Metagenomic Analysis of the Gastrointestinal Microbiota of Gadus morhua callarias L. Originating from a Chemical Munition Dump Site
Abstract
:1. Introduction
2. Materials and Methods
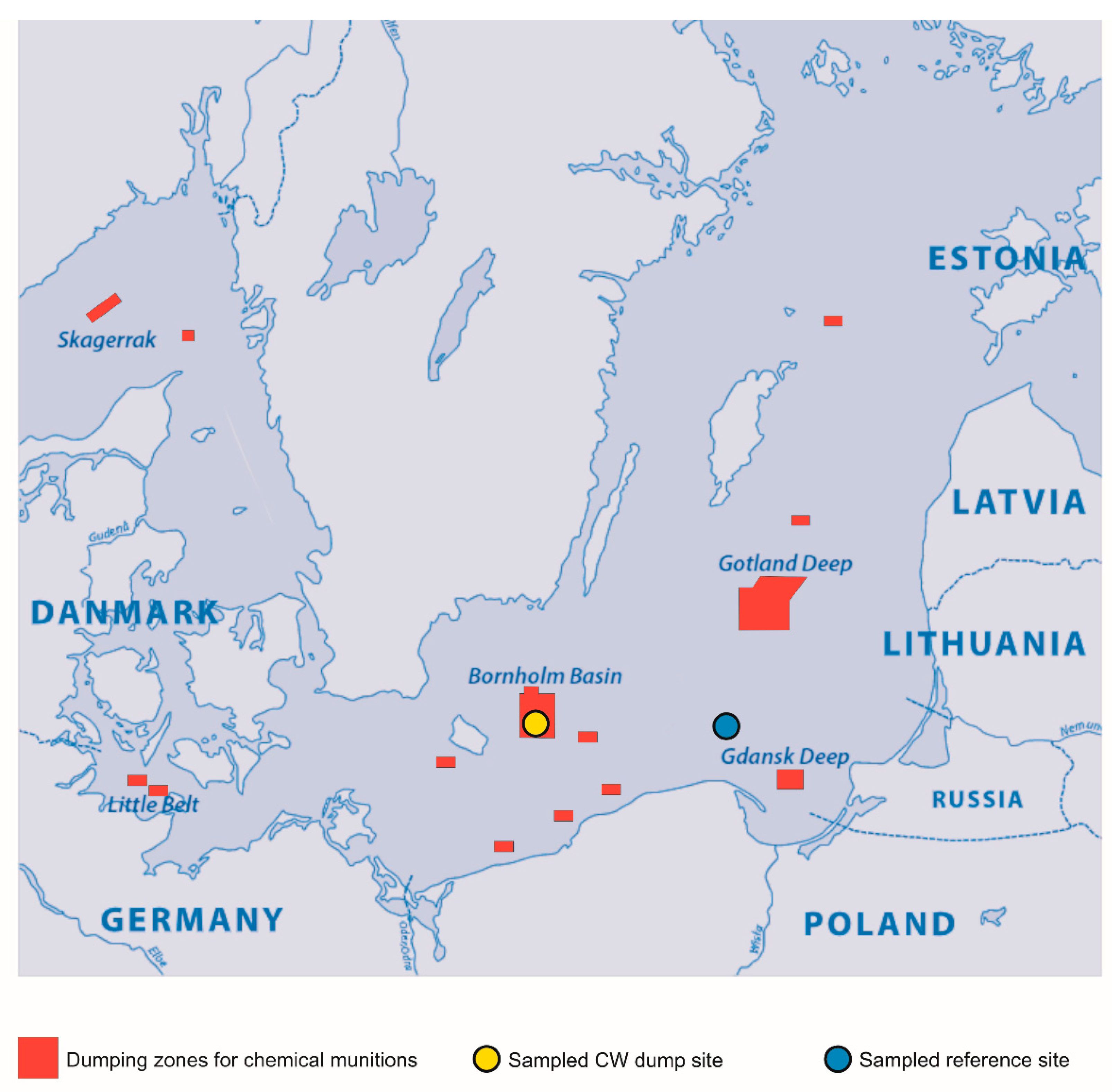
2.1. G. morhua callarias Sampling
2.2. Gastrointestinal Tract and Fecal Matter Extraction
2.3. DNA Extraction and PCR Amplification
2.4. Sequencing and Data Processing
3. Results
3.1. Dominant ASVs
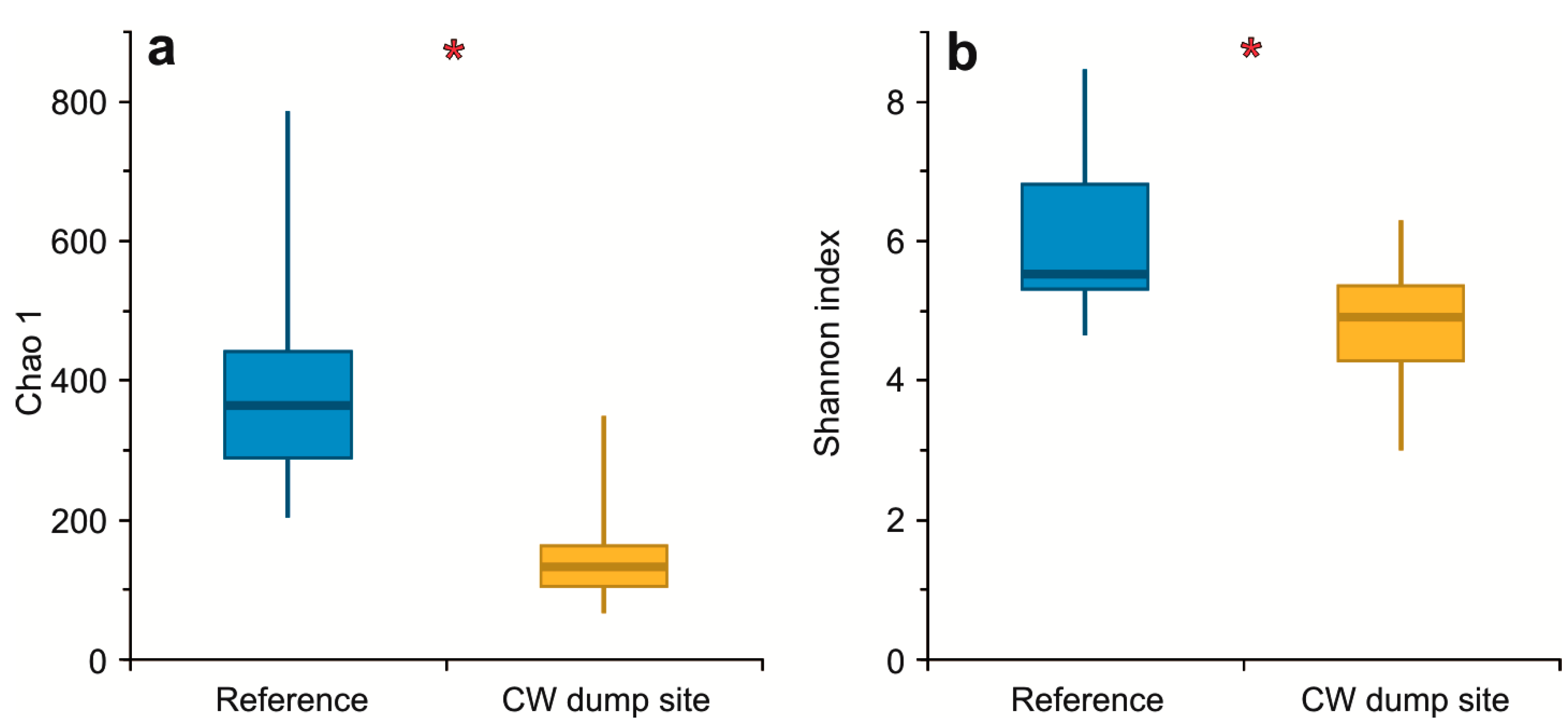
3.2. α Diversity
3.3. β Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenberg, M.I.; Sexton, K.J.; Vearrier, D. Sea-dumped chemical weapons: Environmental risk, occupational hazard. Clin. Toxicol. 2016, 54, 79–91. [Google Scholar] [CrossRef] [PubMed]
- HELCOM. Chemical Munitions Dumped in the Baltic Sea. Report of the ad hoc Expert Group to Update and Review the Existing Information on Dumped Chemical Munitions in the Baltic Sea (HELCOM MUNI). In Proceedings of the 2013 HELCOM Ministerial Meeting, Copenhagen, Denmark, 3 October 2013; p. 142. [Google Scholar]
- Laurin, F. The Baltic and North Sea dumping of chemical weapons: Still a threat. Chall. Old Chem. Munitions Toxic Armament Wastes 1997, 16, 263–278. [Google Scholar]
- Glasby, G.P. Disposal of chemical weapons in the Baltic Sea. Sci. Total Environ. 1997, 206, 267–273. [Google Scholar] [CrossRef]
- Missiaen, T.; Henriet, J.P. Chemical Munition Dump Sites in Coastal Environments; Federal Office for Scientific and Cultural Affairs (OSTC): Brussels, Belgium, 2002. [Google Scholar]
- Sanderson, H.; Fauser, P.; Thomsen, M.; Sørensen, P.B. Screening level fish community risk assessment of chemical warfare agents in the Baltic Sea. J. Hazards Mater. 2008, 154, 846–857. [Google Scholar] [CrossRef]
- Bełdowski, J.; Klusek, Z.; Szubska, M.; Turja, R.; Bulczak, A.; Rak, D.; Brenner, M.; Lang, T.; Kotwicki, L.; Grzelak, K.; et al. Chemical munitions search & assessment—An evaluation of the dumped munitions problem in the Baltic Sea. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2016, 128, 85–95. [Google Scholar] [CrossRef]
- Czub, M.; Kotwicki, L.; Lang, T.; Sanderson, H.; Klusek, Z.; Grabowski, M.; Szubska, M.; Jakacki, J.; Andrzejewski, J.; Rak, D.; et al. Deep sea habitats in the chemical warfare dumping areas of the Baltic Sea. Sci. Total Environ. 2018, 616, 1485–1497. [Google Scholar] [CrossRef]
- Vanninen, P.; Östin, A.; Bełdowski, J.; Pedersen, E.A.; Söderström, M.; Szubska, M.; Grabowski, M.; Siedlewicz, G.; Czub, M.; Popiel, S.; et al. Exposure status of sea-dumped chemical warfare agents in the Baltic Sea. Mar. Environ. Res. 2020, 161, 105112. [Google Scholar] [CrossRef]
- Brzeziński, T.; Czub, M.; Nawała, J.; Gordon, D.; Dziedzic, D.; Dawidziuk, B.; Popiel, S.; Maszczyk, P. The effects of chemical warfare agent Clark I on the life histories and stable isotopes composition of Daphnia magna. Environ. Pollut. 2020, 266, 115142. [Google Scholar] [CrossRef]
- Czub, M.; Nawała, J.; Popiel, S.; Dziedzic, D.; Brzeziński, T.; Maszczyk, P.; Sanderson, H.; Fabisiak, J.; Bełdowski, J.; Kotwicki, L. Acute aquatic toxicity of sulfur mustard and its degradation products to Daphnia magna. Mar. Environ. Res. 2020, 161, 105077. [Google Scholar] [CrossRef]
- Czub, M.; Nawała, J.; Popiel, S.; Brzeziński, T.; Maszczyk, P.; Sanderson, H.; Maser, E.; Gordon, D.; Dziedzic, D.; Dawidziuk, B.; et al. Acute aquatic toxicity of arsenic-based chemical warfare agents to Daphnia magna. Aquat. Toxicol. 2021, 230, 105693. [Google Scholar] [CrossRef]
- Medvedeva, N.; Polyak, Y.; Kankaanpää, H.; Zaytseva, T. Microbial responses to mustard gas dumped in the Baltic Sea. Mar. Environ. Res. 2009, 68, 71–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höher, N.; Turja, R.; Brenner, M.; Nyholm, J.R.; Östin, A.; Leffler, P.; Butrimavičienė, L.; Baršienė, J.; Halme, M.; Karjalainen, M.; et al. Toxic effects of chemical warfare agent mixtures on the mussel Mytilus trossulus in the Baltic Sea: A laboratory exposure study. Mar. Environ. Res. 2019, 145, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Amato, E.; Alcaro, L.; Corsi, I.; Della Torre, C.; Farchi, C.; Focardi, S.; Marino, G.; Tursi, A. An integrated ecotoxicological approach to assess the effects of pollutants released by unexploded chemical ordnance dumped in the southern Adriatic (Mediterranean Sea). Mar. Biol. 2006, 149, 17–23. [Google Scholar] [CrossRef]
- Della Torre, C.; Petochi, T.; Corsi, I.; Dinardo, M.M.; Baroni, D.; Alcaro, L.; Focardi, S.; Tursi, A.; Marino, G.; Frigeri, A.; et al. DNA damage, severe organ lesions and high muscle levels of As and Hg in two benthic fish species from a chemical warfare agent dumping site in the Mediterranean Sea. Sci. Total Environ. 2010, 408, 2136–2145. [Google Scholar] [CrossRef]
- Niemikoski, H.; Söderström, M.; Vanninen, P. Detection of chemical warfare agent-related phenylarsenic compounds in marine biota samples by LC-HESI/MS/MS. Anal. Chem. 2017, 89, 11129–11134. [Google Scholar] [CrossRef]
- Munro, N.B.; Talmage, S.S.; Griffin, G.D.; Waters, L.C.; Watson, A.P.; King, J.F.; Hauschild, V. The sources, fate, and toxicity of chemical warfare agent degradation products. Environ. Health Perspect. 1999, 107, 933–974. [Google Scholar] [CrossRef] [Green Version]
- Amata, R.; Beblo, D.A.; Rosemond, Z.A. Toxicological Profile for Sulfur Mustard; US Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2003; Volume 80. [Google Scholar]
- Niemikoski, H.; Lehtonen, K.K.; Ahvo, A.; Heiskanen, I.; Vanninen, P. Metabolism and cytotoxicity of diphenylarsinic acid, a degradation product of sea-dumped chemical warfare agents, in a rainbow trout liver cell line RTL-W1. Aquat. Toxicol. 2021, 241, 105993. [Google Scholar] [CrossRef]
- Baršienė, J.; Butrimavičienė, L.; Grygiel, W.; Lang, T.; Michailovas, A.; Jackūnas, T. Environmental genotoxicity and cytotoxicity in flounder (Platichthys flesus), herring (Clupea harengus) and Atlantic cod (Gadus morhua) from chemical munitions dumping zones in the southern Baltic Sea. Mar. Environ. Res. 2014, 96, 56–67. [Google Scholar] [CrossRef]
- Bagi, A.; Riiser, E.S.; Molland, H.S.; Star, B.; Haverkamp, T.H.; Sydnes, M.O.; Pampanin, D.M. Gastrointestinal microbial community changes in Atlantic cod (Gadus morhua) exposed to crude oil. BMC Microbiol. 2018, 18, 25. [Google Scholar] [CrossRef]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P. The gut microbiota of marine fish. Front. Microbiol. 2018, 9, 873. [Google Scholar] [CrossRef]
- Nayak, S.K. Probiotics and immunity: A fish perspective. Fish Shellfish Immunol. 2010, 29, 2–14. [Google Scholar] [CrossRef]
- Sullam, K.E.; Essinger, S.D.; Lozupone, C.A.; O’Connor, M.P.; Rosen, G.L.; Knight, R.; Kilham, S.S.; Russell, J.A. Environmental and ecological factors that shape the gut bacterial communities of fish: A meta-analysis. Mol. Ecol. 2012, 21, 3363–3378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, G.D.; Balcázar, J.L. A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol. Med. Microbiol. 2008, 52, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Rawls, J.F.; Samuel, B.S.; Gordon, J.I. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc. Natl. Acad. Sci. USA 2004, 101, 4596–4601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.-L.; Li, S.; Qin, C.-B.; Zhu, Z.-X.; Hu, W.-P.; Yang, L.-P.; Lu, R.-H.; Li, W.-J.; Nie, G.-X. Intestinal microbiota and lipid metabolism responses in the common carp (Cyprinus carpio L.) following copper exposure. Ecotoxicol. Environ. Saf. 2018, 160, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.S.; Park, S.C. Therapeutic effect of intestinal autochthonous Lactobacillus reuteri P16 against waterborne lead toxicity in Cyprinus carpio. Front. Immunol. 2018, 9, 1824. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xia, J.; Pan, Z.; Yang, J.; Wang, W.; Fu, Z. Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environ. Pollut. 2018, 235, 322–329. [Google Scholar] [CrossRef]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef]
- Egan, S.; Gardiner, M. Microbial dysbiosis: Rethinking disease in marine ecosystems. Front. Microbiol. 2016, 7, 991. [Google Scholar] [CrossRef]
- Tomkiewicz, J.; Lehmann, K.M.; John MA, S. Oceanographic influences on the distribution of Baltic cod, Gadus morhua, during spawning in the Bornholm Basin of the Baltic Sea. Fish. Oceanogr. 1998, 7, 48–62. [Google Scholar] [CrossRef]
- Bełdowski, J.; Fabisiak, J.; Popiel, S.; Östin, A.; Olsson, U.; Vanninen, P.; Lastumaki, A.; Lang, T.; Fricke, N.; Brenner, M.; et al. CHEMSEA Findings; Institute of Oceanology Polish Academy of Sciences: Sopot, Poland, 2014. [Google Scholar]
- Bełdowski, J.; Jakacki, J.; Grabowski, M.; Lang, T.; Weber, K.; Kotwicki, L.; Paka, V.; Rak, D.; Golenko, M.; Czub, M.; et al. Best practices in monitoring. In Towards the Monitoring of Dumped Munitions Threat (MODUM). NATO Science for Peace and Security Series C: Environmental Security; Bełdowski, J., Been, R., Turmus, E., Eds.; Springer: Dordrecht, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Lang, T.; Kotwicki, L.; Czub, M.; Grzelak, K.; Weirup, L.; Straumer, K. The health status of fish and benthos communities in chemical munitions dumpsites in the Baltic Sea. In Towards the Monitoring of Dumped Munitions Threat (MODUM); Springer: Dordrecht, The Netherlands, 2018; pp. 129–152. [Google Scholar] [CrossRef]
- Niemikoski, H.; Koske, D.; Kammann, U.; Lang, T.; Vanninen, P. Studying the metabolism of toxic chemical warfare agent-related phenylarsenic chemicals in vitro in cod liver. J. Hazards Mater. 2020, 391, 122221. [Google Scholar] [CrossRef] [PubMed]
- Niemikoski, H.; Straumer, K.; Ahvo, A.; Turja, R.; Brenner, M.; Rautanen, T.; Lang, T.; Lehtonen, K.K.; Vanninen, P. Detection of chemical warfare agent related phenylarsenic compounds and multibiomarker responses in cod (Gadus morhua) from munition dumpsites. Mar. Environ. Res. 2020, 162, 105160. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 3 April 2022).
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Bisanz, J.E. Qiime2R: Importing QIIME2 Artifacts and Associated Data into R Sessions. 2018. Available online: https://github.com/jbisanz/qiime2R (accessed on 15 November 2021).
- McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Chao, A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics 1987, 43, 783–791. [Google Scholar] [CrossRef]
- Guinane, C.M.; Tadrous, A.; Fouhy, F.; Ryan, C.A.; Dempsey, E.M.; Murphy, B.; Andrews, E.; Cotter, P.D.; Stanton, C.; Ross, R. Microbial composition of human appendices from patients following appendectomy. MBio 2013, 4, e00366-12. [Google Scholar] [CrossRef] [Green Version]
- RStudio Team. RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA. 2022. Available online: http://www.rstudio.com/ (accessed on 10 March 2022).
- Tarnecki, A.M.; Burgos, F.A.; Ray, C.L.; Arias, C.R. Fish intestinal microbiome: Diversity and symbiosis unravelled by metagenomics. J. Appl. Microbiol. 2017, 123, 2–17. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Ward, L.R.; Burke, C. Prospects of using marine actinobacteria as probiotics in aquaculture. Appl. Microbiol. Biotechnol. 2008, 81, 419–429. [Google Scholar] [CrossRef]
- Carter, G.R.; Cole, J.R., Jr. (Eds.) Diagnostic Procedure in Veterinary Bacteriology and Mycology; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Zhang, Y.; Wen, B.; David, M.A.; Gao, J.Z.; Chen, Z.Z. Comparative analysis of intestinal microbiota of discus fish (Symphysodon haraldi) with different growth rates. Aquaculture 2021, 540, 736740. [Google Scholar] [CrossRef]
- Hao, Y.T.; Wu, S.G.; Jakovlić, I.; Zou, H.; Li, W.X.; Wang, G.T. Impacts of diet on hindgut microbiota and short-chain fatty acids in grass carp (Ctenopharyngodon idellus). Aquac. Res. 2017, 48, 5595–5605. [Google Scholar] [CrossRef]
- Huang, Q.; Sham, R.C.; Deng, Y.; Mao, Y.; Wang, C.; Zhang, T.; Leung, K.M. Diversity of gut microbiomes in marine fishes is shaped by host-related factors. Mol. Ecol. 2020, 29, 5019–5034. [Google Scholar] [CrossRef] [PubMed]
- Serra, C.R.; Oliva-Teles, A.; Enes, P.; Tavares, F. Gut microbiota dynamics in carnivorous European seabass (Dicentrarchus labrax) fed plant-based diets. Sci. Rep. 2021, 11, 447. [Google Scholar] [CrossRef] [PubMed]
- Sharifah, E.N.; Eguchi, M. Benefits of live phytoplankton, Chlorella vulgaris, as a biocontrol agent against fish pathogen Vibrio anguillarum. Fish. Sci. 2012, 78, 367–373. [Google Scholar] [CrossRef]
- Godoy, F.A.; Miranda, C.D.; Wittwer, G.D.; Aranda, C.P.; Calderon, R. High variability of levels of Aliivibrio and lactic acid bacteria in the intestinal microbiota of farmed Atlantic salmon Salmo salar L. Ann. Microbiol. 2015, 65, 2343–2353. [Google Scholar] [CrossRef]
- He, X.; Chaganti, S.R.; Heath, D.D. Population-specific responses to interspecific competition in the gut microbiota of two Atlantic salmon (Salmo salar) populations. Microb. Ecol. 2018, 75, 140–151. [Google Scholar] [CrossRef]
- Wang, C.; Sun, G.; Li, S.; Li, X.; Liu, Y. Intestinal microbiota of healthy and unhealthy Atlantic salmon Salmo salar L. in a recirculating aquaculture system. J. Oceanol. Limnol. 2018, 36, 414–426. [Google Scholar] [CrossRef]
- Brown, R.M.; Wiens, G.D.; Salinas, I. Analysis of the gut and gill microbiome of resistant and susceptible lines of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2019, 86, 497–506. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [Green Version]
- Sanderson, H.; Fauser, P.; Thomsen, M.; Vanninen, P.; Soderstrom, M.; Savin, Y.; Khalikov, I.; Hirvonen, A.; Niiranen, S.; Missiaen, T.; et al. Environmental hazards of sea-dumped chemical weapons. Environ. Sci. Technol. 2010, 44, 4389–4394. [Google Scholar] [CrossRef] [PubMed]



| Rank | ASV | Relative Abundance (%) Mean ± SD | Difference in Means: Bootstrap Confidence Interval (95%) | |
|---|---|---|---|---|
| Reference | CW Dump Site | |||
| Phylum | Fusobacteriota | 13.5 ± 11.2 | 27.0 ± 24.4 | −28.603~0.252 |
| Firmicutes | 42.6 ± 25.1 | 35.5 ± 21.5 | −11.048~25.784 | |
| Proteobacteria | 14.5 ± 12.1 | 19.5 ± 16.7 | −16.154~6.386 | |
| Actinobacteriota | 6.10 ± 4.66 | 1.35 ± 2.20 | 1.844~7.914 | |
| Spirochaetota | 0.30 ± 0.84 | 5.09 ± 9.29 | −10.093~−0.205 | |
| Family | Fusobacteriaceae (Fusobacteriota) | 13.5 ± 11.2 | 27.0 ± 24.4 | −28.007~0.472 |
| Mycoplasmataceae (Actinobacteriota) | 10.7 ± 11.6 | 7.84 ± 9.21 | −5.194~10.839 | |
| Ruminococcaceae (Firmicutes) | 10.6 ± 9.85 | 12.5 ± 9.66 | −9.385~6.089 | |
| Clostridiaceae (Firmicutes) | 17.3 ± 23.6 | 0.05 ± 0.15 | 5.360~34.340 | |
| Aeromonadaceae (Proteobacteria) | 2.56 ± 1.84 | 8.13 ± 8.91 | −10.573~−1.269 | |
| Lachnospiraceae (Firmicutes) | 2.26 ± 2.57 | 6.02 ± 8.81 | −9.321~0.277 | |
| Rhodobacteraceae (Proteobacteria) | 4.41 ± 9.61 | 0.08 ± 0.13 | 0.962~11.383 | |
| Erysipelotrichaceae (Firmicutes) | 0.57 ± 0.89 | 6.62 ± 8.69 | −10.837~−2.056 | |
| Brachyspiraceae (Spirichaetota) | 0.04 ± 0.10 | 2.27 ± 4.74 | −4.730~−0.080 | |
| Vibrionaceae (Proteobacteria) | 1.41 ± 3.49 | 7.75 ± 11.1 | −13.013~−0.777 | |
| Genus | Cetobacterium (Fusobacteriaceae, Fusobacteriota) | 13.5 ± 11.2 | 27.4 ± 24.4 | −29.601~0.084 |
| Aeromonas (Aeromonadaceae, Proteobacteria) | 2.56 ± 1.84 | 8.19 ± 8.90 | −10.296~−1.185 | |
| Macellibacteroides (Tannerellaceae, Bacteroidota) | 0.04 ± 0.07 | 4.60 ± 15.3 | −13.713~−0.100 | |
| Sulfitobacter (Rhodobacteraceae, Proteobacteria) | 3.78 ± 9.75 | 0.06 ± 0.08 | 0.375~10.765 | |
| Tyzzerella (Lachnospiraceae, Firmicutes) | 0.00 ± 0.00 | 2.11 ± 7.79 | 0.000~6.762 | |
| Escherichia-Shigella (Enterobacteriaceae, Proteobacteria) | 1.72 ± 5.33 | 1.20 ± 2.72 | −2.238~4.428 | |
| Photobacterium (Vibrionaceae, Proteobacteria) | 1.18 ± 3.43 | 3.35 ± 5.74 | −5.842~1.282 | |
| Brevinema (Brevinemataceae, Spirochaetota) | 0.26 ± 0.74 | 2.96 ± 5.34 | −5.582~−0.263 | |
| Aliivibrio (Vibrionaceae, Proteobacteria) | 0.21 ± 0.22 | 4.37 ± 7.65 | −8.126~−0.650 | |
| Candidatus Bacilloplasma (Mycoplasmataceae, Firmicutes) | 1.55 ± 1.95 | 0.44 ± 0.98 | −0.034~2.453 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilczynski, W.; Radlinska, M.; Wysujack, K.; Czub, M.; Brzeziński, T.; Kowalczyk, G.; Bełdowski, J.; Nogueira, P.; Maszczyk, P. Metagenomic Analysis of the Gastrointestinal Microbiota of Gadus morhua callarias L. Originating from a Chemical Munition Dump Site. Toxics 2022, 10, 206. https://doi.org/10.3390/toxics10050206
Wilczynski W, Radlinska M, Wysujack K, Czub M, Brzeziński T, Kowalczyk G, Bełdowski J, Nogueira P, Maszczyk P. Metagenomic Analysis of the Gastrointestinal Microbiota of Gadus morhua callarias L. Originating from a Chemical Munition Dump Site. Toxics. 2022; 10(5):206. https://doi.org/10.3390/toxics10050206
Chicago/Turabian StyleWilczynski, Wojciech, Monika Radlinska, Klaus Wysujack, Michał Czub, Tomasz Brzeziński, Grzegorz Kowalczyk, Jacek Bełdowski, Pedro Nogueira, and Piotr Maszczyk. 2022. "Metagenomic Analysis of the Gastrointestinal Microbiota of Gadus morhua callarias L. Originating from a Chemical Munition Dump Site" Toxics 10, no. 5: 206. https://doi.org/10.3390/toxics10050206
APA StyleWilczynski, W., Radlinska, M., Wysujack, K., Czub, M., Brzeziński, T., Kowalczyk, G., Bełdowski, J., Nogueira, P., & Maszczyk, P. (2022). Metagenomic Analysis of the Gastrointestinal Microbiota of Gadus morhua callarias L. Originating from a Chemical Munition Dump Site. Toxics, 10(5), 206. https://doi.org/10.3390/toxics10050206







