Pesticide Exposure and Risk of Rheumatoid Arthritis: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
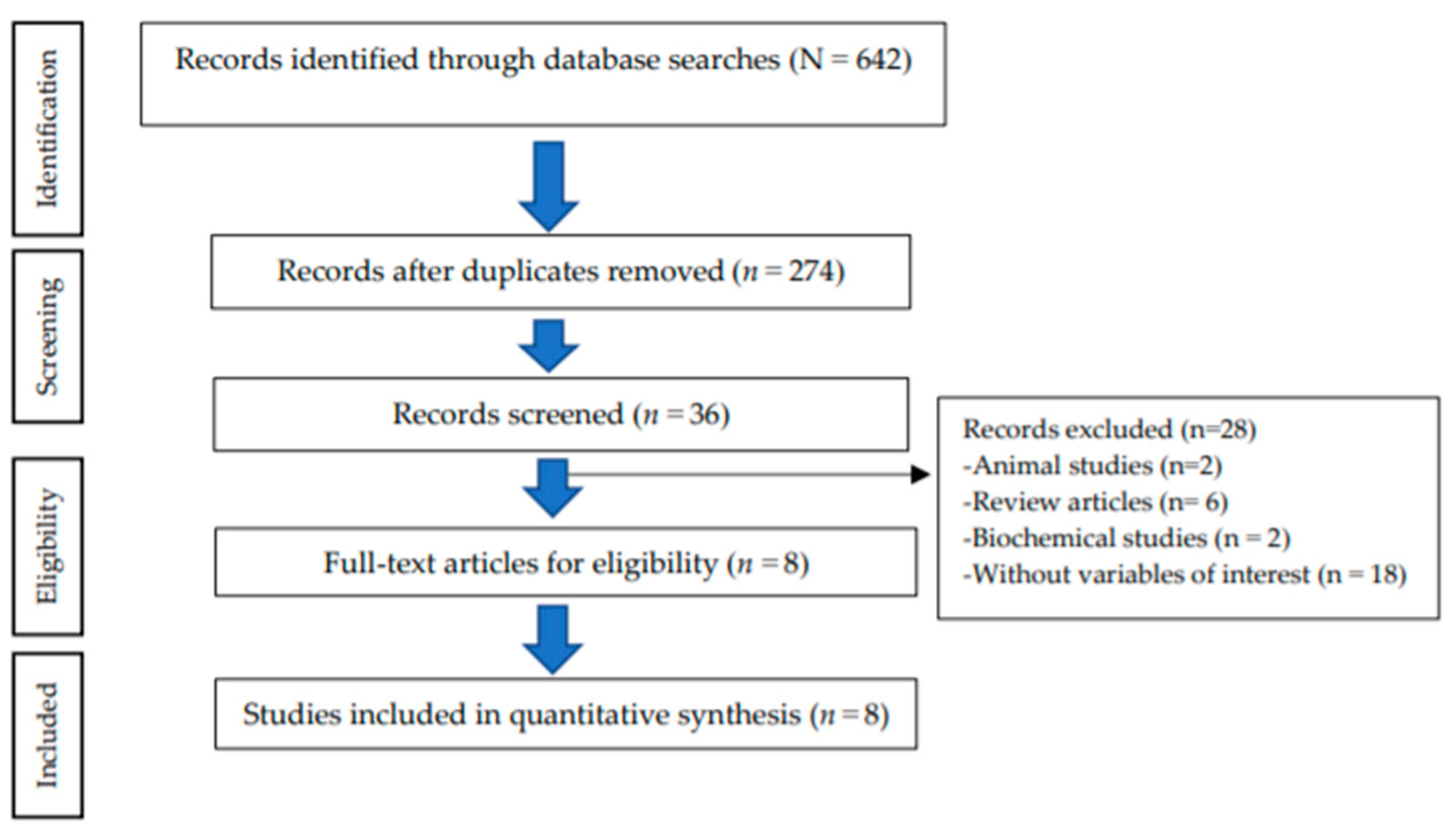
2. Materials and Methods
2.1. Searching Strategy
2.2. Inclusion Criteria
2.3. Data Extraction and Quality Assessment
2.4. Data Analysis
3. Results
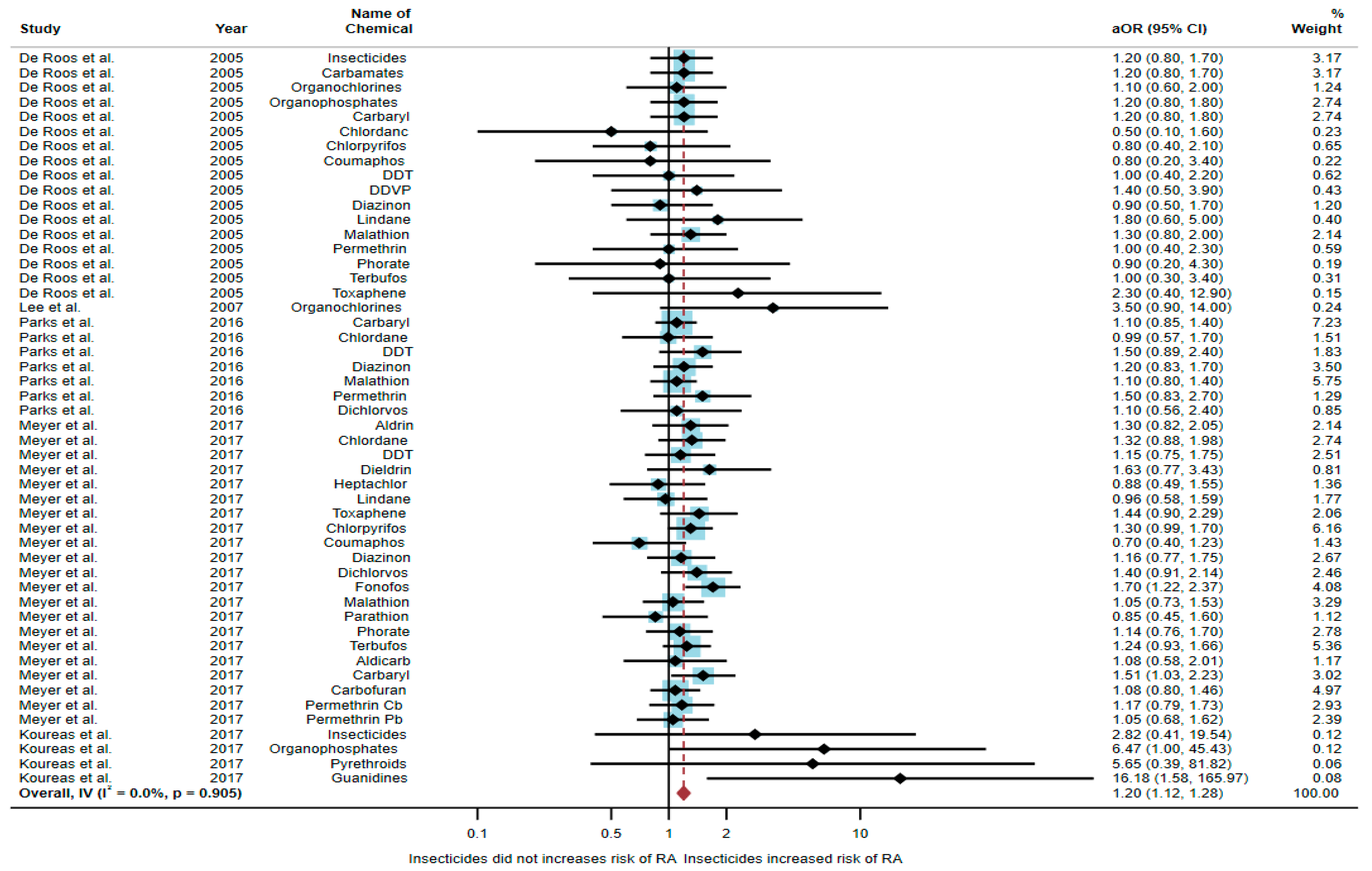
3.1. Association between Exposure to Insecticides and RA Development
3.2. Association between Exposure to Herbicides and RA Development
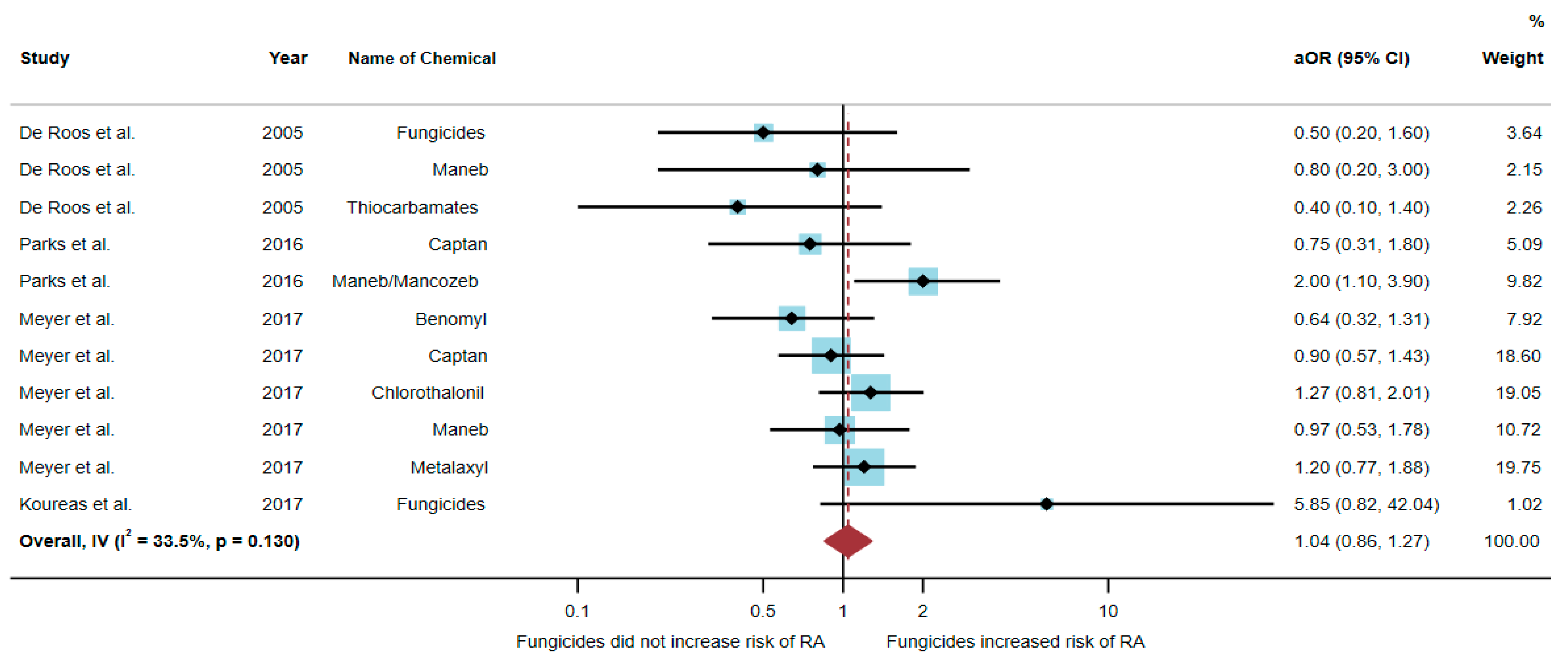
3.3. Association between Exposure to Fungicides and RA Development
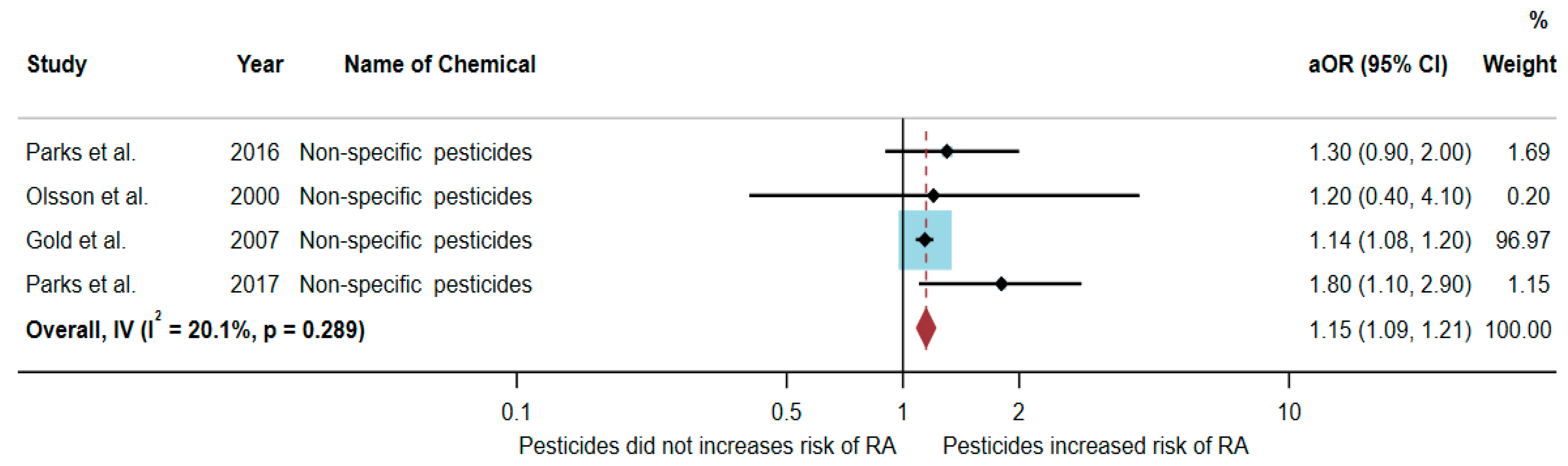
3.4. Association between Exposure to Non-Specific Pesticides and RA Development
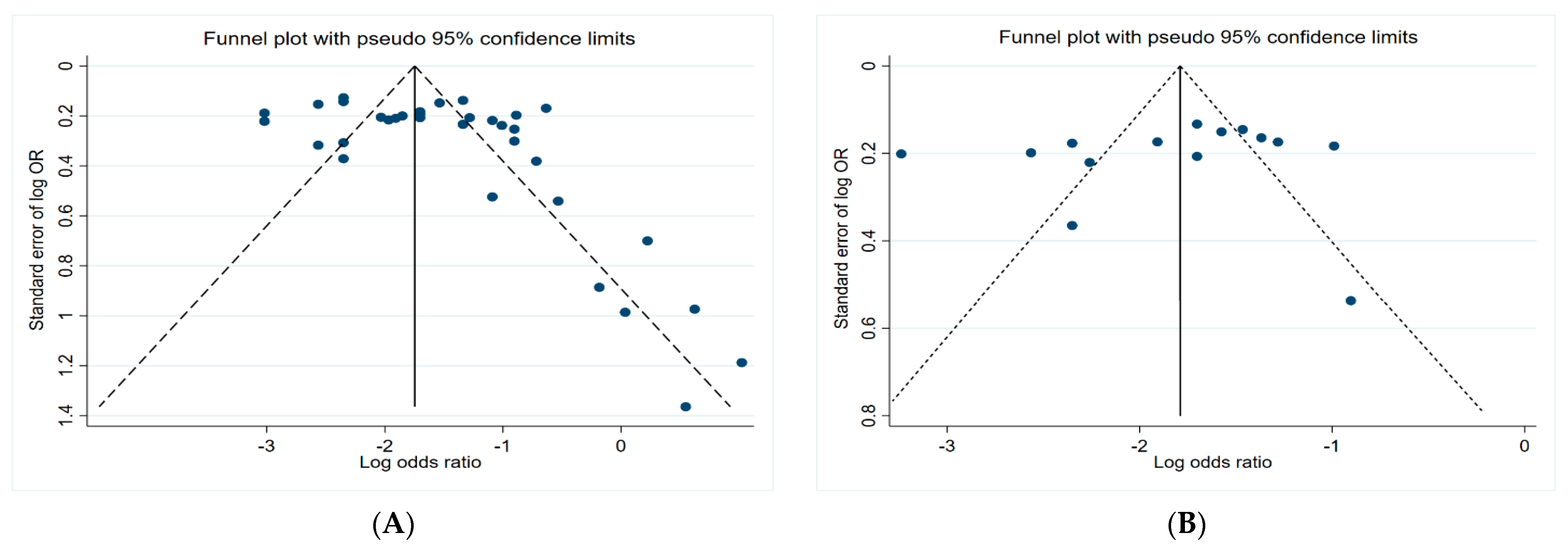
3.5. Funnel Plots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almutairi, K.; Nossent, J.; Preen, D.; Keen, H.; Inderjeeth, C. The global prevalence of rheumatoid arthritis: A meta-analysis based on a systematic review. Rheumatol. Int. 2021, 41, 863–877. [Google Scholar] [CrossRef]
- Choy, E. Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology 2012, 51, 3–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derksen, V.F.A.M.; Huizinga, T.W.J.; van der Woude, D. The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Semin. Immunopathol. 2017, 39, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y. Rheumatoid arthritis. Inflamm. Regen. 2020, 40, 20. [Google Scholar] [CrossRef] [PubMed]
- George, G.; Shyni, G.L.; Raghu, K.G. Current and novel therapeutic targets in the treatment of rheumatoid arthritis. Inflammopharmacology 2020, 28, 1457–1476. [Google Scholar] [CrossRef]
- Meyer, A.; Sandler, D.P.; Freeman, L.E.B.; Hofmann, J.N.; Parks, C.G. Pesticide exposure and risk of rheumatoid arthritis among licensed male pesticide applicators in the Agricultural Health Study. Environ. Health Perspect. 2017, 125, 077010. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.; Sokooti, M.; Galli, C.L.; Moretto, A.; Colosio, C. Pesticide induced immunotoxicity in humans: A comprehensive review of the existing evidence. Toxicology 2013, 307, 123–135. [Google Scholar] [CrossRef]
- Klak, A.; Raciborski, F.; Samel-Kowalik, P. Social implications of rheumatic diseases. Reumatologia 2016, 54, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, P.; Wu, O.; Geue, C.; McIntosh, E.; McInnes, I.B.; Siebert, S. Economic burden of rheumatoid arthritis: A systematic review of literature in biologic era. Ann. Rheum. Dis. 2020, 79, 771–777. [Google Scholar] [CrossRef] [Green Version]
- Boedeker, W.; Watts, M.; Clausing, P.; Marquez, E. The global distribution of acute unintentional pesticide poisoning: Estimations based on a systematic review. BMC Public Health 2020, 20, 1875. [Google Scholar] [CrossRef]
- Akashe, M.M.; Pawade, U.C.; Nikam, A.V. Classification of pesticides: A review. Int. J. Res. Ayurveda Pharm. 2018, 9, 144–150. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Farmers’ exposure to pesticides: Toxicity types and ways of prevention. Toxics 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Gangemi, S.; Miozzi, E.; Teodoro, M.; Briguglio, G.; De Luca, A.; Alibrando, C.; Polito, I.; Libra, M. Occupational exposure to pesticides as a possible risk factor for the development of chronic diseases in humans (Review). Mol. Med. Rep. 2016, 14, 4475–4488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, A.; Ritz, B.; Wesseling, C.; Freeman, L.B. Pesticides and human health. Occup. Environ. Med. 2015, 72, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Chittrakul, J.; Sapbamrer, R.; Sirikul, W. Insecticide exposure and risk of asthmatic symptoms: A systematic review and meta-Analysis. Toxics 2021, 9, 228. [Google Scholar] [CrossRef]
- De Roos, A.J.; Cooper, G.S.; Alavanja, M.C.; Sandler, D.P. Rheumatoid arthritis among women in the Agricultural Health Study: Risk associated with farming activities and exposures. Ann. Epidemiol. 2005, 15, 762–770. [Google Scholar] [CrossRef]
- Lee, D.H.; Steffes, M.; Jacobs, D.R. Positive associations of serum concentration of polychlorinated biphenyls or organochlorine pesticides with self-reported arthritis, especially rheumatoid type, in women. Environ. Health Perspect. 2007, 115, 883–888. [Google Scholar] [CrossRef]
- Guidelines for Reporting Observational Cohort and Cross-Sectional Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 25 December 2021).
- Parks, C.G.; Hoppin, J.A.; De Roos, A.J.; Costenbader, K.H.; Alavanja, M.C.; Sandler, D.P. Rheumatoid arthritis in Agricultural Health Study spouses: Associations with pesticides and other farm exposures. Environ. Health Perspect. 2016, 124, 1728–1734. [Google Scholar] [CrossRef] [Green Version]
- Koureas, M.; Rachiotis, G.; Tsakalof, A.; Hadjichristodoulou, C. Increased frequency of rheumatoid arthritis and allergic rhinitis among pesticide sprayers and associations with pesticide use. Int. J. Environ. Res. Public Health 2017, 14, 865. [Google Scholar] [CrossRef] [Green Version]
- Olsson, A.R.; Skogh, T.; Wingren, G. Occupational determinants for rheumatoid arthritis. Scand. J. Work. Environ. Health 2000, 26, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Gold, L.S.; Ward, M.H.; Dosemeci, M.; De Roos, A.J. Systemic autoimmune disease mortality and occupational exposures. Arthritis Rheum. 2007, 56, 3189–3201. [Google Scholar] [CrossRef] [PubMed]
- Parks, C.G.; Aloisio, A.A.; Sandler, D.P. Childhood residential and agricultural pesticide exposures in relation to adult-onset rheumatoid arthritis in women. Am. J. Epidemiol. 2017, 187, 214–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef]
- Domina, P. Review of toxins associated with autoimmune diseases. ScienceOpen 2021, 1–7. [Google Scholar] [CrossRef]
- Mokarizadeh, A.; Faryabi, M.R.; Rezvanfar, M.A.; Abdollahi, M.A. comprehensive review of pesticides and the immune dysregulation: Mechanisms, evidence and consequences. Toxicol. Mech. Methods 2015, 25, 258–278. [Google Scholar] [CrossRef]
- Dhouib, I.; Jallouli, M.; Annabi, A.; Marzouki, S.; Gharbi, N.; Elfazaa, S.; Lasram, M.M. From immunotoxicity to carcinogenicity: The effects of carbamate pesticides on the immune system. Environ. Sci. Pollut. Res. Int. 2016, 23, 9448–9458. [Google Scholar] [CrossRef]
- Lee, G.H.; Choi, K.C. Adverse effects of pesticides on the functions of immune system. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 235, 108789. [Google Scholar] [CrossRef]
- Goldstein, J.C.; Waterhouse, N.J.; Juin, P.; Evan, G.I.; Green, D.R. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat. Cell Biol. 2000, 2, 156–2162. [Google Scholar] [CrossRef]
- Martin, M.W.; Newcomb, J.; Nunes, J.J.; McGowan, D.C.; Armistead, D.M.; Boucher, C.; Buchanan, J.L.; Buckner, W.; Chai, L.; Elbaum, D.; et al. Novel 2-aminopyrimidine carbamates as potent and orally active inhibitors of Lck: Synthesis, SAR, and in vivo anti-inflammatory activity. J. Med. Chem. 2006, 49, 4981–4991. [Google Scholar] [CrossRef]
- Li, Q.; Hirata, Y.; Piao, S.; Minami, M. The by-products generated during sarin synthesis in the Tokyo sarin disaster induced inhibition of natural killer and cytotoxic T lymphocyte activity. Toxicology 2000, 146, 209–220. [Google Scholar] [CrossRef]
- Li, Q.; Nakadai, A.; Takeda, K.; Kawada, T. Dimethyl 2,2-dichlorovinyl phosphate (DDVP) markedly inhibits activities of natural killer cells, cytotoxic T lymphocytes and lymphokine-activated killer cells via the Fas-ligand/Fas pathway in perforin-knockout (PKO) mice. Toxicology 2004, 204, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Hess, E.V. Environmental chemicals and autoimmune disease: Cause and effect. Toxicology 2002, 181, 65–70. [Google Scholar] [CrossRef]
- Khan, M.F.; Wang, H. Environmental exposures and autoimmune diseases: Contribution of gut microbiome. Front. Immunol. 2020, 10, 3094. [Google Scholar] [CrossRef]
- Salem, I.B.; Boussabbeh, M.; Bacha, H. Dichlorvos-induced toxicity in HCT116 cells: Involvement of oxidative stress and apoptosis. Pestic. Biochem. Physiol. 2015, 119, 62–66. [Google Scholar] [CrossRef]
- Sule, R.O.; Condon, L.; Gomes, A.V. A Common feature of pesticides: Oxidative stress-the role of oxidative stress in pesticide-induced toxicity. Oxid. Med. Cell. Longev. 2022, 2022, 5563759. [Google Scholar] [CrossRef] [PubMed]
- Bossou, Y.M.; Cote, J.; Mantha, M.; Haddad, S.; Achard, S.; Bouchard, M. Impact of pesticide coexposure: An experimental study with binary mixtures of lambda-cyhalothrin (LCT) and captan and its impact on the toxicokinetics of LCT biomarkers of exposure. Arch. Toxicol. 2020, 94, 3045–3058. [Google Scholar] [CrossRef] [PubMed]
- Timchalk, C.; Poet, T.S.; Hinman, M.N.; Busby, A.L.; Kousba, A.A. Pharmacokinetic and pharmacodynamic interaction for a binary mixture of chlorpyrifos and diazinon in the rat. Toxicol. Appl. Pharmacol. 2005, 205, 31–42. [Google Scholar] [CrossRef]


| Authors (Years)/Country | Study Design | Gender | Sample Size | Name of Chemicals | aOR (95% CI) | Confounding Variables |
|---|---|---|---|---|---|---|
| De Roos et al. (2005)/USA [17] | Case–control | Female | 810 | Insecticides Carbamates Organochlorines Organophosphates Carbaryl Chlordane Chlorpyrifos Coumaphos DDT DDVP Diazinon Lindane Malathion Permethrin Phorate Terbufos Toxaphene | 1.2 (0.8–1.7) 1.2 (0.8–1.7) 1.1 (0.6–2.0) 1.2 (0.8–1.8) 1.2 (0.8–1.8) 0.5 (0.1–1.6) 0.8 (0.4–2.1) 0.8 (0.2–3.4) 1.0 (0.4–2.2) 1.4 (0.5–3.9) 0.9 (0.5–1.7) 1.8 (0.6–5.0) 1.3 (0.8–2.0) 1.0 (0.4–2.3) 0.9 (0.2–4.3) 1.0 (0.3–3.4) 2.3 (0.4–12.9) | Birth date, and state |
| Lee et al. (2007)/ Norway [18] | Cross-sectional | Both genders | 1721 | Organochlorines | 3.5 (0.9–14.0) | Age, race, income status, BMI, and cigarette smoking |
| Parks et al. (2016)/USA [20] | Cohort | Female | 23,841 | Carbaryl Chlordane DDT Diazinon Malathion Permethrin Dichlorvos | 1.1 (0.85–1.4) 0.99 (0.57–1.7) 1.5 (0.89–2.4) 1.2 (0.83–1.7) 1.1 (0.80–1.4) 1.5 (0.83–2.7) 1.1 (0.56–2.4) | Age, state, and smoking pack-years |
| Meyer et al. (2017)/USA [6] | Case–control | Male | 26,354 | Aldrin Chlordane DDT Dieldrin Heptachlor Lindane Toxaphene Chlorpyrifos Coumaphos Diazinon Dichlorvos Fonofos Malathion Parathion Phorate Terbufos Aldicarb Carbaryl Carbofuran Permethrin a Permethrin b | 1.30 (0.82–2.05) 1.32 (0.88–1.98) 115 (0.75–1.75) 1.63 (0.77–3.43) 0.88 (0.49–1.55) 0.96 (0.58–1.59) 1.44 (0.90–2.29) 1.30 (0.99–1.70) 0.70 (0.40–1.23) 1.16 (0.77–1.75) 1.40 (0.91–2.14) 1.70 (1.22–2.37) 1.05 (0.73–1.53) 0.85 (0.45–1.60) 1.14 (0.76–1.70) 1.24 (0.93–1.66) 1.08 (0.58–2.01) 1.51 (1.03–2.23) 1.08 (0.80–1.46) 1.17 (0.79–1.73) 1.05 (0.68–1.62) | Age, state of enrollment, pack-years smoking, and education level |
| Koureas et al. (2017)/Greece [21] | Cross- sectional | Male | 170 | Insecticides Organophosphates Pyrethroids Guanidines | 2.82 (0.41–19.54) 6.47 (1.00–45.43) 5.65 (0.39–81.82) 16.18 (1.58–165.97) | Age, smoker, alcohol consumption, and use of a tractor on a farm |
| Authors (Years)/Country | Study Design | Gender | Sample Size | Name of Chemicals | aOR (95% CI) | Confounding Variables |
|---|---|---|---|---|---|---|
| De Roos et al. (2005)/USA [17] | Case–control | Female | 810 | Herbicides Phenoxyacetic acids Triazines 2,4-D Alachlor Atrazine Cyanazine Glyphosate Imazethapyr Metolachlor | 1.1 (0.8–1.6) 0.5 (0.3–0.9) 0.5 (0.2–1.3) 0.5 (0.3–0.9) 0.5 (0.1–1.6) 0.5 (0.2–1.6) 0.8 (0.2–3.4) 1.2 (0.8–1.8) 1.5 (0.5–4.1) 0.4 (0.1–1.7) | Birth date and state |
| Parks et al. (2016)/USA [20] | Cohort | Female | 23,841 | 2,4-D Atrazine Glyphosate Imazethapyr Trifluralin Dicamba | 0.75 (0.51–1.1) 0.65 (0.32–1.3) 1.2 (0.95–1.6) 1.1 (0.55–2.3) 0.57 (0.28–1.1) 0.68 (0.32–1.5) | Age, state, and pack-years smoking |
| Meyer et al. (2017)/USA [6] | Case–control | Male | 26,354 | 2,4-D 2,4,5-T 2,4,5-TP Alachlor Atrazine Butylate Chlorimuron ethyl Cyanazine Dicamba EPTC Glyphosate Imazethapyr Metolachlor Metribuzin Paraquat Pendimethalin Trifluralin | 1.16 (0.83–1.64) 1.11 (0.72–1.71) 1.00 (0.46–2.15) 1.26 (0.95–1.68) 1.29 (0.94–1.79) 1.04 (0.70–1.54) 1.45 (1.01–2.07) 0.96 (0.69–1.31) 0.90 (0.65–1.25) 0.62 (0.40–0.96) 0.90 (0.65–1.24) 1.32 (0.94–1.86) 0.90 (0.67–1.20) 1.08 (0.73–1.59) 0.92 (0.57–1.49) 0.98 (0.69–1.40) 1.23 (0.92–1.66) | Age, state of enrollment, pack-years smoking, and education level |
| Koureas et al. (2017)/Greece [21] | Cross- sectional | Male | 170 | Herbicides Paraquat | 3.51 (0.45–27.20) 0.69 (0.09–5.03) | Age, smoker, alcohol consumption, and use of a tractor on a farm |
| Authors (Years)/Country | Study Design | Gender | Sample Size | Name of Chemicals | aOR (95% CI) | Confounding Variables |
|---|---|---|---|---|---|---|
| De Roos et al. (2005)/USA [17] | Case–control | Female | 810 | Fungicides Maneb Thiocarbamates | 0.5 (0.2–1.6) 0.8 (0.2–3.0) 0.4 (0.1–1.4) | Birth date and state |
| Parks et al. (2016)/USA [20] | Cohort | Female | 23,841 | Captan Maneb/Mancozeb | 0.75 (0.31–1.8) 2.0 (1.1–3.9) | Age, state, and pack-years smoking |
| Meyer et al. (2017)/USA [6] | Case–control | Male | 26,354 | Benomyl Captan Chlorothalonil Maneb Metalaxyl | 0.64 (0.32–1.31) 0.90 (0.57–1.43) 1.27 (0.81–2.01) 0.97 (0.53–1.78) 1.20 (0.77–1.88) | Age, state of enrollment, pack-years smoking, and education level |
| Koureas et al. (2017)/Greece [21] | Cross-sectional | Male | 170 | Fungicides | 5.85 (0.82–42.04) | Age, smoker, alcohol consumption, and use of a tractor on a farm |
| Authors (Years)/ Country | Study Design | Gender | Sample Size | Adjusted OR (95% CI) | Confounding Variables |
|---|---|---|---|---|---|
| Parks et al. (2016)/USA [20] | Cohort | Female | 23,841 | 1.3 (0.9–2.0) | Age, state, and pack-years smoking |
| Olsson et al. (2000)/Sweden [22] | Case–control | Male | 350 | 1.2 (0.4–4.1) | Age, smoking, and occupation |
| Gold et al. (2007)/USA [23] | Case–control | Both genders | 296,362 | 1.14 (1.08–1.20) | Age, sex, race, region, and socioeconomic status |
| Parks et al. (2017)/USA [24] | Cohort | Female | 49,343 | 1.8 (1.1–2.9) | Age, race, education level, packyears of smoking, and childhood socioeconomic status |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chittrakul, J.; Sapbamrer, R.; Sirikul, W. Pesticide Exposure and Risk of Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Toxics 2022, 10, 207. https://doi.org/10.3390/toxics10050207
Chittrakul J, Sapbamrer R, Sirikul W. Pesticide Exposure and Risk of Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Toxics. 2022; 10(5):207. https://doi.org/10.3390/toxics10050207
Chicago/Turabian StyleChittrakul, Jiraporn, Ratana Sapbamrer, and Wachiranun Sirikul. 2022. "Pesticide Exposure and Risk of Rheumatoid Arthritis: A Systematic Review and Meta-Analysis" Toxics 10, no. 5: 207. https://doi.org/10.3390/toxics10050207
APA StyleChittrakul, J., Sapbamrer, R., & Sirikul, W. (2022). Pesticide Exposure and Risk of Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Toxics, 10(5), 207. https://doi.org/10.3390/toxics10050207







