Enhanced Cd Phytoextraction by Solanum nigrum L. from Contaminated Soils Combined with the Application of N Fertilizers and Double Harvests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Basic Physicochemical Properties of Soil and the Pot Experiment
2.2. Chemical Analysis
2.3. Analysis of Enzyme Activity
2.4. Data Processing and Statistical Analysis
3. Results
3.1. Effects of Different Types of N Fertilizers on Shoot Phytoextraction of Cd in S. nigrum
3.2. Effects of Different Types of N Fertilizers on Root and Shoot Biomasses in S. nigrum
3.3. Effects of Different Types of N Fertilizers on H2O2 and MDA Contents in S. nigrum Shoots
3.4. Effects of Different Types of N Fertilizers on Proline Concentration and the Activity of CAT, POD and SOD in S. nigrum Shoots
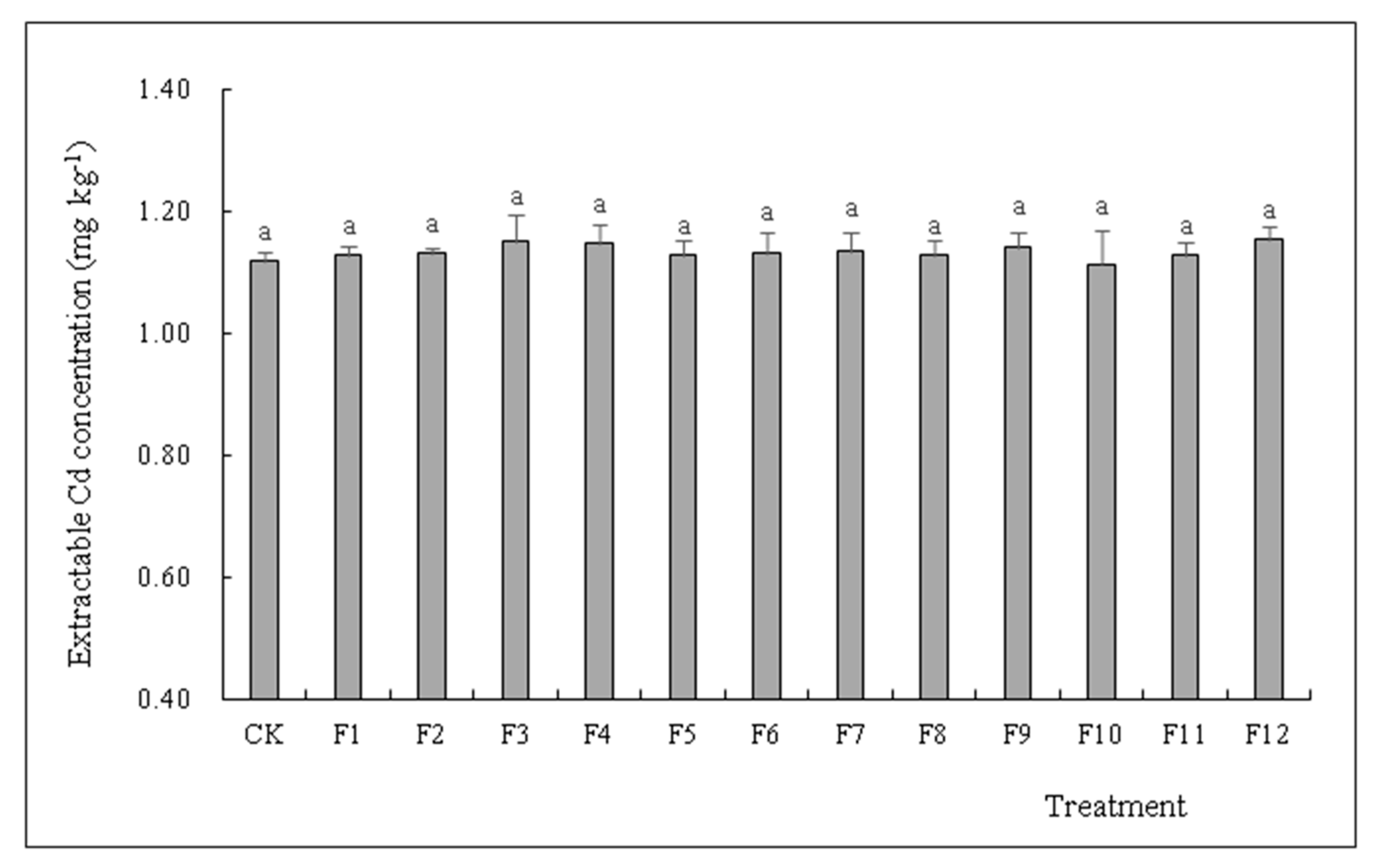
3.5. Effects of Different Types of N Fertilizers on Extractable Cd Concentration in S. nigrum
3.6. Effects of Different Types of N Fertilizers on Soil pH in S. nigrum Cultivation
4. Discussion
4.1. Effects of Different Fertilizers on S. nigrum Cd Phytoextraction in Relation to Single and Double Harvests
4.2. Effects of Different Fertilizers on H2O2, MDA and Proline Contents and Antioxidant Enzyme Activities in S. nigrum in Relation to Single and Successive Harvests
4.3. Effects of Different Fertilizers on S. nigrum Extractable Cd Concentration in Relation to Single and Double Harvests
4.4. Effects of Different Fertilizers on the pH Value in Comparison to Single and Double Harvests of S. nigrum
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, X.H.; Xue, N.D.; Han, Z.G. A meta-analysis of heavy metals pollution in farmland and urban soils in China over the past 20 years. J. Environ. Sci. 2021, 101, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.X.; Song, Y.F. Remediation of Contaminated Soils: Principles and Methods; Science Press: Beijing, China, 2004. [Google Scholar]
- Feng, Y.; Wang, Q.; Meng, Q.; Liu, Y.J.; Pan, F.S.; Luo, S.; Wu, Y.J.; Ma, L.Y.; Yang, X.E. Chromosome doubling of Sedum alfredii Hance: A novel approach for improving phytoremediation efficiency. J. Environ. Sci. 2019, 86, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, L.H.; Yang, Y.; Peng, L.; Luo, S.; Zeng, Q.R. Phytoremediation of Heavy Metals under an Oil Crop Rotation and Treatment of Biochar from Contaminated Biomass for Safe Use. Chemosphere 2020, 247, 125856. [Google Scholar] [CrossRef] [PubMed]
- Rees, F.; Germain, C.; Sterckeman, T.; Morel, J.L. Plant growth and metal uptake by a non-hyperaccumulating species (Lolium perenne) and a Cd–Zn hyperaccumulator (Noccaea caerulescens) in contaminated soils amended with biochar. Plant Soil 2015, 395, 57–73. [Google Scholar] [CrossRef]
- Dou, X.K.; Dai, H.P.; Skuza, L.; Wei, S.H. Strong accumulation capacity of hyperaccumulator Solanum nigrum L. for low or insoluble Cd compounds in soil and its implication for phytoremediation. Chemosphere 2020, 260, 127564. [Google Scholar] [CrossRef]
- Wu, C.; Liao, B.; Wang, S.L.; Zhang, J.; Li, J.T. Pb and Zn accumulation in a Cd-hyperaccumulator (Viola baoshanensis). Int. J. Phytoremediat. 2010, 12, 574–585. [Google Scholar] [CrossRef]
- Liu, J.; Duan, C.; Zhang, X.; Zhu, Y.; Lu, X. Potential of Leersia hexandra Swartz for phytoextraction of Cr from soil. J. Hazard Mater. 2011, 188, 85–91. [Google Scholar] [CrossRef]
- Guo, J.K.; Lv, X.; Jia, H.L.; Hua, L.; Ren, X.H.; Muhammad, H.; Wei, T.; Ding, Y.Z. Effects of EDTA and plant growth-promoting rhizobacteria on plant growth and heavy metal uptake of hyperaccumulatro Sedum alfredii Hance. J. Environ. Sci. 2020, 88, 361–369. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, L.; Liu, Q.; Topalovic, O.; Wang, Q.; Yang, X.; Feng, Y. Pseudomonas fluorescens accelerates a reverse and long-distance transport of cadmium and sucrose in the hyperaccumulator plant Sedum alfredii. Chemosphere 2020, 256, 127156. [Google Scholar] [CrossRef]
- Bani, A.; Pavlova, D.K.; Benizri, E.; Shallari, S.; Echevarria, G. Relationship between the Ni hyperaccumulator Alyssum murale and the parasitic plant Orobanche nowackiana from serpentines in albania. Ecol. Res. 2018, 33, 549–559. [Google Scholar] [CrossRef]
- Singh, N.; Srivastava, S.; Rathaur, S.; Singh, N. Assessing the bioremediation potential of arsenic tolerant bacterial strains in rice rhizosphere interface. J. Environ. Sci. 2016, 48, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.F.; Liao, S.J.; Tu, S.X.; Zhu, D.W.; Xie, T.; Wang, G. Surfactants enhanced soil arsenic phytoextraction efficiency by Pteris vittata L. Bullet. Environ. Contaminat. Toxicol. 2020, 104, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Liu, Y.; Zeng, G.; Zheng, B.; Tan, X.; Liu, H.; Xie, J.; Gan, C.; Liu, W. Cadmium accumulation and tolerance of Macleaya cordata: A newly potential plant for sustainable phytoremediation in Cd-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 10189–10199. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, S.B. Responses of soil aggregates and bacterial communities to soil-Pb immobilization induced by biofertilizer. Chemosphere 2019, 220, 828–836. [Google Scholar] [CrossRef]
- Li, T.Q.; Tao, Q.; Liang, C.F.; Yang, X.E. Elevated CO2 concentration increase the mobility of Cd and Zn in the rhizosphere of hyperaccumulator Sedum alfredii. Environ. Sci. Pollut. Res. 2014, 21, 5899–5908. [Google Scholar] [CrossRef]
- Wang, S.Q.; Wei, S.H.; Ji, D.D.; Bai, J.Y. Co-planting Cd contaminated field using hyperaccumulator Solanum nigrum L. through interplant with low accumulation welsh onion. Int. J. Phytoremediat. 2015, 17, 879–884. [Google Scholar] [CrossRef]
- Abid, R.; Manzoor, M.; De Oliveira, L.M.; da Silva, E.; Rathinasabapathi, B.; Rensing, C.; Ma, L.Q. Interactive effects of As, Cd and Zn on their uptake and oxidative stress in As-hyperaccumulator Pteris vittata. Environ. Pollut. 2019, 248, 756–762. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, W.; Liang, X.; Zhou, K.; Lin, X. Increased bound putrescine accumulation contributes to the maintenance of antioxidant enzymes and higher aluminum tolerance in wheat. Environ. Pollut. 2019, 252, 941–949. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Guo, Q.J.; Yang, J.X.; Chen, T.B.; Zhu, G.X.; Peters, M.; Wei, R.F.; Tian, L.Y.; Wang, C.Y.; Tan, D.Y.; et al. Cadmium accumulation and tolerance of two castor cultivars in relation to antioxidant systems. J. Environ. Sci. 2014, 26, 2048–2055. [Google Scholar] [CrossRef]
- Hui, F.Q.; Liu, J.; Gao, Q.K.; Lou, B.G. Piriformospora indica confers cadmium tolerance in Nicotiana tabacum. J. Environ. Sci. 2015, 37, 184–191. [Google Scholar] [CrossRef]
- Wei, S.H.; Ji, D.D.; Twardowska, I.; Li, Y.M.; Zhu, J.G. Effect of different nitrogenous nutrients on the cadmium hyperaccumulation efficiency of Rorippa globosa (Turcz.) Thell. Environ. Sci. Pollut. Res. 2015, 22, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Dai, H.P.; Dou, X.K.; Zhang, Q.R.; Wei, S.H. Effect and mechanism of commonly used four nitrogen fertilizers and three organic fertilizers on Solanum nigrum L. hyperaccumulating Cd. Environ. Sci. Pollut. Res. 2019, 26, 12940–12947. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.N.; Balardin, R.S.; Stefanello, M.T.; Pezzini, D.T.; Gulart, C.A.; de Ramos, J.P.; Farias, J.G. Physiological, biochemical, and nutritional parameters of wheat exposed to fungicide and foliar fertilizer. Semin. Ciências Agrárias 2016, 37, 1243–1254. [Google Scholar] [CrossRef] [Green Version]
- Sangoi, L.; Junior, G.J.P.; Vargas, V.P.; Vieira, J.; Schmitt, A.; Zoldan, S.R.; Carniel, G. Nitrogen side-dress as a strategy to reduce defoliation demages at different growth stages of maize. Semin. Ciências Agrárias 2014, 35, 671–682. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.H.; Zhou, Q.X. Phytoremediation of cadmium-contaminated soils by Rorippa globosa using two-phase planting. Environ. Sci. Pollut. Res. 2006, 13, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.H.; Sun, T.; Song, Y.; Ackland, M.L.; Liu, Y. Strategies for enhancing the phytoremediation of cadmium-contaminated agricultural soils by Solanum nigrum L. Environ. Pollut. 2011, 159, 762–768. [Google Scholar] [CrossRef]
- National Standard of China GB15618-2018; Soil Environmental Quality—Risk Control Standard for Soil Contamination of Agricultural Land (On trial). Ministry of Ecology and Environment: Beijing, China, 2018.
- Lu, R.K. Analysis Method of Soil Agricultural Chemistry; Chinese Agricultural S &T Press: Beijing, China, 2000; Volume 107, pp. 147–150. [Google Scholar]
- Zhu, Z.; Huang, Y.; Wu, X.; Liu, Z.; Zou, J.; Chen, Y. Increased antioxidative capacity and decreased cadmium uptake contribute to hemin-induced alleviation of cadmium toxicity in Chinese cabbage seedlings. Ecotoxicol. Environ. Saf. 2019, 177, 47–57. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldern, R.P.; Teare, T.D. Rapid determination of proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, J.; Chen, X.; Gao, Z.; Xuan, W.; Xu, S.; Shen, W. Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa. New Phyto. 2008, 177, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.H.; Zhou, Q.X.; Koval, P.V. Flowering stage characteristics of cadmium hyperaccumulator Solanum nigrum L. and their significance to phytoremediation. Sci. Total Environ. 2006, 369, 441–446. [Google Scholar] [CrossRef]
- Fässler, E.; Robinson, B.H.; Stauffer, W.; Gupta, S.K.; Papritz, A.; Schulin, R. Phytomanagement of metal-contaminated agricultural land using sunflower, maize and tobacco. Agric. Ecosyst. Environ. 2010, 136, 49–58. [Google Scholar] [CrossRef]
- Ye, X.X.; Hu, H.; Li, H.; Xiong, Q.; Gao, H. Combined nitrogen fertilizer and wheat straw increases the cadmium phytoextraction efficiency of Tagetes patula. Ecotoxicol. Environ. Saf. 2019, 170, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.; Echevarria, G.; Morel, J.L. Phytoextraction of cadmium with Thlaspi caerulescens. Plant Soil 2003, 249, 27–35. [Google Scholar] [CrossRef]
- Liu, W.X.; Zhang, C.J.; Hu, P.J.; Luo, Y.M.; Wu, L.H.; Sale, P.; Tang, C.X. Influence of nitrogen form on the phytoextraction of cadmium by a newly discovered hyperaccumulator Carpobrotus rossii. Environ. Sci. Pollut. Res. 2016, 23, 1246–1253. [Google Scholar] [CrossRef]
- Xie, H.L.; Jiang, R.F.; Zhang, F.S.; McGrath, S.P.; Zhao, F.J. Effect of nitrogen form on the rhizosphere dynamics and uptake of cadmium and zinc by the hyperaccumulator Thlaspi caerulescens. Plant Soil 2009, 318, 205–215. [Google Scholar] [CrossRef]
- Peng, Y.L.; Wang, Y.S.; Fei, J.; Sun, C.C.; Cheng, H. Ecophysiological differences between three mangrove seedlings (Kandelia obovata, Aegiceras corniculatum, and Avicennia marina) exposed to chilling stress. Ecotoxicol. Environ. Saf. 2015, 24, 1722–1732. [Google Scholar] [CrossRef]
- Jalloh, M.A.; Chen, J.; Zhen, F.; Zhang, G. Effect of different N fertilizer forms on antioxidant capacity and grain yield of rice growing under Cd stress. J. Hazard Mater. 2009, 162, 1081–1085. [Google Scholar] [CrossRef]
- Giansoldati, V.; Tassi, E.; Morelli, E.; Gabellieri, E.; Pedron, F.; Barbafieri, M. Nitrogen fertilizer improves boron phytoextraction by Brassica juncea grown in contaminated sediments and alleviates plant stress. Chemosphere 2012, 87, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Rizwan, M.; Li, M.; Song, F.; Zhou, S.; He, X.; Xiong, S. Comparative efficacy of organic and inorganic silicon fertilizers on antioxidant response, Cd/Pb accumulation and health risk assessment in wheat (Triticum aestivum L.). Environ. Pollut. 2019, 255, 113146–113154. [Google Scholar] [CrossRef] [PubMed]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Bahtiyar, M.; Kucukoduk., M. The humic acid-induced changes in the water status, chlorophyll fluorescence and antioxidant defense systems of wheat leaves with cadmium stress. Ecotoxicol. Environ. Saf. 2018, 155, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nature Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Li, Y.; Xu, Y.; Liang, X.; Wang, L. In situ stabilization remediation of cadmium (Cd) and lead (Pb) co-contaminated paddy soil using bentonite. Appl. Clay Sci. 2015, 105, 200–206. [Google Scholar] [CrossRef]
- Guo, J.; Qin, S.; Rengel, Z.; Gao, W.; Nie, Z.; Liu, H.; Zhao, P. Cadmium stress increases antioxidant enzyme activities and decreases endogenous hormone concentrations more in Cd-tolerant than Cd-sensitive wheat varieties. Ecotoxicol. Environ. Saf. 2019, 172, 380–387. [Google Scholar] [CrossRef]
- Naeem, A.; Zia-ur-Rehman, M.; Akhtar, T.; Zia, M.H.; Aslam, M. Silicon nutrition lowers cadmium content of wheat cultivars by regulating transpiration rate and activity of antioxidant enzymes. Environ. Pollut. 2018, 242, 126–135. [Google Scholar] [CrossRef]
- Esmaeili, H.; Karami, A.; Maggi, F. Essential oil composition, total phenolic and flavonoids contents, and antioxidant activity of Oliveria decumbens Vent.(Apiaceae) at different phenological stages. J. Clean. Prod. 2018, 198, 91–95. [Google Scholar] [CrossRef]
- Chen, Y.H.; Hu, L.; Liu, X.L.; Deng, Y.W.; Liu, M.J.; Xu, B.; Wang, G. Influences of king grass (Pennisetum sinese Roxb)-enhanced approaches for phytoextraction and microbial communities in multi-metal contaminated soil. Geoderma 2017, 307, 253–266. [Google Scholar] [CrossRef]
- Li, J.T.; Baker, A.J.; Ye, Z.H.; Wang, H.B.; Shu, W.S. Phytoextraction of Cd-contaminated soils: Current status and future challenges. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2113–2152. [Google Scholar] [CrossRef] [Green Version]



| No. | Treatment | Dose (g∙kg−1) | Added Total N (mg∙kg−1) | Harvest Time |
|---|---|---|---|---|
| CK | Control, no N addition | 0.00 | 0.00 | at maturation stage |
| F1 | NH4HCO3 | 1.68 | 300.00 | at the first florescence stage |
| F2 | NH4HCO3 | 1.68 | 300.00 | at the second florescence stage |
| F3 | NH4HCO3 | 3.36 | 600.00 | at maturation stage |
| F4 | NH4Cl | 1.14 | 300.00 | at the first florescence stage |
| F5 | NH4Cl | 1.14 | 300.00 | at the second florescence stage |
| F6 | NH4Cl | 2.28 | 600.00 | at maturation stage |
| F7 | (NH4)2SO4 | 1.41 | 300.00 | at the first florescence stage |
| F8 | (NH4)2SO4 | 1.41 | 300.00 | at the second florescence stage |
| F9 | (NH4)2SO4 | 2.82 | 600.00 | at maturation stage |
| F10 | CH4N2O | 0.65 | 300.00 | at the first florescence stage |
| F11 | CH4N2O | 0.65 | 300.00 | at the second florescence stage |
| F12 | CH4N2O | 1.30 | 600.00 | at maturation stage |
| Treatment | Roots (mg∙kg−1) | Shoots (mg∙kg−1) | Shoot Cd Extraction (μg∙Plant−1) |
|---|---|---|---|
| CK | 20.11 ± 0.55 a | 20.61 ± 0.63 a | 17.66 ± 0.57 e |
| F1 | 19.66 ± 0.37 a | 20.38 ± 0.31 a | 25.29 ± 0.56 d |
| F2 | 19.94 ± 0.66 a | 21.05 ± 0.26 a | 23.03 ± 0.49 d |
| F3 | 19.53 ± 0.24 a | 21.08 ± 0.25 a | 38.86 ± 0.58 b |
| F4 | 20.27 ± 0.42 a | 21.00 ± 0.45 a | 27.01 ± 0.68 d |
| F5 | 20.65 ± 0.27 a | 21.05 ± 0.23 a | 24.21 ± 0.28 d |
| F6 | 19.73 ± 0.41 a | 20.27 ± 0.34 a | 37.76 ± 0.79 b |
| F7 | 20.35 ± 0.68 a | 21.09 ± 0.21 a | 34.41 ± 0.30 c |
| F8 | 19.82 ± 0.41 a | 21.00 ± 0.30 a | 33.61 ± 0.39 c |
| F9 | 19.81 ± 0.49 a | 21.07 ± 0.05 a | 50.83 ± 0.55 a |
| F10 | 20.26 ± 0.75 a | 21.35 ± 0.32 a | 33.54 ± 0.33 c |
| F11 | 19.75 ± 0.58 a | 20.80 ± 0.23 a | 33.06 ± 0.35 c |
| F12 | 19.92 ± 0.24 a | 20.84 ± 0.48 a | 50.16 ± 1.02 a |
| Treatment | Roots (g∙Plant−1) | Shoots (g∙Plant−1) |
|---|---|---|
| CK | 0.16 ± 0.01 e | 0.86 ± 0.02 e |
| F1 | 0.37 ± 0.01 cd | 1.24 ± 0.02 d |
| F2 | 0.35 ± 0.01 d | 1.09 ± 0.01 d |
| F3 | 0.41 ± 0.01 c | 1.84 ± 0.02 b |
| F4 | 0.39 ± 0.02 c | 1.29 ± 0.01 d |
| F5 | 0.37 ± 0.02 cd | 1.15 ± 0.01 d |
| F6 | 0.42 ± 0.01 c | 1.86 ± 0.01 b |
| F7 | 0.61 ± 0.01 b | 1.63 ± 0.03 c |
| F8 | 0.57 ± 0.01 b | 1.60 ± 0.03 c |
| F9 | 0.69 ± 0.02 a | 2.41 ± 0.01 a |
| F10 | 0.61 ± 0.01 b | 1.59 ± 0.02 c |
| F11 | 0.58 ± 0.02 b | 1.59 ± 0.02 c |
| F12 | 0.70 ± 0.01 a | 2.41 ± 0.02 a |
| Treatment | H2O2 (mg g−1 FW) | MDA (μmol g−1 FW) |
|---|---|---|
| CK | 0.46 ± 0.03 a | 6.27 ± 0.04 a |
| F1 | 0.31 ± 0.02 c | 5.57 ± 0.03 c |
| F2 | 0.32 ± 0.02 c | 5.55 ± 0.03 c |
| F3 | 0.39 ± 0.03 b | 6.00 ± 0.05 b |
| F4 | 0.31 ± 0.01 c | 5.51 ± 0.02 c |
| F5 | 0.30 ± 0.02 c | 5.53 ± 0.03 c |
| F6 | 0.38 ± 0.02 b | 6.02 ± 0.07 b |
| F7 | 0.33 ± 0.03 c | 5.50 ± 0.03 c |
| F8 | 0.29 ± 0.02 c | 5.52 ± 0.04 c |
| F9 | 0.37 ± 0.02 b | 6.04 ± 0.04 b |
| F10 | 0.30 ± 0.01 c | 5.49 ± 0.04 c |
| F11 | 0.34 ± 0.02 c | 5.52 ± 0.04 c |
| F12 | 0.38 ± 0.04 b | 6.03 ± 0.05 b |
| Treatment | Root (mg∙kg−1) | Shoot (mg∙kg−1) | Shoot Extraction (μg∙Plant−1) | Root (g∙Plant−1) | Shoot (g∙Plant−1) | H2O2 (mg∙g−1) | MDA (μmol∙g−1) | Proline (mg∙g−1) | CAT (U g−1∙min−1) | POD (U g−1∙min−1) | SOD (U∙g−1) | Extractable Cd Concentration (mg∙kg−1) | pH Value of Soil |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F (FT) | 1.609 | 1.526 | 914.06 * | 1324.7 * | 1579.7 * | 0.325 | 1.404 | 0.335 | 0.212 | 0.329 | 0.18 | 0.035 | 0.021 |
| F (HM) | 2.03 | 0.884 | 2852.6 * | 166.4 * | 5376.2 * | 43.5 * | 663.2 * | 101.2 * | 58.9 * | 109.7 * | 48.5 * | 0.926 | 1.374 |
| F (FT × HM) | 1.061 | 1.988 | 18.29 * | 8.048 * | 27.3 * | 0.966 | 1.015 | 0.15 | 0.746 | 0.237 | 0.211 | 0.472 | 1.957 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Dai, H.; Skuza, L.; Wei, S. Enhanced Cd Phytoextraction by Solanum nigrum L. from Contaminated Soils Combined with the Application of N Fertilizers and Double Harvests. Toxics 2022, 10, 266. https://doi.org/10.3390/toxics10050266
Yang W, Dai H, Skuza L, Wei S. Enhanced Cd Phytoextraction by Solanum nigrum L. from Contaminated Soils Combined with the Application of N Fertilizers and Double Harvests. Toxics. 2022; 10(5):266. https://doi.org/10.3390/toxics10050266
Chicago/Turabian StyleYang, Wei, Huiping Dai, Lidia Skuza, and Shuhe Wei. 2022. "Enhanced Cd Phytoextraction by Solanum nigrum L. from Contaminated Soils Combined with the Application of N Fertilizers and Double Harvests" Toxics 10, no. 5: 266. https://doi.org/10.3390/toxics10050266
APA StyleYang, W., Dai, H., Skuza, L., & Wei, S. (2022). Enhanced Cd Phytoextraction by Solanum nigrum L. from Contaminated Soils Combined with the Application of N Fertilizers and Double Harvests. Toxics, 10(5), 266. https://doi.org/10.3390/toxics10050266








