Effect of Hypoxic Stress and Levels of Mn on the Physiology and Biochemistry of Phyllostachys praecox
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Hydroponics Experiment
2.3. Determination of Leaf Chlorophyll Contents (SPAD)
2.4. Determination of Plant Root Morphology
2.5. Determination of Plant Root Vitality
2.6. Determination of Concentrations of Lipid Peroxidation
2.7. Assay of Antioxidant Enzymes
2.8. Determination of Proline Concentration
2.9. Mineral Element Analysis
2.10. Determination of Anaerobic Respiratory Enzyme Activity
2.11. Statistical Analysis
3. Results
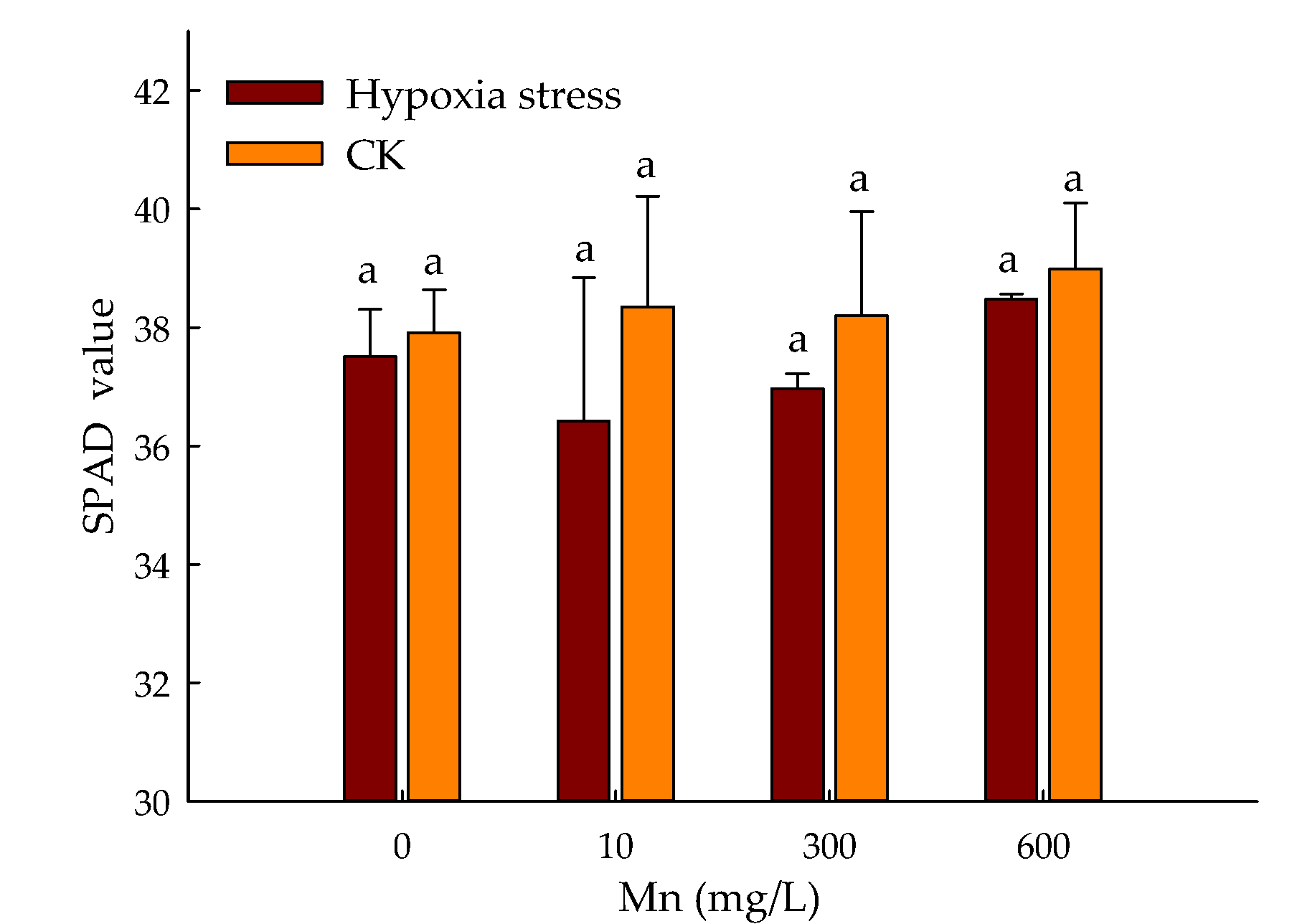
3.1. Effect of Hypoxic Stress and Mn on SPAD Values of P. praecox in Hydroponics Environment
3.2. Effect of Hypoxia Stress and Level of Mn on Root Morphology of P. praecox in Hydroponics
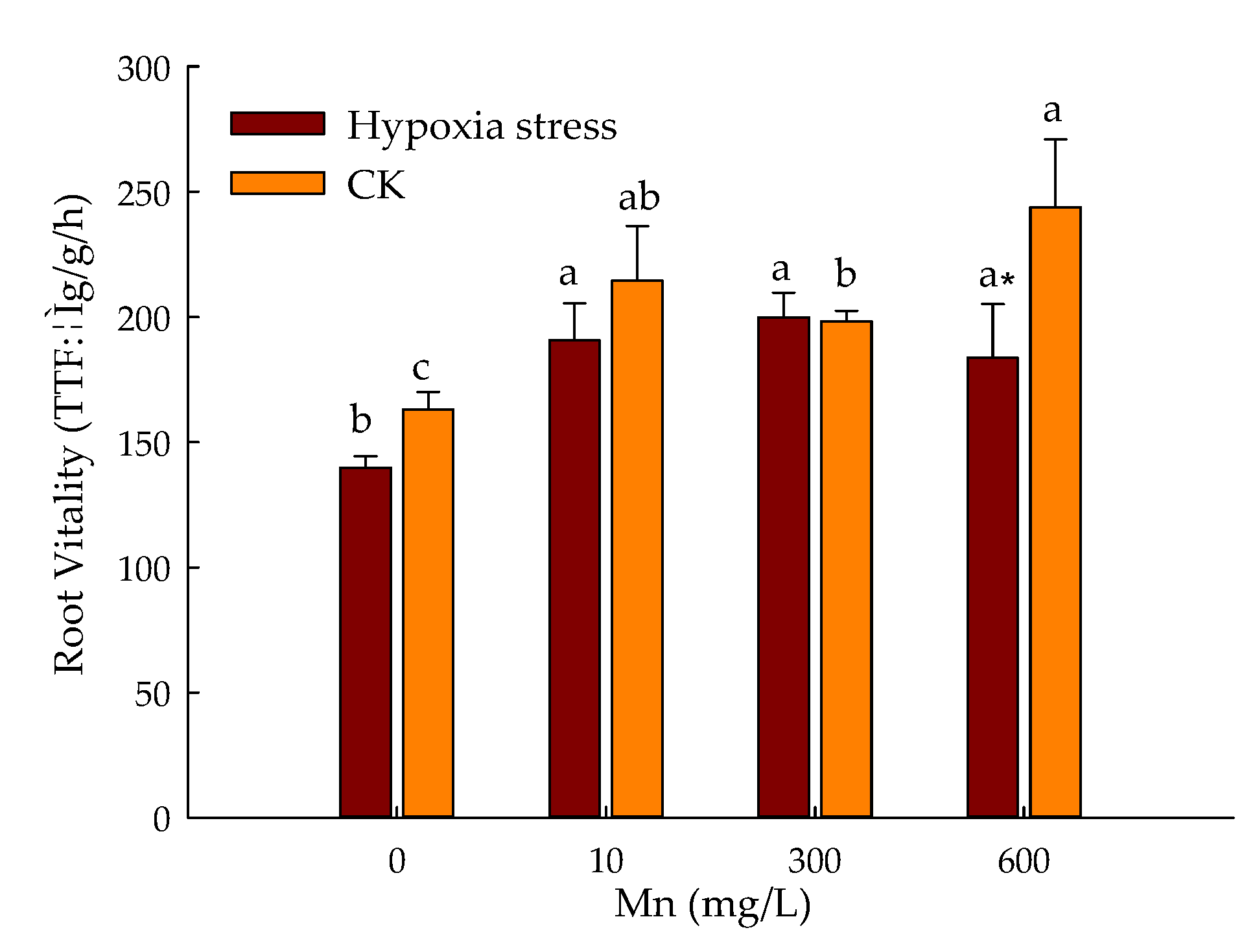
3.3. Effect of Hypoxic Stress and Level of Mn on Root Vitality of P. praecox in Hydroponics
3.4. Effect of Hypoxic Stress and Levels of Mn on Antioxidation System of P. praecox in Hydroponics Environment
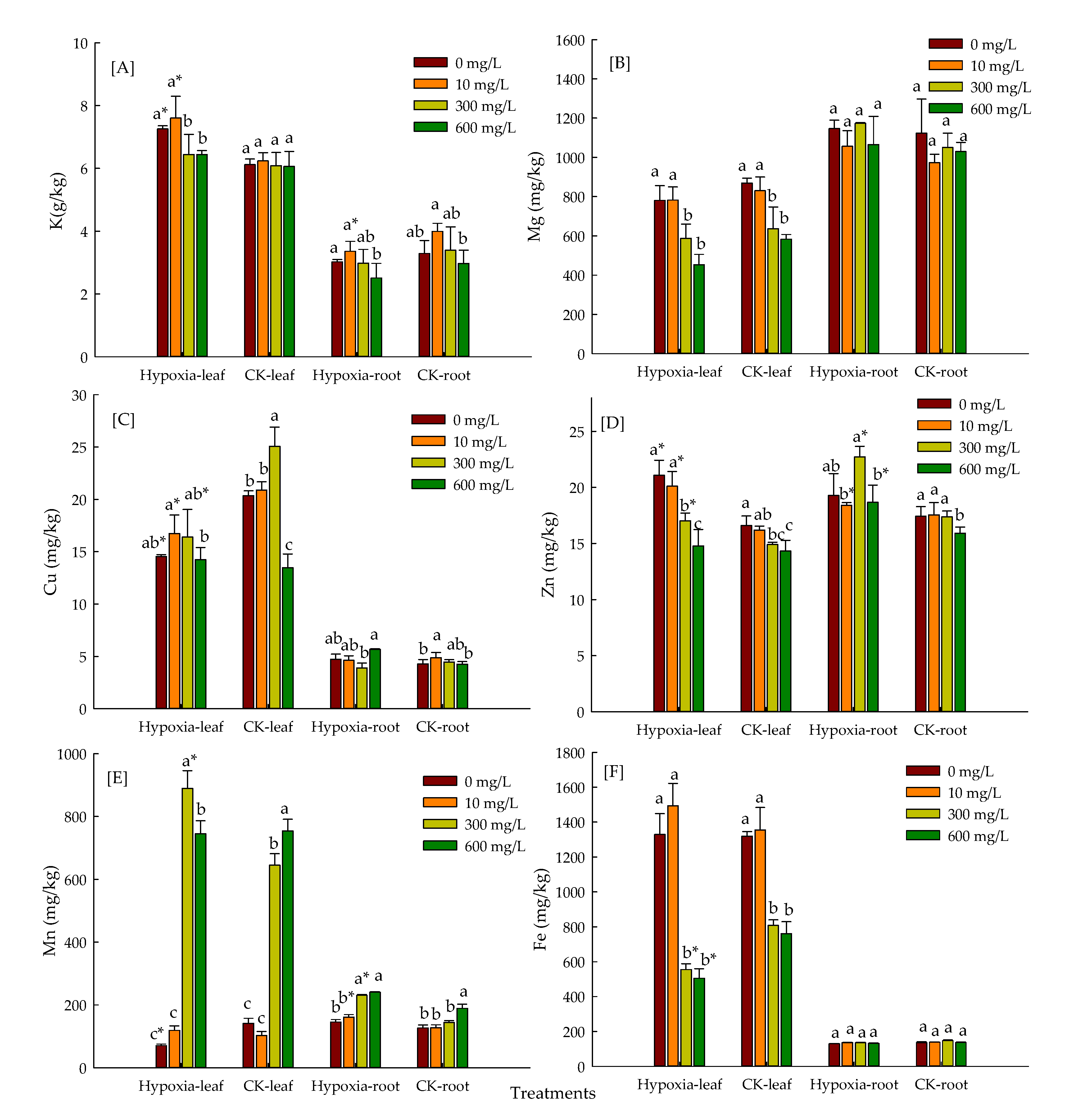
3.5. Effect of Hypoxic Stress and Levels of Mn on Mineral Element Absorption of P. praecox in Hydroponics Environment
3.6. Effect of Hypoxic Stress and Levels of Mn on Anaerobic Respiratory Enzyme Activity of P. praecox in Hydroponics Environment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TCA | Trichloroacetic acid |
| TBA | Thiobarbituric acid |
| MDA | Malonaldehyde |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| PDC | Pyruvate decarboxylase |
| LDH | Lactic dehydrogenase |
| ADH | Alcohol dehydrogenase |
References
- Yamauchi, T.; Colmer, T.D.; Pedersen, O.; Nakazono, M. Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiol. 2018, 176, 1118–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Niu, W.; Cao, X.; Wang, J.; Zhang, M.; Duan, X.; Zhang, Z. Effect of soil aeration on root morphology and photosynthetic characteristics of potted tomato plants (Solanum lycopersicum) at different nacl salinity levels. BMC Plant Biol. 2019, 19, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Noah, I.; Friedman, S. Aeration of clayey soils by injecting air through subsurface drippers: Lysimetric and field experiments. Agric. Water Manag. 2016, 176, 222–233. [Google Scholar] [CrossRef]
- Li, Y.; Niu, W.; Wang, J.; Liu, L.; Zhang, M.; Xu, J. Effects of artificial soil aeration volume and frequency on soil enzyme activity and microbial abundance when cultivating greenhouse tomato. Soil Sci. Soc. Am. J. 2016, 80, 1208–1221. [Google Scholar] [CrossRef]
- Mehmood, S.; Ahmed, W.; Alatalo, J.M.; Mahmood, M.; Imtiaz, M.; Ditta, A.; Ali, E.F.; Abdelrahman, H.; Slaný, M.; Antoniadis, V. Herbal plants-and rice straw-derived biochars reduced metal mobilization in fishpond sediments and improved their potential as fertilizers. Sci. Total Environ. 2022, 826, 154043. [Google Scholar] [CrossRef] [PubMed]
- Melo, T.M.; Schauerte, M.; Bluhm, A.; Slaný, M.; Paller, M.; Bolan, N.; Bosch, J.; Fritzsche, A.; Rinklebe, J. Ecotoxicological effects of per-and polyfluoroalkyl substances (PFAS) and of a new PFAS adsorbing organoclay to immobilize PFAS in soils on earthworms and plants. J. Hazard. Mater. 2022, 433, 128771. [Google Scholar] [CrossRef] [PubMed]
- Licausi, F.; Pucciariello, C.; Perata, P. New role for an old rule: N-end rule-mediated degradation of ethylene responsive factor proteins governs low oxygen response in plants. J. Integr. Plant Biol. 2013, 55, 31–39. [Google Scholar] [CrossRef]
- Pan, R.; Jiang, W.; Wang, Q.; Xu, L.; Shabala, S.; Zhang, W. Differential response of growth and photosynthesis in diverse cotton genotypes under hypoxia stress. Photosynthetica 2019, 57, 772–779. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Zhuang, S.; Zhang, Y.; Qian, Z. Exogenously applied spermidine alleviates hypoxia stress in Phyllostachys praecox seedlings via changes in endogenous hormones and gene expression. BMC Plant Biol. 2022, 22, 200. [Google Scholar] [CrossRef]
- Weits, D.A.; Kunkowska, A.B.; Kamps, N.C.; Portz, K.M.; Packbier, N.K.; Venza, Z.N.; Gaillochet, C.; Lohmann, J.U.; Pedersen, O.; Van Dongen, J.T. An apical hypoxic niche sets the pace of shoot meristem activity. Nature 2019, 569, 714. [Google Scholar] [CrossRef]
- Shukla, V.; Lombardi, L.; Iacopino, S.; Pencik, A.; Novak, O.; Perata, P.; Giuntoli, B.; Licausi, F. Endogenous hypoxia in lateral root primordia controls root architecture by antagonizing auxin signaling in arabidopsis. Mol. Plant 2019, 12, 538–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millaleo, R.; Reyes-Díaz, M.; Ivanov, A.; Mora, M.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Chen, S.; Mi, Y.; Zhou, Z.; Ahammed, G.J. Effects of hypoxia stress and different level of Mn2+ on antioxidant enzyme of tomato seedlings. Am. J. Plant Sci. 2010, 1, 24. [Google Scholar] [CrossRef]
- Ducic, T.; Polle, A. Transport and detoxification of manganese and copper in plants. Braz. J. Plant Physiol. 2005, 17, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Horst, W.J.; Fecht, M.; Naumann, A.; Wissemeier, A.H.; Maier, P. Physiology of manganese toxicity and tolerance in Vigna unguiculata (L.) walp. J. Plant Nutr. Soil Sci. 1999, 162, 263–274. [Google Scholar] [CrossRef]
- Zhou, G.; Zhuang, S.; Jiang, P.; Xu, Q.; Qin, H.; Wong, M.; Cao, Z. Soil organic carbon accumulation in intensively managed Phyllostachys praecox stands. Bot. Rev. 2011, 77, 296–303. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, P.; Chang, S.X.; Wu, J.; Lin, L. Organic mulch and fertilization affect soil carbon pools and forms under intensively managed bamboo (Phyllostachys praecox) forests in southeast china. J. Soils Sediments 2010, 10, 739–747. [Google Scholar] [CrossRef]
- Liu, C.; Liang, M.; Nie, Y.; Tang, J.; Siddique, K.H. The conversion of tropical forests to rubber plantations accelerates soil acidification and changes the distribution of soil metal ions in topsoil layers. Sci. Total Environ. 2019, 696, 134082. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, P.; Li, Y.; Wu, J.; Xu, K.; Hill, S.; Wang, H. Chemistry of decomposing mulching materials and the effect on soil carbon dynamics under a Phyllostachys praecox bamboo stand. J. Soils Sediments 2013, 13, 24–33. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; Jiang, C.; Wu, M.; Song, R.; Gui, R.; Jia, J.; Li, Z. Improved nutrient status affects soil microbial biomass, respiration, and functional diversity in a lei bamboo plantation under intensive management. J. Soils Sediments 2017, 17, 917–926. [Google Scholar] [CrossRef]
- Xu, M.; Zhuang, S.; Gui, R. Soil hypoxia induced by an organic-material mulching technique stimulates the bamboo rhizome up-floating of phyllostachys praecox. Sci. Rep. 2017, 7, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagola, M.; Ortiz, R.; Irigoyen, I.; Bustince, H.; Barrenechea, E.; Aparicio-Tejo, P.; Lamsfus, C.; Lasa, B. New method to assess barley nitrogen nutrition status based on image colour analysis: Comparison with spad-502. Comput. Electron. Agric. 2009, 65, 213–218. [Google Scholar] [CrossRef]
- Liu, D.; Li, S.; Islam, E.; Chen, J.-R.; Wu, J.-S.; Ye, Z.-Q.; Peng, D.-L.; Yan, W.-B.; Lu, K.-P. Lead accumulation and tolerance of moso bamboo (phyllostachys pubescens) seedlings: Applications of phytoremediation. J. Zhejiang Univ. Sci. B 2015, 16, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, F.; Wang, S.; Xin, H. Toxicity of cuo nanoparticles to structure and metabolic activity of allium cepa root tips. Bull. Environ. Contam. Toxicol. 2016, 97, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shafi, M.; Li, S.; Wang, Y.; Wu, J.; Ye, Z.; Peng, D.; Yan, W.; Liu, D. Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of moso bamboo (phyllostachys pubescens). Sci. Rep. 2015, 5, 13554. [Google Scholar] [CrossRef] [Green Version]
- Peng, D.; Shafi, M.; Wang, Y.; Li, S.; Yan, W.; Chen, J.; Ye, Z.; Liu, D. Effect of Zn stresses on physiology, growth, Zn accumulation, and chlorophyll of Phyllostachys pubescens. Environ. Sci. Pollut. Res. 2015, 22, 14983–14992. [Google Scholar] [CrossRef]
- Zhong, B.; Chen, J.; Shafi, M.; Guo, J.; Wang, Y.; Wu, J.; Ye, Z.; He, L.; Liu, D. Effect of lead (pb) on antioxidation system and accumulation ability of moso bamboo (phyllostachys pubescens). Ecotoxicol. Environ. Saf. 2017, 138, 71–77. [Google Scholar] [CrossRef]
- Meng, Q.; Zou, J.; Zou, J.; Jiang, W.; Liu, D. Effect of Cu2+ concentration on growth, antioxidant enzyme activity and malondialdehyde content in garlic (Allium sativum L.). Acta Biol. Cracov Bot. 2007, 49, 95–101. [Google Scholar]
- Li, S.; Chen, J.; Islam, E.; Wang, Y.; Wu, J.; Ye, Z.; Yan, W.; Peng, D.; Liu, D. Cadmium-induced oxidative stress, response of antioxidants and detection of intracellular cadmium in organs of moso bamboo (phyllostachys pubescens) seedlings. Chemosphere 2016, 153, 107–114. [Google Scholar] [CrossRef]
- Mustroph, A.; Albrecht, G. Tolerance of crop plants to oxygen deficiency stress: Fermentative activity and photosynthetic capacity of entire seedlings under hypoxia and anoxia. Physiol. Plant. 2003, 117, 508–520. [Google Scholar] [CrossRef]
- WatersI, M.S.; Greenway, H.; Colmer, T. Effects of anoxia on wheat seedlings. Ii. Influence of o 2 supply prior to anoxia on tolerance to anoxia, alcoholic fermentation, and sugar levels. J. Exp. Bot. 1991, 42, 1437–1447. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yuan, Y.; Shu, S.; Li, S.; He, L.; Li, H.; Du, N.; Sun, J.; Guo, S. Effects of exogenous putrescine on chlorophyll fluorescence imaging and heat dissipation capacity in cucumber (Cucumis sativus L.) under salt stress. J. Plant Growth Regul. 2014, 33, 798–808. [Google Scholar] [CrossRef]
- He, L.; Yu, L.; Li, B.; Du, N.; Guo, S. The effect of exogenous calcium on cucumber fruit quality, photosynthesis, chlorophyll fluorescence, and fast chlorophyll fluorescence during the fruiting period under hypoxic stress. BMC Plant Biol. 2018, 18, 180. [Google Scholar] [CrossRef]
- Lin, K.; Huang, M.; Weng, J.; Kao, S. Photosynthetic activity of red and green leaf sectors in coleus blumei plants as sensed by chlorophyll fluorescence. Photosynthetica 2019, 57, 659–667. [Google Scholar] [CrossRef] [Green Version]
- Shenker, M.; Plessner, O.E.; Tel-Or, E. Manganese nutrition effects on tomato growth, chlorophyll concentration, and superoxide dismutase activity. J. Plant Physiol. 2004, 161, 202. [Google Scholar] [CrossRef]
- Christensen, P.B.; Revsbech, N.P.; Sand-Jensen, K. Microsensor analysis of oxygen in the rhizosphere of the aquatic macrophyte Littorella uniflora (L.) ascherson. Plant Physiol. 1994, 105, 847–852. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Xu, C.; Zhang, W.; Zhang, X.; Li, F.; Chen, J.; Wang, D. Effects of rhizosphere dissolved oxygen content and nitrogen form on root traits and nitrogen accumulation in rice. Rice Sci. 2011, 18, 304–310. [Google Scholar]
- Pradhan, S.; Varade, S.; Kar, S. Influence of soil water conditions on growth and root porosity of rice. Plant Soil 1973, 38, 501–507. [Google Scholar] [CrossRef]
- Kläring, H.-P.; Zude, M. Sensing of tomato plant response to hypoxia in the root environment. Sci. Hortic. 2009, 122, 17–25. [Google Scholar] [CrossRef]
- Chen, X.; Kou, M.; Tang, Z.; Zhang, A.; Li, H.; Wei, M. Responses of root physiological characteristics and yield of sweet potato to humic acid urea fertilizer. PLoS ONE 2017, 12, e0189715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, W.; Drew, M.C. Root growth and metabolism under oxygen deficiency. In Plant Roots; CRC Press: Boca Raton, FL, USA, 2002; pp. 1139–1187. [Google Scholar]
- Li, J.; Chen, S.; Liu, A.; Wang, Z.; Liu, D.; Wang, F.; Ahammed, G. Combined effects of hypoxia and excess Mn2+ on oxidative stress and antioxidant enzymes in tomato seedlings. Russ. J. Plant Physiol. 2012, 59, 670–678. [Google Scholar] [CrossRef]
- Hu, Z.; Xie, Y.; Jin, G.; Fu, J.; Li, H. Growth responses of two tall fescue cultivars to pb stress and their metal accumulation characteristics. Ecotoxicology 2015, 24, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Bauddh, K.; Singh, R.P. Growth, tolerance efficiency and phytoremediation potential of Ricinus communis (L.) and Brassica juncea (L.) in salinity and drought affected cadmium contaminated soil. Ecotoxicol. Environ. Saf. 2012, 85, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, P.H.; Rafique, S.; Singh, N. Response of maize (Zea mays l.) genotypes to excess soil moisture stress: Morpho-physiological effects and basis of tolerance. Eur. J. Agron. 2003, 19, 383–399. [Google Scholar] [CrossRef]
- Pittman, J.K. Managing the manganese: Molecular mechanisms of manganese transport and homeostasis. New Phytol. 2005, 167, 733–742. [Google Scholar] [CrossRef]
- Sieprawska, A.; Filek, M.; Tobiasz, A.; Walas, S.; Dudek-Adamska, D.; Grygo-Szymanko, E. Trace elements’ uptake and antioxidant response to excess of manganese in in vitro cells of sensitive and tolerant wheat. Acta Physiol. Plant. 2016, 38, 55. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Liu, D.; Liu, G.; Liu, H.; Kurtenbach, R. Relationship between polyamines and anaerobic respiration of wheat seedling root under water-logging stress. Russ. J. Plant Physiol. 2018, 65, 874–881. [Google Scholar] [CrossRef]
- Chen, H.; Qualls, R.G. Anaerobic metabolism in the roots of seedlings of the invasive exotic lepidium latifolium. Environ. Exp. Bot. 2003, 50, 29–40. [Google Scholar] [CrossRef]
- Zhou, C.; Bai, T.; Wang, Y.; Wu, T.; Zhang, X.; Xu, X.; Han, Z. Morpholoical and enzymatic responses to waterlogging in three prunus species. Sci. Hortic. 2017, 221, 62–67. [Google Scholar] [CrossRef]


| Mn (μmol/L) | Length (cm) | Surf Area (cm2) | Avg Diam (mm) | Root Volume (cm3) | Root Tips | |
|---|---|---|---|---|---|---|
| Hypoxia | 0 | 37.76 ± 1.92 ab | 8.74 ± 0.39 b | 0.75 ± 0.01 b | 0.21 ± 0.08 a | 99.00 ± 39.60 a |
| 10 | 34.51 ± 1.49 b | 9.15 ± 0.11 a | 0.80 ± 0.11 a | 0.23 ± 0.04 a | 67.50 ± 20.51 b | |
| 300 | 38.19 ± 5.24 ab | 10.43 ± 1.85 a | 0.87 ± 0.04 a | 0.23 ± 0.05 a | 80.00 ± 9.90 ab | |
| 600 | 44.68 ± 4.57 a | 10.79 ± 2.72 a | 0.76 ± 0.11 ab | 0.26 ± 0.02 a | 100.00 ± 1.41 a | |
| CK | 0 | 42.85 ± 2.12 a | 12.71 ± 0.71 b | 0.86 ± 0.05 ab | 0.28 ± 0.01 b | 106.00 ± 14.85 |
| 10 | 46.53 ± 3.88 a | 14.80 ± 2.25 ab | 0.66 ± 0.01 b | 0.29 ± 0.06 ab | 111.00 ± 22.63 a | |
| 300 | 47.64 ± 0.96 a | 12.33 ± 0.47 b | 0.86 ± 0.05 ab | 0.27 ± 0.03 b | 105.00 ± 13.44 a | |
| 600 | 51.29 ± 7.42 a | 17.92 ± 0.47 a | 0.91 ± 0.16 a | 0.35 ± 0.03 a | 124.50 ± 16.26 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Rukh, G.; Ye, Z.; Xie, X.; Ruan, Z.; Liu, D. Effect of Hypoxic Stress and Levels of Mn on the Physiology and Biochemistry of Phyllostachys praecox. Toxics 2022, 10, 290. https://doi.org/10.3390/toxics10060290
Ma J, Rukh G, Ye Z, Xie X, Ruan Z, Liu D. Effect of Hypoxic Stress and Levels of Mn on the Physiology and Biochemistry of Phyllostachys praecox. Toxics. 2022; 10(6):290. https://doi.org/10.3390/toxics10060290
Chicago/Turabian StyleMa, Jiawei, Gul Rukh, Zhengqian Ye, Xiaocui Xie, Zhongqiang Ruan, and Dan Liu. 2022. "Effect of Hypoxic Stress and Levels of Mn on the Physiology and Biochemistry of Phyllostachys praecox" Toxics 10, no. 6: 290. https://doi.org/10.3390/toxics10060290
APA StyleMa, J., Rukh, G., Ye, Z., Xie, X., Ruan, Z., & Liu, D. (2022). Effect of Hypoxic Stress and Levels of Mn on the Physiology and Biochemistry of Phyllostachys praecox. Toxics, 10(6), 290. https://doi.org/10.3390/toxics10060290





