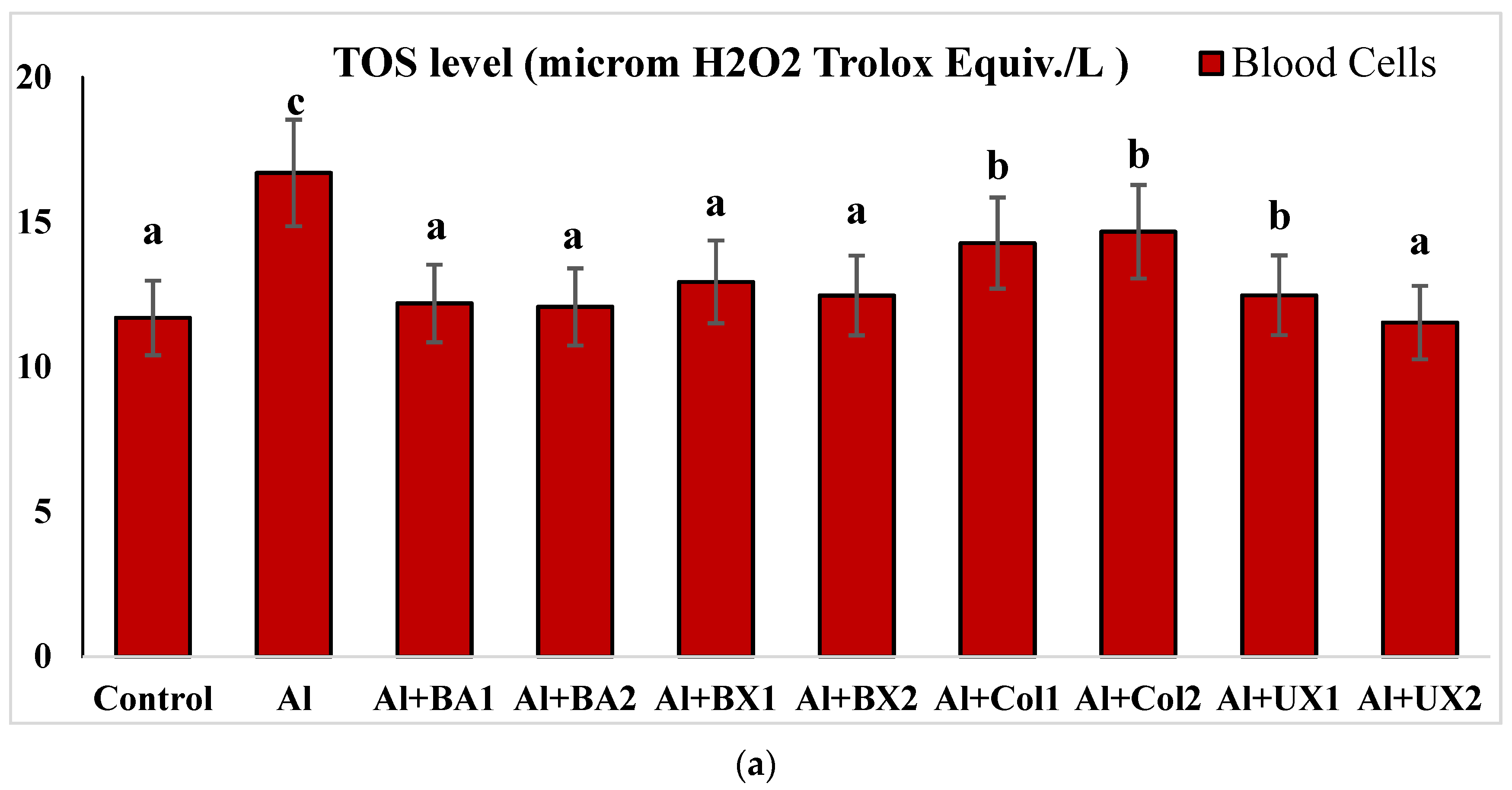
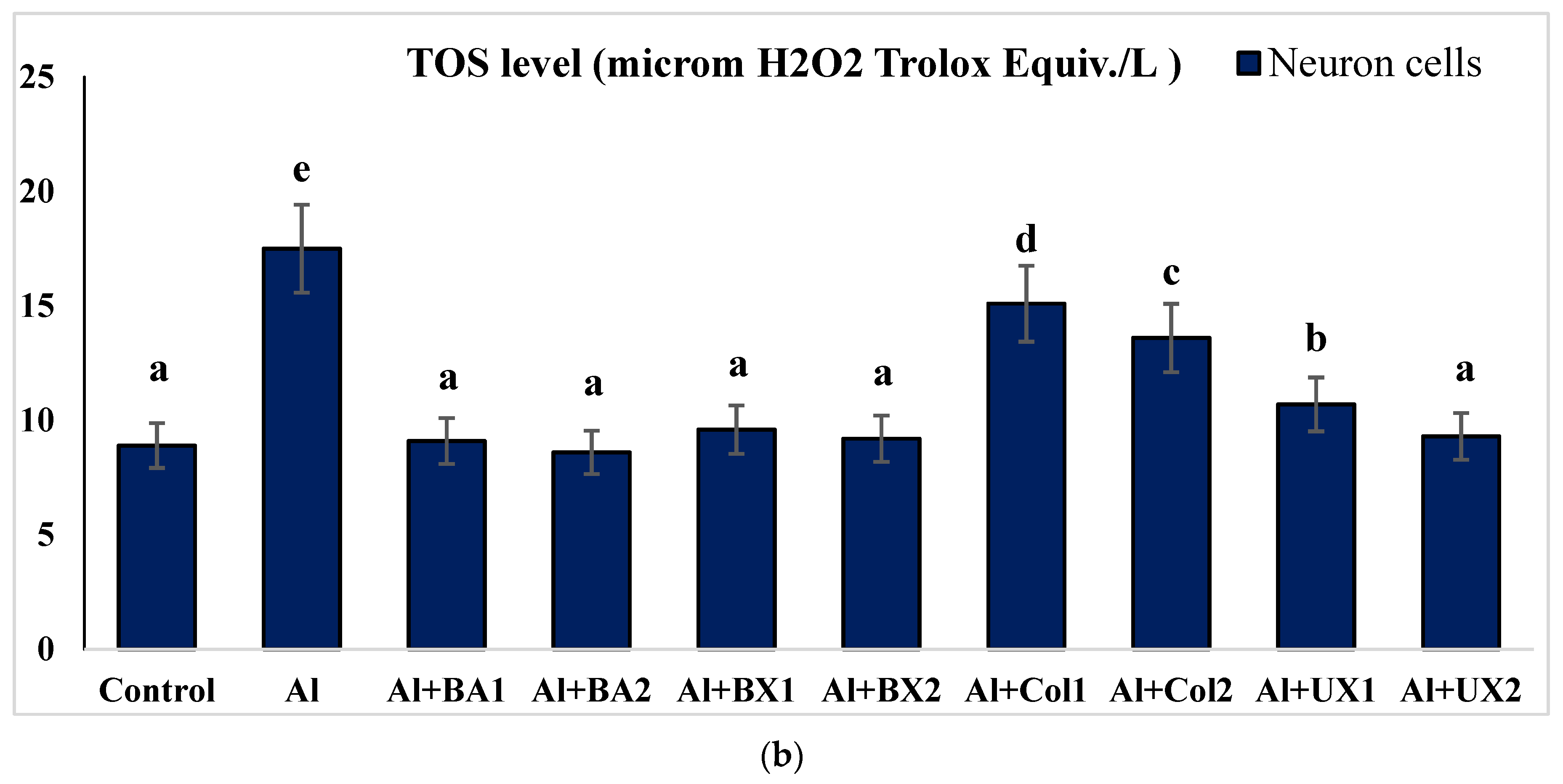
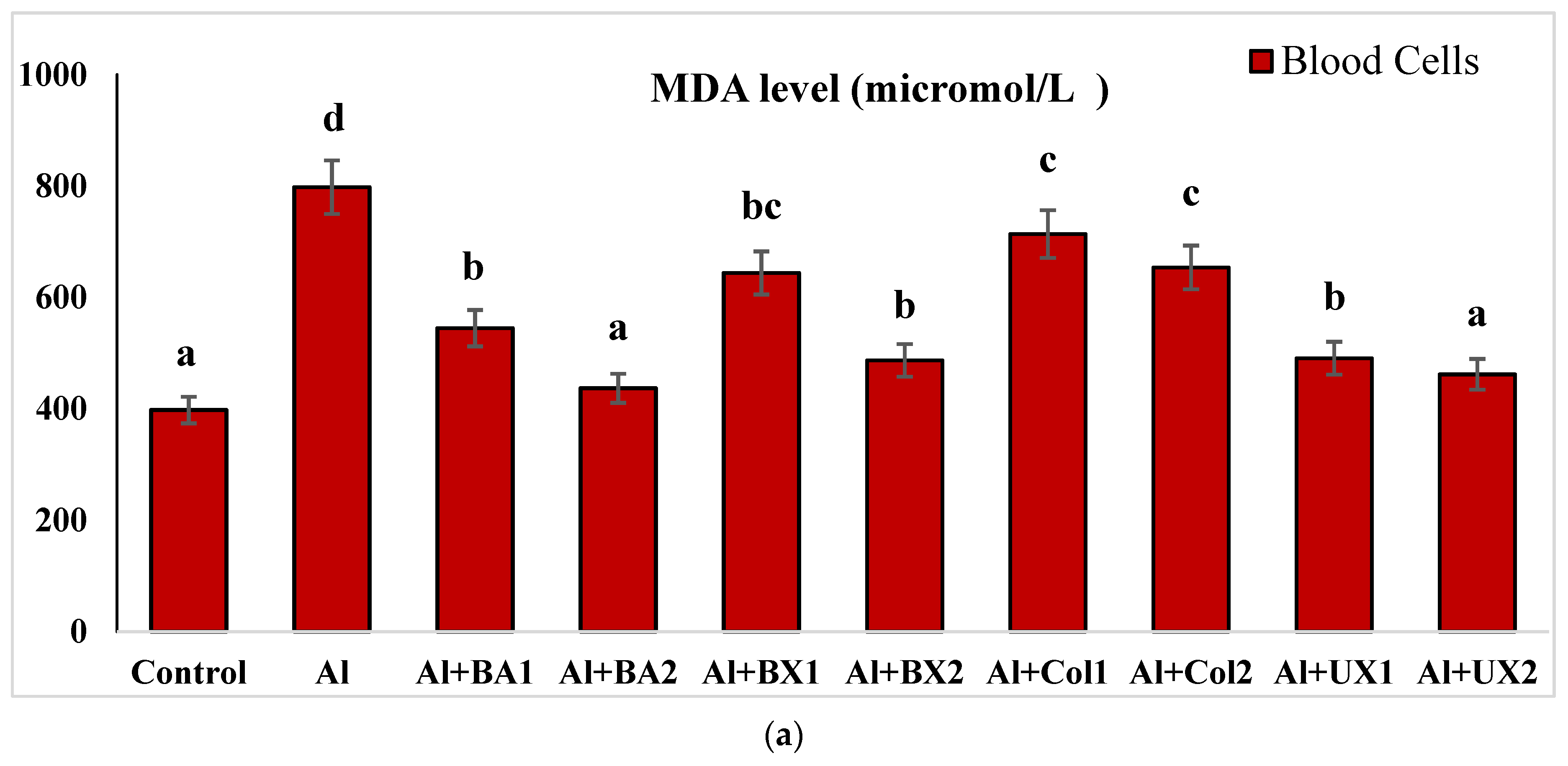
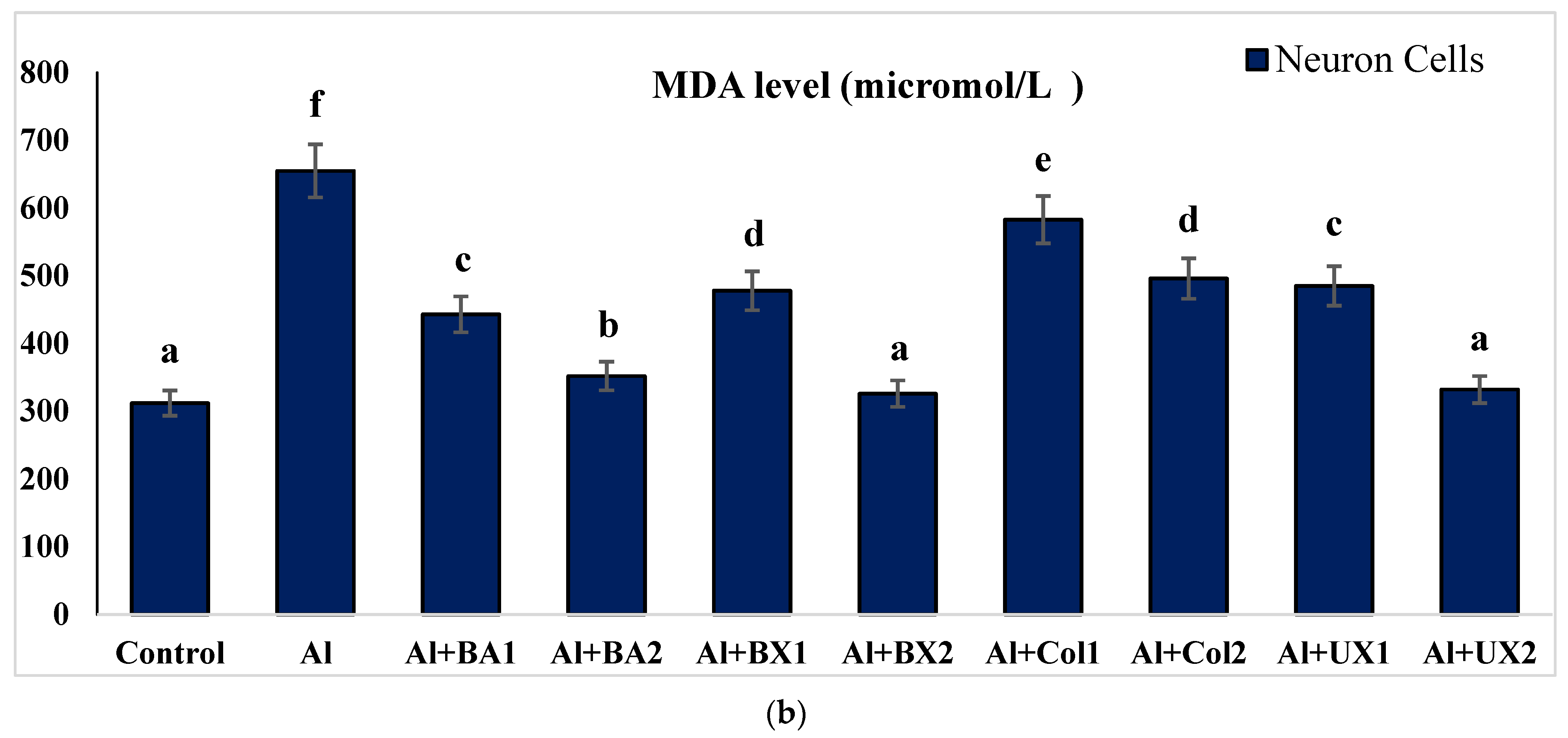




| Treatments (as ppm) | p | |||||||
|---|---|---|---|---|---|---|---|---|
| Blood Cells | PRCNs | |||||||
| BA | BX | Col | UX | BA | BX | Col | UX | |
| 0.01 | 1.00 | 0.97 | 0.98 | 1.00 | 1.00 | 1.00 | 0.97 | 1.00 |
| 0.02 | 1.00 | 0.95 | 0.96 | 1.00 | 1.00 | 1.00 | 0.93 | 1.00 |
| 0.05 | 1.00 | 0.96 | 0.94 | 1.00 | 0.96 | 1.00 | 0.90 | 0.97 |
| 0.1 | 0.97 | 0.95 | 0.96 | 0.98 | 0.93 | 0.96 | 0.86 | 0.90 |
| 0.2 | 0.95 | 0.92 | 0.91 | 0.96 | 0.90 | 0.94 | 0.90 | 0.86 |
| 0.5 | 0.94 | 0.90 | 0.84 | 0.94 | 0.85 | 0.90 | 0.72 | 0.79 |
| 1 | 0.94 | 0.85 | 0.81 | 0.95 | 0.82 | 0.86 | 0.78 | 0.77 |
| 2 | 0.90 | 0.84 | 0.79 | 0.92 | 0.74 | 0.88 | 0.68 | 0.84 |
| 5 | 0.86 | 0.81 | 0.76 | 0.87 | 0.72 | 0.82 | 0.66 | 0.70 |
| 10 | 0.85 | 0.79 | 0.73 | 0.85 | 0.78 | 0.77 | 0.61 | 0.66 |
| 20 | 0.83 | 0.74 | 0.69 | 0.83 | 0.71 | 0.75 | 0.53 | 0.67 |
| AlCl3 | 0.00 * | 0.00 * | ||||||
| Treatments (as ppm) | p | |||||||
|---|---|---|---|---|---|---|---|---|
| Blood Cells | PRCNs | |||||||
| BA | BX | Col | UX | BA | BX | Col | UX | |
| 0.01 | 0.87 | 0.94 | 0.96 | 0.92 | 0.91 | 0.82 | 0.93 | 0.86 |
| 0.02 | 0.79 | 0.86 | 0.91 | 0.83 | 0.88 | 0.87 | 0.90 | 0.71 |
| 0.05 | 0.76 | 0.72 | 0.87 | 0.65 | 0.79 | 0.74 | 0.83 | 0.74 |
| 0.1 | 0.67 | 0.55 | 0.79 | 0.62 | 0.66 | 0.72 | 0.78 | 0.67 |
| 0.2 | 0.59 | 0.49 * | 0.72 | 0.54 | 0.70 | 0.65 | 0.74 | 0.63 |
| 0.5 | 0.04 * | 0.06 * | 0.65 | 0.04 * | 0.56 | 0.53 | 0.60 | 0.56 |
| 1 | 0.04 * | 0.33 * | 0.59 | 0.04 * | 0.51 | 0.02 * | 0.53 | 0.04 * |
| 2 | 0.04 * | 0.21 * | 0.53 | 0.03 * | 0.04 * | 0.02 * | 0.04 * | 0.04 * |
| 5 | 0.03 * | 0.19 * | 0.45 * | 0.02 * | 0.04 * | 0.02 * | 0.04 * | 0.02 * |
| 10 | 0.02 * | 0.02 * | 0.04 * | 0.02 * | 0.03 * | 0.02 * | 0.04 * | 0.03 * |
| 20 | 0.01 * | 0.06 * | 0.03 | 0.01 * | 0.07 * | 0.02 * | 0.54 | 0.03 * |
| Control | 0.00 * | 0.00 * | ||||||
| Groups | MN/1000 Cell | CA/Cell | Total Damage Score | NDI (%) |
|---|---|---|---|---|
| Control | 3.82 ± 0.26 | 0.18 ± 0.02 | 32.25 ± 4.76 | 1.22 ± 0.05 |
| Al (20 mg/L) | 14.21 ± 1.55 * | 1.14 ± 0.09 * | 167.75 ± 14.65 * | 1.09 ± 0.03 * |
| BA1 (5 mg/L) | 2.72 ± 0.14 | 0.18 ± 0.02 | 34.00 ± 3.65 | 1.20 ± 0.02 |
| BA2 (10 mg/L) | 2.85 ± 0.26 | 0.16 ± 0.01 | 30.75 ± 3.35 | 1.18 ± 0.03 |
| BX1 (5 mg/L) | 3.76 ± 0.44 | 0.20 ± 0.03 | 33.25 ± 3.48 | 1.24 ± 0.04 |
| BX2 10 mg/L) | 3.68 ± 0.29 | 0.22 ± 0.02 | 34.50 ± 2.65 | 1.22 ± 0.03 |
| Col1 (5 mg/L) | 3.91 ± 0.33 | 0.18 ± 0.02 | 34.75 ± 4.15 | 1.20 ± 0.03 |
| Col2 10 mg/L) | 4.01 ± 0.37 | 0.21 ± 0.03 | 36.50 ± 3.70 | 1.19 ± 0.02 |
| UX1 (5 mg/L) | 3.94 ± 0.41 | 0.17 ± 0.02 | 30.25 ± 3.45 | 1.24 ± 0.03 |
| UX2 10 mg/L) | 3.56 ± 0.34 | 0.18 ± 0.03 | 35.50 ± 4.20 | 1.21 ± 0.03 |
| BA1 + Al | 6.42 ± 0.48 * | 0.44 ± 0.02 * | 79.50 ± 6.80 * | 1.15 ± 0.02 |
| BA2 + Al | 5.74 ± 0.45 * | 0.48 ± 0.03 * | 71.25 ± 6.22 * | 1.17 ± 0.02 |
| BX1 + Al | 5.90 ± 0.32 * | 0.64 ± 0.04 * | 89.00 ± 9.38 * | 1.13 ± 0.03 * |
| BX2 + Al | 6.23 ± 0.38 * | 0.57 ± 0.03 * | 87.75 ± 9.04 * | 1.16 ± 0.02 |
| Col1 + Al | 8.91 ± 0.61 * | 0.81 ± 0.05 * | 149.25 ± 12.66 * | 1.12 ± 0.02 * |
| Col2 + Al | 8.44 ± 0.55 * | 0.75 ± 0.06 * | 135.00 ± 15.42 * | 1.12 ± 0.03 * |
| UX1 + Al | 5.50 ± 0.35 * | 0.57 ± 0.04 * | 68.50 ± 7.20 * | 1.16 ± 0.03 |
| UX2 + Al | 5.73 ± 0.46 * | 0.47 ± 0.04 * | 44.25 ± 4.60 * | 1.18 ± 0.03 |
| Groups | MNPKE/1000 PKE | CA/Cell | Total Damage Score |
|---|---|---|---|
| Control | 13.40 ± 1.18 | 0.66 ± 0.08 | 19.35 ± 2.44 |
| Al (20 mg/L) | 27.78 ± 1.86 * | 4.58 ± 0.16 * | 66.35 ± 7.98 * |
| BA1 (5 mg/L) | 12.65 ± 0.96 | 0.54 ± 0.12 | 21.40 ± 1.74 |
| BA2 (10 mg/L) | 12.89 ± 1.32 | 0.52 ± 0.16 | 20.34 ± 2.55 |
| BX1 (5 mg/L) | 12.20 ± 0.89 | 0.58 ± 0.11 | 19.33 ± 1.80 |
| BX2 10 mg/L) | 12.93 ± 1.16 | 0.51 ± 0.18 | 20.08 ± 2.14 |
| Col1 (5 mg/L) | 12.84 ± 1.09 | 0.62 ± 0.21 | 20.80 ± 1.92 |
| Col2 10 mg/L) | 13.15 ± 1.33 | 0.65 ± 0.19 | 21.66 ± 2.15 |
| UX1 (5 mg/L) | 12.74 ± 0.92 | 0.54 ± 0.13 | 21.85 ± 1.92 |
| UX2 10 m/L) | 12.96 ± 0.78 | 0.61 ± 0.22 | 20.66 ± 2.38 |
| BA1 + Al | 17.04 ± 1.53 * | 2.70 ± 0.28 * | 30.72 ± 2.78 * |
| BA2 + Al | 18.45 ± 1.22 * | 3.16 ± 0.24 * | 37.85 ± 3.25 * |
| BX1 + Al | 16.70 ± 1.42 * | 2.40 ± 0.19 * | 27.20 ± 2.82 * |
| BX2 + Al | 17.35 ± 1.30 * | 2.67 ± 0.16 * | 34.41 ± 3.16 * |
| Col1 + Al | 19.86 ± 1.50 * | 2.86 ± 0.22 * | 42.36 ± 3.40 * |
| Col2 + Al | 23.75 ± 1.72 * | 3.68 ± 0.25 * | 45.08 ± 4.13 * |
| UX1 + Al | 17.92 ± 1.05 * | 3.38 ± 0.26 * | 34.23 ± 3.15 * |
| UX2 + Al | 21.55 ± 1.77 * | 4.23 ± 0.24 * | 42.76 ± 4.07 * |
| Degeneration in Neurons | Necrosis in Neurons | Hyperemia in Vessels | |
|---|---|---|---|
| Control | - | - | - |
| Al (4.2 mg/kg) | ++++ | ++++ | ++++ |
| BA (3.25 mg/kg) | - | - | - |
| BA (6.50 mg/kg) | - | - | - |
| BX (3.25 mg/kg) | - | - | - |
| BX (6.50 mg/kg) | - | - | - |
| Col (3.25 mg/kg) | - | - | - |
| Col (6.50 mg/kg) | - | - | - |
| UX (3.25 mg/kg) | - | - | - |
| UX (6.50 mg/kg) | - | - | - |
| BA (3.25 mg/kg) + Al (4.2 mg/kg) | ++ | ++ | ++ |
| BA (6.50 mg/kg) + Al (4.2 mg/kg) | +++ | +++ | +++ |
| BX (3.25 mg/kg) + Al (4.2 mg/kg) | + | - | + |
| BX (6.50 mg/kg) + Al (4.2 mg/kg) | ++ | ++ | +++ |
| Col (3.25 mg/kg) + Al (4.2 mg/kg) | ++ | ++ | ++ |
| Col (6.50 mg/kg) + Al (4.2 mg/kg) | +++ | +++ | +++ |
| UX (3.25 mg/kg) + Al (4.2 mg/kg) | + | ++ | ++ |
| UX (6.50 mg/kg + Al (4.2 mg/kg) | +++ | +++ | +++ |
| Degeneration in Neurons | Necrosis in Neurons | Hyperemia in Vessels | |
|---|---|---|---|
| Control | - | - | - |
| Al (4.2 mg/kg) | +++++ | +++++ | +++++ |
| BA (3.25 mg/kg) | - | - | - |
| BA (6.50 mg/kg) | - | - | - |
| BX (3.25 mg/kg) | - | - | - |
| BX (6.50 mg/kg) | - | - | - |
| Col (3.25 mg/kg) | - | - | - |
| Col (6.50 mg/kg) | - | - | - |
| UX (3.25 mg/kg) | - | - | - |
| UX (6.50 mg/kg) | - | - | - |
| BA (3.25 mg/kg) + Al (4.2 mg/kg) | ++ | ++ | +++ |
| BA (6.50 mg/kg) + Al (4.2 mg/kg) | ++++ | ++++ | ++++ |
| BX (3.25 mg/kg) + Al (4.2 mg/kg) | ++ | + | ++ |
| BX (6.50 mg/kg) + Al (4.2 mg/kg) | ++++ | ++++ | +++ |
| Col (3.25 mg/kg) + Al (4.2 mg/kg) | +++ | +++ | +++ |
| Col (6.50 mg/kg) + Al (4.2 mg/kg) | ++++ | ++++ | ++++ |
| UX (3.25 mg/kg) + Al (4.2 mg/kg) | +++ | ++ | +++ |
| UX (6.50 mg/kg + Al (4.2 mg/kg) | +++ | ++++ | ++++ |
| Vacuolar Degeneration | Nuclear Pyknosis | Mitochondrial Damage | Number of Secondary Lysosomes | |
|---|---|---|---|---|
| Control | - | - | - | - |
| Al (4.2 mg/kg) | +++++ | +++++ | +++++ | +++++ |
| BA (3.25 mg/kg) | - | - | - | - |
| BA (6.50 mg/kg) | - | - | - | - |
| BX (3.25 mg/kg) | - | - | - | - |
| BX (6.50 mg/kg) | - | - | - | - |
| Col (3.25 mg/kg) | - | - | - | - |
| Col (6.50 mg/kg) | - | - | - | - |
| UX (3.25 mg/kg) | - | - | - | - |
| UX (6.50 mg/kg) | - | - | - | - |
| BA (3.25 mg/kg) + Al (4.2 mg/kg) | ++ | ++ | +++ | +++ |
| BA (6.50 mg/kg) + Al (4.2 mg/kg) | ++++ | ++++ | ++++ | ++++ |
| BX (3.25 mg/kg) + Al (4.2 mg/kg) | ++ | + | ++ | ++ |
| BX (6.50 mg/kg) + Al (4.2 mg/kg) | ++++ | ++++ | ++++ | ++++ |
| Col (3.25 mg/kg) + Al (4.2 mg/kg) | +++ | +++ | +++ | +++ |
| Col (6.50 mg/kg) + Al (4.2 mg/kg) | ++++ | ++++ | ++++ | ++++ |
| UX (3.25 mg/kg) + Al (4.2 mg/kg) | +++ | +++ | +++ | +++ |
| UX (6.50 mg/kg + Al (4.2 mg/kg) | ++++ | ++++ | ++++ | ++++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turkez, H.; Yıldırım, S.; Sahin, E.; Arslan, M.E.; Emsen, B.; Tozlu, O.O.; Alak, G.; Ucar, A.; Tatar, A.; Hacimuftuoglu, A.; et al. Boron Compounds Exhibit Protective Effects against Aluminum-Induced Neurotoxicity and Genotoxicity: In Vitro and In Vivo Study. Toxics 2022, 10, 428. https://doi.org/10.3390/toxics10080428
Turkez H, Yıldırım S, Sahin E, Arslan ME, Emsen B, Tozlu OO, Alak G, Ucar A, Tatar A, Hacimuftuoglu A, et al. Boron Compounds Exhibit Protective Effects against Aluminum-Induced Neurotoxicity and Genotoxicity: In Vitro and In Vivo Study. Toxics. 2022; 10(8):428. https://doi.org/10.3390/toxics10080428
Chicago/Turabian StyleTurkez, Hasan, Serkan Yıldırım, Elvan Sahin, Mehmet Enes Arslan, Bugrahan Emsen, Ozlem Ozdemir Tozlu, Gonca Alak, Arzu Ucar, Abdulgani Tatar, Ahmet Hacimuftuoglu, and et al. 2022. "Boron Compounds Exhibit Protective Effects against Aluminum-Induced Neurotoxicity and Genotoxicity: In Vitro and In Vivo Study" Toxics 10, no. 8: 428. https://doi.org/10.3390/toxics10080428
APA StyleTurkez, H., Yıldırım, S., Sahin, E., Arslan, M. E., Emsen, B., Tozlu, O. O., Alak, G., Ucar, A., Tatar, A., Hacimuftuoglu, A., Keles, M. S., Geyikoglu, F., Atamanalp, M., Saruhan, F., & Mardinoglu, A. (2022). Boron Compounds Exhibit Protective Effects against Aluminum-Induced Neurotoxicity and Genotoxicity: In Vitro and In Vivo Study. Toxics, 10(8), 428. https://doi.org/10.3390/toxics10080428









