Chronic Perigestational Exposure to Chlorpyrifos Induces Perturbations in Gut Bacteria and Glucose and Lipid Markers in Female Rats and Their Offspring
Abstract
1. Introduction
2. Materials and Methods
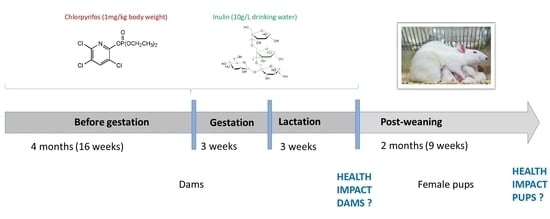
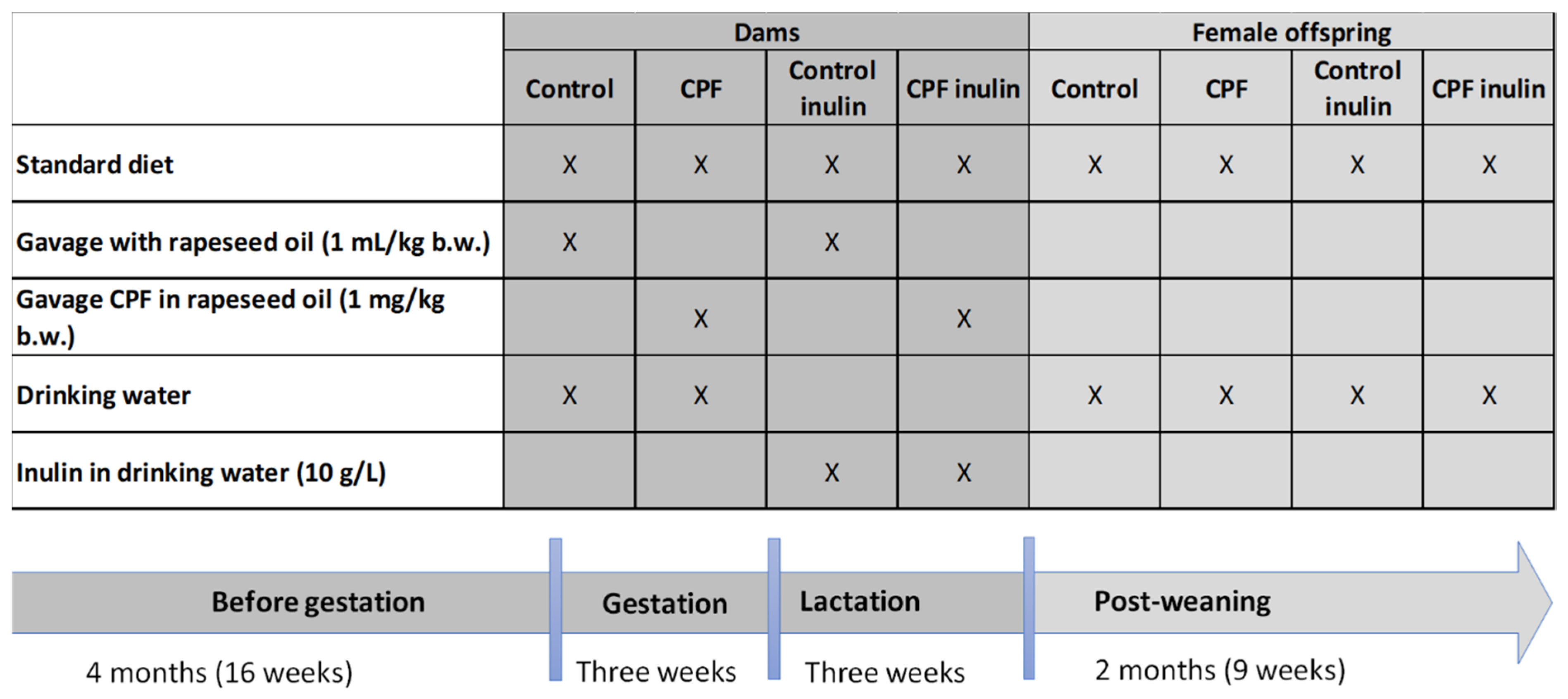
2.1. Experimental Conditions
2.2. Metabolic Perturbations
2.3. Disruption of Key Bacteria
2.3.1. Concentrations of Selected Intestinal Bacteria and Bacterial Translocation
2.3.2. Microbial Metabolites
2.3.3. Serum Lipopolysaccharide (LPS)
2.4. Statistical Analyses
3. Results
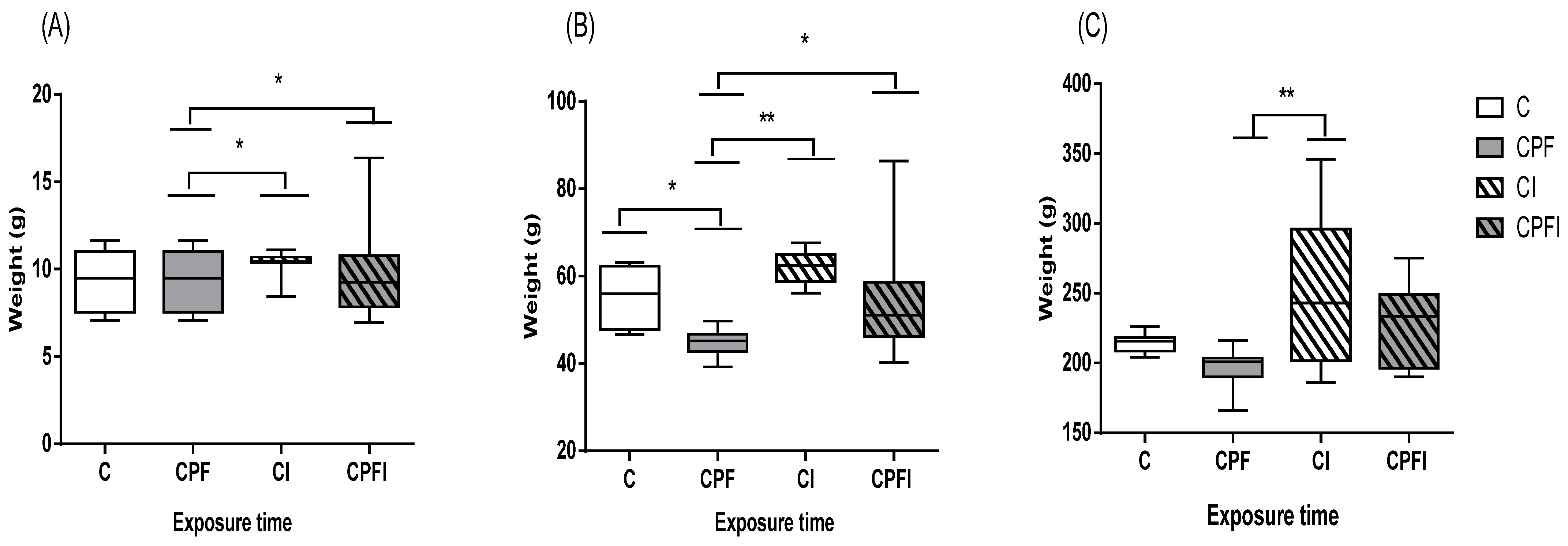
3.1. Animal Weight and Weight Gain
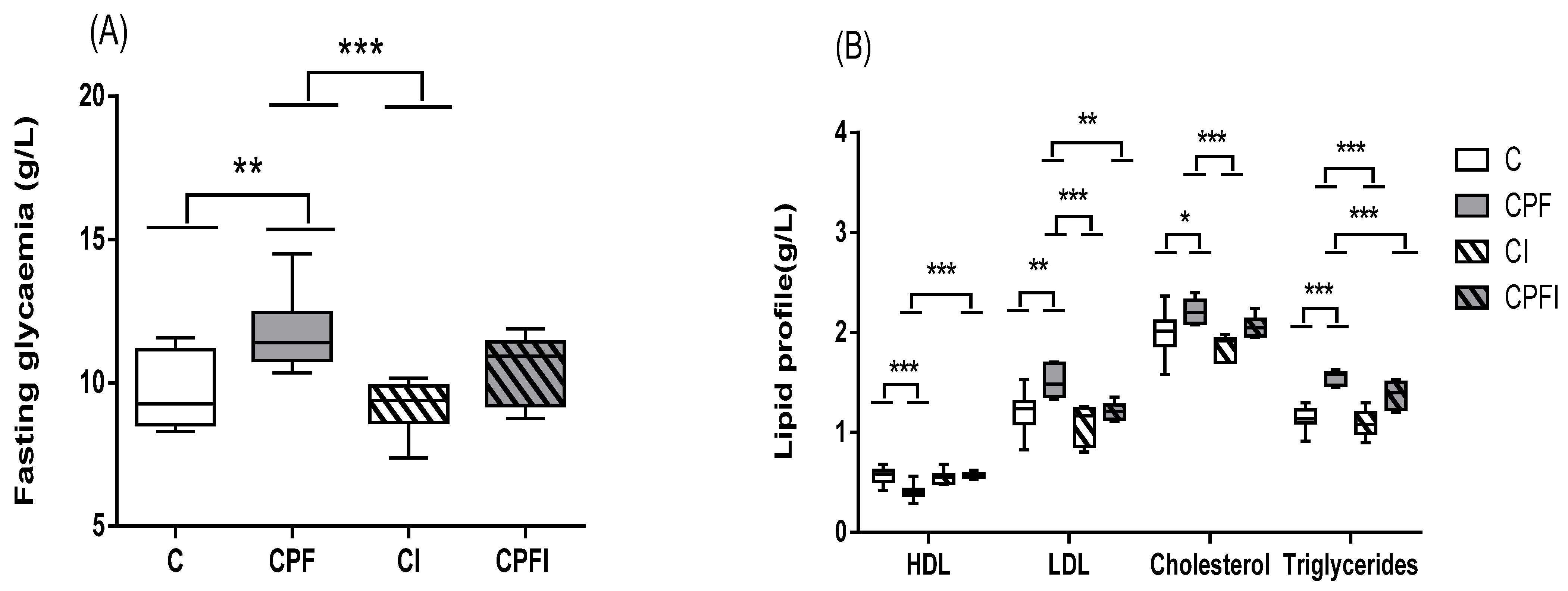
3.2. Metabolic Perturbations
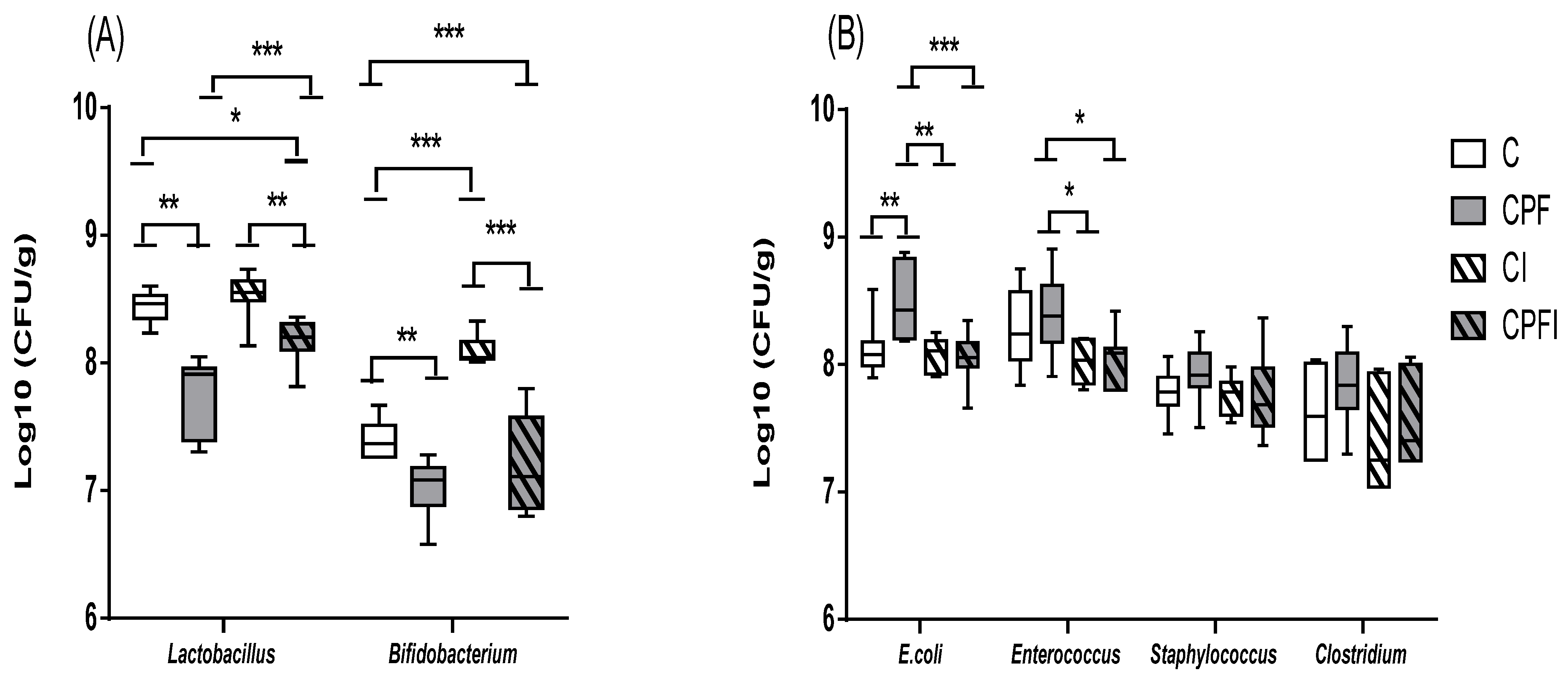
3.3. Disturbances of the Selected Intestinal Bacteria
3.3.1. Concentrations of Selected Intestinal Bacteria
3.3.2. Bacterial Metabolites
3.3.3. Bacterial Translocation
4. Discussion
4.1. Animal Weight and Weight Gain
4.2. Metabolic Perturbations
4.3. Disturbances of the Selected Intestinal Bacteria
4.4. Nutritional Prevention
4.5. Perigestational Modulation of CPF
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| B.W. | body weight |
| C | Control |
| ChE | choline esterase |
| CI | control + inulin |
| CNS | central nervous system |
| CPF | chlorpyrifos |
| CPFI | CPF + inulin |
| CRP | C-reactive protein |
| DETP | diethyl thiophosphonate |
| DOHaD | Developmental origin of Health and Disease |
| EPA | United States Environmental Protection Agency |
| GC | gas chromatography |
| GLP1 | glucose-like protein-1 |
| HDL | high-density lipoprotein |
| IGF1 | insulin growth factor-1 |
| IL6 | interleukin-6 |
| LDL | low-density lipoprotein |
| LPS | lipopolysaccharide |
| NOAEL | no observed adverse effect level |
| OP | organophosphate |
| PND | post-natal day |
| PNSE | National Health and Environment Plans |
| SCFA | short chain fatty acid |
| SEM | standard error of the mean |
| T2D | type 2 diabetes |
| TCPy | 3,5,6-trichloro-2-pyridinol |
| TG | triglyceride |
| WHO | World Health Organization |
References
- Wang, X.; Xing, H.; Li, X.; Xu, S.; Wang, X. Effects of atrazine and chlorpyrifos on the mRNA levels of IL-1 and IFN-γ2b in immune organs of common carp. Fish. Shellfish Immunol. 2011, 31, 126–133. [Google Scholar] [CrossRef] [PubMed]
- OCSPP. Opp Pesticides Industry Sales and Usage 2006 and 2007 Market Estimates; OCSPP: Arlington, TX, USA, 2015. [Google Scholar]
- World Health Organization; Safety International Programme on Chemical. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2009; World Health Organization: Geneva, Switzerland, 2010; ISBN 978-92-4-154796-3.
- Solomon, K.R.; Williams, W.M.; Mackay, D.; Purdy, J.; Giddings, J.M.; Giesy, J.P. Properties and uses of chlorpyrifos in the United States. Rev. Environ. Contam. Toxicol. 2014, 231, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Sales of Pesticides in the EU. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20200603-1 (accessed on 23 September 2021).
- Choudhary, S.; Yamini, N.R.; Yadav, S.K.; Kamboj, M.L.; Sharma, A. A review: Pesticide residue: Cause of many animal health problems. J. Entomol. Zool. Stud. 2018, 6, 330–333. [Google Scholar]
- Guichard, L.; Dedieu, F.; Jeuffroy, M.-H.; Meynard, J.M.; Reau, R.; Savini, I. Le plan Ecophyto de réduction d’usage des pesticides en France: Décryptage d’un échec et raisons d’espérer. Cah. Agric. 2017, 26, 1–12. [Google Scholar] [CrossRef]
- Renaudie, O. Les plans nationaux santé-environnement: Conciliation ou concurrence entre deux politiques publiques? Rev. Droit Sanit. Soc. 2019, HS, 23–35. [Google Scholar]
- Combarnous, Y. Endocrine Disruptor Compounds (EDCs) and agriculture: The case of pesticides. Comptes Rendus Biol. 2017, 340, 406–409. [Google Scholar] [CrossRef]
- Jepson, P.C.; Murray, K.; Bach, O.; Bonilla, M.; Neumeister, L. A Global Guideline for Pesticide Selection to Reduce Risks, and Establish a Minimum Pesticides List; Social Science Research Network: Rochester, NY, USA, 2019. [Google Scholar]
- Alamgir Zaman Chowdhury, M.; Fakhruddin, A.N.M.; Nazrul Islam, M.; Moniruzzaman, M.; Gan, S.H.; Khorshed Alam, M. Detection of the residues of nineteen pesticides in fresh vegetable samples using gas chromatography–mass spectrometry. Food Control 2013, 34, 457–465. [Google Scholar] [CrossRef]
- Carr, R.L.; Ho, L.L.; Chambers, J.E. Selective toxicity of chlorpyrifos to several species of fish during an environmental exposure: Biochemical mechanisms. Environ. Toxicol. Chem. 1997, 16, 2369–2374. [Google Scholar] [CrossRef]
- Casida, J.E.; Quistad, G.B. Organophosphate toxicology: Safety aspects of nonacetylcholinesterase secondary targets. Chem. Res. Toxicol. 2004, 17, 983–998. [Google Scholar] [CrossRef]
- He, M.-J.; Lu, J.-F.; Wang, J.; Wei, S.-Q.; Hageman, K.J. Phthalate esters in biota, air and water in an agricultural area of western China, with emphasis on bioaccumulation and human exposure. Sci. Total Environ. 2020, 698, 134264. [Google Scholar] [CrossRef]
- Grewal, A.S.; Grewal, A.S.; Singla, A.; Kamboj, P.; Dua, J.S.; Internationals, O. Pesticide Residues in Food Grains, Vegetables and Fruits: A Hazard to Human Health. J. Med. Chem. Toxicol. 2017, 2, 40–46. [Google Scholar] [CrossRef]
- Chishti, Z.; Hussain, S.; Arshad, K.R.; Khalid, A.; Arshad, M. Microbial degradation of chlorpyrifos in liquid media and soil. J. Environ. Manag. 2013, 114, 372–380. [Google Scholar] [CrossRef]
- Das, S.; Adhya, T.K. Degradation of chlorpyrifos in tropical rice soils. J. Environ. Manag. 2015, 152, 36–42. [Google Scholar] [CrossRef]
- Kumar, A.; Correll, R.; Grocke, S.; Bajet, C. Toxicity of selected pesticides to freshwater shrimp, Paratya australiensis (Decapoda: Atyidae): Use of time series acute toxicity data to predict chronic lethality. Ecotoxicol. Environ. Saf. 2010, 73, 360–369. [Google Scholar] [CrossRef]
- Li, D.; Huang, Q.; Lu, M.; Zhang, L.; Yang, Z.; Zong, M.; Tao, L. The organophosphate insecticide chlorpyrifos confers its genotoxic effects by inducing DNA damage and cell apoptosis. Chemosphere 2015, 135, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Hites, R.A. The Rise and Fall of Chlorpyrifos in the United States. Environ. Sci. Technol. 2021, 55, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Ur Rahman, S.; Xuebin, Q.; Yasin, G.; Cheng, H.; Mehmood, F.; Zain, M.; Shehzad, M.; Ahmad, M.I.; Riaz, L.; Rahim, A.; et al. Role of silicon on root morphological characters of wheat (Triticum aestivum L.) plants grown under Cd-contaminated nutrient solution. Acta Physiol. Plant 2021, 43, 60. [Google Scholar] [CrossRef]
- Olisah, C.; Okoh, O.O.; Okoh, A.I. Occurrence of organochlorine pesticide residues in biological and environmental matrices in Africa: A two-decade review. Heliyon 2020, 6, e03518. [Google Scholar] [CrossRef]
- Mulder, T.A.; van den Dries, M.A.; Korevaar, T.I.M.; Ferguson, K.K.; Peeters, R.P.; Tiemeier, H. Organophosphate pesticides exposure in pregnant women and maternal and cord blood thyroid hormone concentrations. Environ. Int. 2019, 132, 105124. [Google Scholar] [CrossRef]
- Araki, A.; Miyashita, C.; Mitsui, T.; Goudarzi, H.; Mizutani, F.; Chisaki, Y.; Itoh, S.; Sasaki, S.; Cho, K.; Moriya, K.; et al. Prenatal organochlorine pesticide exposure and the disruption of steroids and reproductive hormones in cord blood: The Hokkaido study. Environ. Int. 2018, 110, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, E.; Oltramare, C.; Nfon Dibié, J.-J.; Konaté, Y.; de Alencastro, L.F. Assessment of human exposure to pesticides by hair analysis: The case of vegetable-producing areas in Burkina Faso. Environ. Int. 2018, 111, 317–331. [Google Scholar] [CrossRef]
- Iglesias-González, A.; Hardy, E.M.; Appenzeller, B.M.R. Cumulative exposure to organic pollutants of French children assessed by hair analysis. Environ. Int. 2020, 134, 105332. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Revised Human Health Risk Assessment on Chlorpyrifos. Available online: https://www.epa.gov/ingredients-used-pesticide-products/revised-human-health-risk-assessment-chlorpyrifos (accessed on 28 June 2021).
- Perez-Fernandez, C.; Morales-Navas, M.; Aguilera-Sáez, L.M.; Abreu, A.C.; Guardia-Escote, L.; Fernández, I.; Garrido-Cárdenas, J.A.; Colomina, M.T.; Giménez, E.; Sánchez-Santed, F. Medium and long-term effects of low doses of Chlorpyrifos during the postnatal, preweaning developmental stage on sociability, dominance, gut microbiota and plasma metabolites. Environ. Res. 2020, 184, 109341. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-P.; Liang, Y.-J.; Long, D.-X.; Chen, J.-X.; Hou, W.-Y.; Wu, Y.-J. Metabolic profiles of serum from rats after subchronic exposure to chlorpyrifos and carbaryl. Chem. Res. Toxicol. 2009, 22, 1026–1033. [Google Scholar] [CrossRef]
- Xu, M.-Y.; Sun, Y.-J.; Wang, P.; Xu, H.-Y.; Chen, L.-P.; Zhu, L.; Wu, Y.-J. Metabolomics analysis and biomarker identification for brains of rats exposed subchronically to the mixtures of low-dose cadmium and chlorpyrifos. Chem. Res. Toxicol. 2015, 28, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Lassiter, T.L.; Brimijoin, S. Rats gain excess weight after developmental exposure to the organophosphorothionate pesticide, chlorpyrifos. Neurotoxicol. Teratol. 2008, 30, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Slotkin Theodore, A.; Brown Kathleen, K.; Seidler Frederic, J. Developmental Exposure of Rats to Chlorpyrifos Elicits Sex-Selective Hyperlipidemia and Hyperinsulinemia in Adulthood. Environ. Health Perspect. 2005, 113, 1291–1294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lasram, M.M.; Dhouib, I.B.; Annabi, A.; El Fazaa, S.; Gharbi, N. A review on the molecular mechanisms involved in insulin resistance induced by organophosphorus pesticides. Toxicology 2014, 322, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Peris-Sampedro, F.; Salazar, J.G.; Cabré, M.; Reverte, I.; Domingo, J.L.; Sánchez-Santed, F.; Colomina, M.T. Impaired retention in AβPP Swedish mice six months after oral exposure to chlorpyrifos. Food Chem. Toxicol. 2014, 72, 289–294. [Google Scholar] [CrossRef]
- Fang, B.; Li, J.W.; Zhang, M.; Ren, F.Z.; Pang, G.F. Chronic chlorpyrifos exposure elicits diet-specific effects on metabolism and the gut microbiome in rats. Food Chem. Toxicol. 2018, 111, 144–152. [Google Scholar] [CrossRef]
- Li, J.; Ren, F.; Li, Y.; Luo, J.; Pang, G. Chlorpyrifos Induces Metabolic Disruption by Altering Levels of Reproductive Hormones. J. Agric. Food Chem. 2019, 67, 10553–10562. [Google Scholar] [CrossRef]
- Peris-Sampedro, F.; Cabré, M.; Basaure, P.; Reverte, I.; Domingo, J.L.; Teresa Colomina, M. Adulthood dietary exposure to a common pesticide leads to an obese-like phenotype and a diabetic profile in apoE3 mice. Environ. Res. 2015, 142, 169–176. [Google Scholar] [CrossRef]
- Reygner, J.; Lichtenberger, L.; Elmhiri, G.; Dou, S.; Bahi-Jaber, N.; Rhazi, L.; Depeint, F.; Bach, V.; Khorsi-Cauet, H.; Abdennebi-Najar, L. Inulin Supplementation Lowered the Metabolic Defects of Prolonged Exposure to Chlorpyrifos from Gestation to Young Adult Stage in Offspring Rats. PLoS ONE 2016, 11, e0164614. [Google Scholar] [CrossRef]
- Goel, A.; Dani, V.; Dhawan, D.K. Protective effects of zinc on lipid peroxidation, antioxidant enzymes and hepatic histoarchitecture in chlorpyrifos-induced toxicity. Chem. Biol. Interact. 2005, 156, 131–140. [Google Scholar] [CrossRef]
- Meggs, W.J.; Brewer, K.L. Weight gain associated with chronic exposure to chlorpyrifos in rats. J. Med. Toxicol. 2007, 3, 89–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uchendu, C.; Ambali, S.F.; Ayo, J.O.; Esievo, K.A.N. Body weight and hematological changes induced by chronic exposure to low levels of chlorpyrifos and deltamethrin combination in rats: The effect of alpha-lipoic acid. Comp. Clin. Pathol. 2018, 27, 1383–1388. [Google Scholar] [CrossRef]
- Whyatt, R.M.; Rauh, V.; Barr, D.B.; Camann, D.E.; Andrews, H.F.; Garfinkel, R.; Hoepner, L.A.; Diaz, D.; Dietrich, J.; Reyes, A.; et al. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ. Health Perspect. 2004, 112, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.J.; Shenoy, S.S. Intestinal permeability of chlorpyrifos using the single-pass intestinal perfusion method in the rat. Toxicology 2003, 184, 125–133. [Google Scholar] [CrossRef]
- Gao, J.; Naughton, S.X.; Beck, W.D.; Hernandez, C.M.; Wu, G.; Wei, Z.; Yang, X.; Bartlett, M.G.; Terry, A.V. Chlorpyrifos and chlorpyrifos oxon impair the transport of membrane bound organelles in rat cortical axons. NeuroToxicology 2017, 62, 111–123. [Google Scholar] [CrossRef]
- Joly Condette, C.; Khorsi-Cauet, H.; Morlière, P.; Zabijak, L.; Reygner, J.; Bach, V.; Gay-Quéheillard, J. Increased Gut Permeability and Bacterial Translocation after Chronic Chlorpyrifos Exposure in Rats. PLoS ONE 2014, 9, e102217. [Google Scholar] [CrossRef]
- Velmurugan, G.; Ramprasath, T.; Gilles, M.; Swaminathan, K.; Ramasamy, S. Gut Microbiota, Endocrine-Disrupting Chemicals, and the Diabetes Epidemic. Trends Endocrinol. Metab. 2017, 28, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Cani, P.D.; Everard, A.; Duparc, T. Gut microbiota, enteroendocrine functions and metabolism. Curr. Opin. Pharmacol. 2013, 13, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Tomas, J.; Reygner, J.; Mayeur, C.; Ducroc, R.; Bouet, S.; Bridonneau, C.; Cavin, J.-B.; Thomas, M.; Langella, P.; Cherbuy, C. Early colonizing Escherichia coli elicits remodeling of rat colonic epithelium shifting toward a new homeostatic state. ISME J. 2015, 9, 46–58. [Google Scholar] [CrossRef]
- Condette, C.J.; Bach, V.; Mayeur, C.; Gay-Quéheillard, J.; Khorsi-Cauet, H. Chlorpyrifos Exposure During Perinatal Period Affects Intestinal Microbiota Associated With Delay of Maturation of Digestive Tract in Rats. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 30–40. [Google Scholar] [CrossRef]
- Condette, C.J.; Gay-Quéheillard, J.; Léké, A.; Chardon, K.; Delanaud, S.; Bach, V.; Khorsi-Cauet, H. Impact of chronic exposure to low doses of chlorpyrifos on the intestinal microbiota in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME) and in the rat. Environ. Sci. Pollut. Res. Int. 2013, 20, 2726–2734. [Google Scholar] [CrossRef]
- Reygner, J.; Joly Condette, C.; Bruneau, A.; Delanaud, S.; Rhazi, L.; Depeint, F.; Abdennebi-Najar, L.; Bach, V.; Mayeur, C.; Khorsi-Cauet, H. Changes in Composition and Function of Human Intestinal Microbiota Exposed to Chlorpyrifos in Oil as Assessed by the SHIME® Model. Int. J. Environ. Res. Public Health 2016, 13, 1088. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Jin, C.; Pan, Z.; Sun, L.; Fu, Z.; Jin, Y. Chronic exposure to low concentrations of lead induces metabolic disorder and dysbiosis of the gut microbiota in mice. Sci. Total Environ. 2018, 631–632, 439–448. [Google Scholar] [CrossRef]
- Réquilé, M.; Gonzàlez Alvarez, D.O.; Delanaud, S.; Rhazi, L.; Bach, V.; Depeint, F.; Khorsi-Cauet, H. Use of a combination of in vitro models to investigate the impact of chlorpyrifos and inulin on the intestinal microbiota and the permeability of the intestinal mucosa. Environ. Sci. Pollut. Res. 2018, 25, 22529–22540. [Google Scholar] [CrossRef]
- Tirelli, V.; Catone, T.; Turco, L.; Di Consiglio, E.; Testai, E.; De Angelis, I. Effects of the pesticide clorpyrifos on an in vitro model of intestinal barrier. Toxicol. Vitr. 2007, 21, 308–313. [Google Scholar] [CrossRef]
- Steffen, E.K.; Berg, R.D. Relationship between cecal population levels of indigenous bacteria and translocation to the mesenteric lymph nodes. Infect. Immun. 1983, 39, 1252–1259. [Google Scholar] [CrossRef]
- Sarron, E.; Pérot, M.; Barbezier, N.; Delayre-Orthez, C.; Gay-Quéheillard, J.; Anton, P.M. Early exposure to food contaminants reshapes maturation of the human brain-gut-microbiota axis. World J. Gastroenterol. 2020, 26, 3145. [Google Scholar] [CrossRef] [PubMed]
- Djekkoun, N.; Lalau, J.-D.; Bach, V.; Depeint, F.; Khorsi-Cauet, H. Chronic oral exposure to pesticides and their consequences on metabolic regulation: Role of the microbiota. Eur. J. Nutr. 2021, 60, 4131–4149. [Google Scholar] [CrossRef]
- Cani, P.D.; Joly, E.; Horsmans, Y.; Delzenne, N.M. Oligofructose promotes satiety in healthy human: A pilot study. Eur. J. Clin. Nutr. 2006, 60, 567–572. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Holle, A.V.; François, P.; de Vos Willem, M.; et al. Responses of Gut Microbiota and Glucose and Lipid Metabolism to Prebiotics in Genetic Obese and Diet-Induced Leptin-Resistant Mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef]
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef]
- Geurts, L.; Neyrinck, A.M.; Delzenne, N.M.; Knauf, C.; Cani, P.D. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: Novel insights into molecular targets and interventions using prebiotics. Benef. Microbes. 2014, 5, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Cochran, R.C.; Kishiyama, J.; Aldous, C.; Carr, W.C.; Pfeifer, K.F. Chlorpyrifos: Hazard assessment based on a review of the effects of short-term and long-term exposure in animals and humans. Food Chem. Toxicol. 1995, 33, 165–172. [Google Scholar] [CrossRef]
- Guibourdenche, M.; El Khayat El Sabbouri, H.; Bonnet, F.; Djekkoun, N.; Khorsi-Cauet, H.; Corona, A.; Guibourdenche, J.; Bach, V.; Anton, P.M.; Gay-Quéheillard, J. Perinatal exposure to chlorpyrifos and/or a high-fat diet is associated with liver damage in male rat offspring. Cells Dev. 2021, 166, 203678. [Google Scholar] [CrossRef] [PubMed]
- El Khayat El Sabbouri, H.; Gay-Quéheillard, J.; Joumaa, W.H.; Delanaud, S.; Guibourdenche, M.; Darwiche, W.; Djekkoun, N.; Bach, V.; Ramadan, W. Does the perigestational exposure to chlorpyrifos and/or high-fat diet affect respiratory parameters and diaphragmatic muscle contractility in young rats? Food Chem. Toxicol. 2020, 140, 111322. [Google Scholar] [CrossRef]
- American Society of Microbiology. Manual of Clinical Microbiology, 10th ed.; American Society of Microbiology: Washington, DC, USA, 2011; ISBN 978-1-55581-672-8. [Google Scholar]
- Lecerf, J.-M.; Dépeint, F.; Clerc, E.; Dugenet, Y.; Niamba, C.N.; Rhazi, L.; Cayzeele, A.; Abdelnour, G.; Jaruga, A.; Younes, H.; et al. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br. J. Nutr. 2012, 108, 1847–1858. [Google Scholar] [CrossRef]
- De Felice, A.; Greco, A.; Calamandrei, G.; Minghetti, L. Prenatal exposure to the organophosphate insecticide chlorpyrifos enhances brain oxidative stress and prostaglandin E2 synthesis in a mouse model of idiopathic autism. J. Neuroinflamm. 2016, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-W.; Fang, B.; Pang, G.-F.; Zhang, M.; Ren, F.-Z. Age- and diet-specific effects of chronic exposure to chlorpyrifos on hormones, inflammation and gut microbiota in rats. Pestic. Biochem. Physiol. 2019, 159, 68–79. [Google Scholar] [CrossRef]
- Xu, M.-Y.; Sun, Y.-J.; Wang, P.; Yang, L.; Wu, Y.-J. Metabolomic biomarkers in urine of rats following long-term low-dose exposure of cadmium and/or chlorpyrifos. Ecotoxicol. Environ. Saf. 2020, 195, 110467. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-Y.; Wang, P.; Sun, Y.-J.; Yang, L.; Wu, Y.-J. Joint toxicity of chlorpyrifos and cadmium on the oxidative stress and mitochondrial damage in neuronal cells. Food Chem. Toxicol. 2017, 103, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, J.; Pang, G.; Ren, F.; Fang, B. Effects of Diethyl Phosphate, a Non-Specific Metabolite of Organophosphorus Pesticides, on Serum Lipid, Hormones, Inflammation, and Gut Microbiota. Molecules 2019, 24, 2003. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Dani, V.; Dhawan, D.K. Zinc mediates normalization of hepatic drug metabolizing enzymes in chlorpyrifos-induced toxicity. Toxicol. Lett. 2007, 169, 26–33. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, S.; Wang, S.; Wang, W.; Xing, H.; Xu, S. Chlorpyrifos induced oxidative stress to promote apoptosis and autophagy through the regulation of miR-19a-AMPK axis in common carp. Fish. Shellfish Immunol. 2019, 93, 1093–1099. [Google Scholar] [CrossRef]
- Ramirez-Vargas, M.A.; Flores-Alfaro, E.; Uriostegui-Acosta, M.; Alvarez-Fitz, P.; Parra-Rojas, I.; Moreno-Godinez, M.E. Effects of Exposure to Malathion on Blood Glucose Concentration: A Meta-Analysis. Environ. Sci. Pollut. Res. 2018, 25, 3233–3242. [Google Scholar] [CrossRef]
- Silva, J.G.; Boareto, A.C.; Schreiber, A.K.; Redivo, D.D.B.; Gambeta, E.; Vergara, F.; Morais, H.; Zanoveli, J.M.; Dalsenter, P.R. Chlorpyrifos induces anxiety-like behavior in offspring rats exposed during pregnancy. Neurosci. Lett. 2017, 641, 94–100. [Google Scholar] [CrossRef]
- Acker, C.I.; Nogueira, C.W. Chlorpyrifos acute exposure induces hyperglycemia and hyperlipidemia in rats. Chemosphere 2012, 89, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.K.R.; Rajini, P.S. Hyperglycemic and stressogenic effects of monocrotophos in rats: Evidence for the involvement of acetylcholinesterase inhibition. Exp. Toxicol. Pathol. 2012, 64, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Lasram, M.M.; Annabi, A.B.; Rezg, R.; Elj, N.; Slimen, S.; Kamoun, A.; El-Fazaa, S.; Gharbi, N. Effect of short-time malathion administration on glucose homeostasis in Wistar rat. Pestic. Biochem. Physiol. 2008, 92, 114–119. [Google Scholar] [CrossRef]
- Sullivan, J.F.; Jetton, M.M.; Hahn, H.K.; Burch, R.E. Enhanced lipid peroxidation in liver microsomes of zinc-deficient rats. Am. J. Clin. Nutr. 1980, 33, 51–56. [Google Scholar] [CrossRef]
- Akhtar, N.; Srivastava, M.K.; Raizada, R.B. Assessment of chlorpyrifos toxicity on certain organs in rat, Rattus norvegicus. J. Environ. Biol. 2009, 30, 1047–1053. [Google Scholar] [PubMed]
- Ambali, S.F.; Ayo, J.O.; Esievo, K.A.N.; Ojo, S.A. Hemotoxicity Induced by Chronic Chlorpyrifos Exposure in Wistar Rats: Mitigating Effect of Vitamin C. Vet. Med. Int. 2011, 2011, e945439. [Google Scholar] [CrossRef]
- Uchendu, C.; Ambali, S.F.; Ayo, J.O.; Esievo, K.A.N. Chronic co-exposure to chlorpyrifos and deltamethrin pesticides induces alterations in serum lipids and oxidative stress in Wistar rats: Mitigating role of alpha-lipoic acid. Environ. Sci. Pollut. Res. 2018, 25, 19605–19611. [Google Scholar] [CrossRef]
- Lukowicz, C.; Ellero-Simatos, S.; Régnier, M.; Polizzi, A.; Lasserre, F.; Montagner, A.; Yannick, L.; Jamin Emilien, L.; Martin, J.-F.; Naylies, C.; et al. Metabolic Effects of a Chronic Dietary Exposure to a Low-Dose Pesticide Cocktail in Mice: Sexual Dimorphism and Role of the Constitutive Androstane Receptor. Environ. Health Perspect. 2018, 126, 067007. [Google Scholar] [CrossRef]
- Newairy, A.A.; Abdou, H.M. Effect of propolis consumption on hepatotoxicity and brain damage in male rats exposed to chlorpyrifos. Afr. J. Biotechnol. 2013, 12, 5232–5243. [Google Scholar] [CrossRef]
- Dong, T.; Guan, Q.; Hu, W.; Zhang, M.; Zhang, Y.; Chen, M.; Wang, X.; Xia, Y. Prenatal exposure to glufosinate ammonium disturbs gut microbiome and induces behavioral abnormalities in mice. J. Hazard. Mater. 2020, 389, 122152. [Google Scholar] [CrossRef]
- Gao, B.; Mahbub, R.; Lu, K. Sex-Specific Effects of Organophosphate Diazinon on the Gut Microbiome and Its Metabolic Functions. Environ. Health Perspect. 2017, 125, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Perez-Fernandez, C.; Morales-Navas, M.; Guardia-Escote, L.; Garrido-Cárdenas, J.A.; Colomina, M.T.; Giménez, E.; Sánchez-Santed, F. Long-term effects of low doses of Chlorpyrifos exposure at the preweaning developmental stage: A locomotor, pharmacological, brain gene expression and gut microbiome analysis. Food Chem. Toxicol. 2020, 135, 110865. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Wang, G.; Han, R.; Xie, X. Effects of chlorpyrifos on the gut microbiome and urine metabolome in mouse (Mus musculus). Chemosphere 2016, 153, 287–293. [Google Scholar] [CrossRef]
- Blaut, M. Gut microbiota and energy balance: Role in obesity. Proc. Nutr. Soc. 2015, 74, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Kim, C.Y.; Kaur, A.; Lamothe, L.; Shaikh, M.; Keshavarzian, A.; Hamaker, B.R. Dietary fibre-based SCFA mixtures promote both protection and repair of intestinal epithelial barrier function in a Caco-2 cell model. Food Funct. 2017, 8, 1166–1173. [Google Scholar] [CrossRef]
- Jin, C.; Xia, J.; Wu, S.; Tu, W.; Pan, Z.; Fu, Z.; Wang, Y.; Jin, Y. Insights Into a Possible Influence on Gut Microbiota and Intestinal Barrier Function During Chronic Exposure of Mice to Imazalil. Toxicol. Sci. 2018, 162, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Nasuti, C.; Coman, M.M.; Olek, R.A.; Fiorini, D.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Fedeli, D.; Gabbianelli, R. Changes on fecal microbiota in rats exposed to permethrin during postnatal development. Environ. Sci. Pollut. Res. Int. 2016, 23, 10930–10937. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Jin, C.; Wang, Y.; Fu, Z.; Jin, Y. Exposure to the fungicide propamocarb causes gut microbiota dysbiosis and metabolic disorder in mice. Environ. Pollut. 2018, 237, 775–783. [Google Scholar] [CrossRef]
- Carroll, I.M.; Maharshak, N. Enteric bacterial proteases in inflammatory bowel disease- pathophysiology and clinical implications. World J. Gastroenterol. 2013, 19, 7531–7543. [Google Scholar] [CrossRef]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Yajima, M.; Nakayama, M.; Hatano, S.; Yamazaki, K.; Aoyama, Y.; Yajima, T.; Kuwata, T. Bacterial translocation in neonatal rats: The relation between intestinal flora, translocated bacteria, and influence of milk. J. Pediatr. Gastroenterol. Nutr. 2001, 33, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Delzenne, N.M. The Role of the Gut Microbiota in Energy Metabolism and Metabolic Disease. Curr. Pharm. Des. 2009, 15, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104 (Suppl. S2), S1–S63. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Yousefi, S.; Hoseinifar, S.H. Protective effects of prebiotic in zebrafish, Danio rerio, under experimental exposure to Chlorpyrifos. Int. J. Aquat. Biol. 2018, 6, 49–54. [Google Scholar] [CrossRef]
- Ooi, L.-G.; Liong, M.-T. Cholesterol-Lowering Effects of Probiotics and Prebiotics: A Review of in Vivo and in Vitro Findings. Int. J. Mol. Sci. 2010, 11, 2499–2522. [Google Scholar] [CrossRef] [PubMed]
- Dikeman, C.L.; Murphy, M.R.; Fahey, G.C., Jr. Dietary Fibers Affect Viscosity of Solutions and Simulated Human Gastric and Small Intestinal Digesta. J. Nutr. 2006, 136, 913–919. [Google Scholar] [CrossRef]
- Levrat, M.-A.; Rémésy, C.; Demigné, C. High Propionic Acid Fermentations and Mineral Accumulation in the Cecum of Rats Adapted to Different Levels of Inulin. J. Nutr. 1991, 121, 1730–1737. [Google Scholar] [CrossRef]
- Liu, F.; Prabhakar, M.; Ju, J.; Long, H.; Zhou, H.-W. Effect of inulin-type fructans on blood lipid profile and glucose level: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2017, 71, 9–20. [Google Scholar] [CrossRef]
- Song, X.; Zhong, L.; Lyu, N.; Liu, F.; Li, B.; Hao, Y.; Xue, Y.; Li, J.; Feng, Y.; Ma, Y.; et al. Inulin Can Alleviate Metabolism Disorders in ob/ob Mice by Partially Restoring Leptin-related Pathways Mediated by Gut Microbiota. Genom. Proteom. Bioinform. 2019, 17, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, H.; Xiao, X.; Hu, L.; Xin, F.; Yu, X. Inulin-type fructan improves diabetic phenotype and gut microbiota profiles in rats. PeerJ 2018, 6, e4446. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Fukuda, S.; Hase, K.; Nishiumi, S.; Izumi, Y.; Yoshida, M.; Hagiwara, T.; Kawashima, R.; Yamazaki, M.; Oshio, T.; et al. Microbiota-derived lactate accelerates colon epithelial cell turnover in starvation-refed mice. Nat. Commun. 2013, 4, 1654. [Google Scholar] [CrossRef]
- Sasaki, H.; Miyakawa, H.; Watanabe, A.; Nakayama, Y.; Lyu, Y.; Hama, K.; Shibata, S. Mice Microbiota Composition Changes by Inulin Feeding with a Long Fasting Period under a Two-Meals-Per-Day Schedule. Nutrients 2019, 11, E2802. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sam, A.G. The Organic Premium of Baby Food Estimated with National Level Scanner Data; Social Science Research Network: Rochester, NY, USA, 2020. [Google Scholar]
- Maguire, K.B.; Owens, N.N.; Simon, N.B. Focus on Babies: A Note on Parental Attitudes and Preferences for Organic Babyfood. J. Agribus. 2006, 24, 187–195. [Google Scholar]
- Maguire, K.B.; Owens, N.; Simon, N.B. Focus on Babies: Evidence on Parental Attitudes towards Pesticide Risks; AgEcon Search: Minneapolis, MN, USA, 2004. [Google Scholar]
- Berton, T.; Mayhoub, F.; Chardon, K.; Duca, R.-C.; Lestremau, F.; Bach, V.; Tack, K. Development of an analytical strategy based on LC–MS/MS for the measurement of different classes of pesticides and theirs metabolites in meconium: Application and characterisation of foetal exposure in France. Environ. Res. 2014, 132, 311–320. [Google Scholar] [CrossRef]
- Haraux, E.; Tourneux, P.; Kouakam, C.; Stephan-Blanchard, E.; Boudailliez, B.; Leke, A.; Klein, C.; Chardon, K. Isolated hypospadias: The impact of prenatal exposure to pesticides, as determined by meconium analysis. Environ. Int. 2018, 119, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Béranger, R.; Hardy, E.M.; Binter, A.-C.; Charles, M.-A.; Zaros, C.; Appenzeller, B.M.R.; Chevrier, C. Multiple pesticides in mothers’ hair samples and children’s measurements at birth: Results from the French national birth cohort (ELFE). Int. J. Hyg. Environ. Health 2020, 223, 22–33. [Google Scholar] [CrossRef]
| Before Gestation (g) | Gestation (g) | Lactation(g) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1st Month | 4th Month | 21st Day of Pregnancy | 21st Day of Lactation | |||||
| Control | 268.7 ± 28.28 | F value = 0.980 p value = 0.44 | 308.7 ± 31.75 | F value = 1.289 p value = 0.32 | 396.6 ± 88.79 | F value = 0.347 p value = 0.793 | 334.6 ± 17.47 | F value = 3.140 p value = 0.096 |
| CPF | 253.5 ± 23.86 | 286.7 ± 30.20 | 413.5 ± 105.35 | 311.0 ± 19.89 | ||||
| Control + Inulin | 260.7 ± 33.62 | 297.2 ± 35.77 | 443.0 ± 68.06 | 392.0 ± 56.56 | ||||
| CPFI | 283.0 ± 10.42 | 325.0 ± 11.63 | 447.0 ± 46.61 | 390.7 ± 42.46 | ||||
| Glucose (g/L) | Cholesterol (g/L) | Triglycerides (g/L) | HDL (g/L) | LDL (g/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 7.8 ± 0.83 | F value = 40.018 p value = 0.0001 | 1.7 ± 0.25 | F value = 6.480 p value = 0.02 | 1.4 ± 0.02 | F value = 8.573 p value = 0.01 | 0.6 ± 0.02 | F value = 3.817 p value = 0.066 | 0.8 ± 0.27 | F value = 5.549 p value = 0.029 |
| CPF | 11.6 ± 0.08 | 2.4 ± 0.24 | 1.8 ± 0.15 | 0.4 ± 0.08 | 1.6 ± 0.32 | |||||
| Control + inulin | 7.4 ± 0.15 | 1.4 ± 0.17 | 1.3 ± 0.12 | 0.5 ± 0.10 | 0.5 ± 0.25 | |||||
| CPFI | 9.4 ± 0.40 | 2.0 ± 0.39 | 1.6 ± 0.12 | 0.6 ± 0.04 | 1.0 ± 0.36 | |||||
| Beneficial Flora (CFU/g) | ||||
|---|---|---|---|---|
| Lactobacillus | Bifidobacterium | |||
| Control | 8.2 ± 0.09 | F value = 4.672 p value = 0.04 | 7.2 ± 0.61 | F value = 2.192 p value = 0.177 |
| CPF | 7.2 ± 0.22 | 6.5 ± 0.32 | ||
| Control + inulin | 8.3 ± 0.66 | 7.6 ± 0.53 | ||
| CPFI | 7.9 ± 0.48 | 7.0 ± 0.60 | ||
| Potentially Pathogenic Flora (CFU/g) | ||||||||
|---|---|---|---|---|---|---|---|---|
| E.coli | Enterococcus | Staphylococcus | Clostridium | |||||
| Control | 7.9 ± 0.26 | F value = 7.662 p value = 0.013 | 7.6 ± 0.10 | F value = 13.012 p value = 0.003 | 7.3 ± 0.28 | F value = 3.293 p value = 0.088 | 6.7 ± 0.35 | F value = 0.935 p value = 0.473 |
| CPF | 8.3 ± 0.04 | 8.3 ± 0.14 | 8.2 ± 0.10 | 7.1 ± 0.20 | ||||
| Control + inulin | 7.4 ± 0.31 | 7.4 ± 0.03 | 7.2 ± 0.28 | 7.0 ± 0.17 | ||||
| CPFI | 7.9 ± 0.19 | 7.3 ± 0.33 | 7.5 ± 0.70 | 7.0 ± 0.36 | ||||
| Direct Effect (Dams) | Indirect Effect (Offspring) | ||||
|---|---|---|---|---|---|
| CPF | Inulin on CPF | CPF | Inulin on CPF | ||
| Metabolic | Weight | - | - | Loss (at PND21 only) | Recovery |
| Glucose | Increased | Recovery | Increased | - | |
| Cholesterol | Increased (total) Increased (LDL) Decreased (HDL) | Recovery (total) - (LDL) Recovery (HDL) | Increased (total) Increased (LDL) Decreased (HDL) | - (total) Recovery (LDL) Recovery (HDL) | |
| Triglycerides | Increased | - | Increased | Recovery | |
| Bacterial | Selected bacteria (+) | Decreased (Lactobacillus) - (Bifidobacterium) | - (Lactobacillus) - (Bifidobacterium) | Decreased (Lactobacillus) Decreased (Bifidobacterium) | Recovery (Lactobacillus) - (Bifidobacterium) |
| Selected bacteria (−) | Increased (E. coli) Increased (Enterococcus) | - (E. coli) Recovery (Enterococcus) | Increased (E. coli) Increased (Enterococcus) | Recovery (E. coli) Recovery (Enterococcus) | |
| Metabolic ratio | - | - | Increased Acetate Decreased Butyrate | Recovery | |
| Translocation | - | - | Increased | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djekkoun, N.; Depeint, F.; Guibourdenche, M.; El Khayat El Sabbouri, H.; Corona, A.; Rhazi, L.; Gay-Queheillard, J.; Rouabah, L.; Hamdad, F.; Bach, V.; et al. Chronic Perigestational Exposure to Chlorpyrifos Induces Perturbations in Gut Bacteria and Glucose and Lipid Markers in Female Rats and Their Offspring. Toxics 2022, 10, 138. https://doi.org/10.3390/toxics10030138
Djekkoun N, Depeint F, Guibourdenche M, El Khayat El Sabbouri H, Corona A, Rhazi L, Gay-Queheillard J, Rouabah L, Hamdad F, Bach V, et al. Chronic Perigestational Exposure to Chlorpyrifos Induces Perturbations in Gut Bacteria and Glucose and Lipid Markers in Female Rats and Their Offspring. Toxics. 2022; 10(3):138. https://doi.org/10.3390/toxics10030138
Chicago/Turabian StyleDjekkoun, Narimane, Flore Depeint, Marion Guibourdenche, Hiba El Khayat El Sabbouri, Aurélie Corona, Larbi Rhazi, Jerome Gay-Queheillard, Leila Rouabah, Farida Hamdad, Véronique Bach, and et al. 2022. "Chronic Perigestational Exposure to Chlorpyrifos Induces Perturbations in Gut Bacteria and Glucose and Lipid Markers in Female Rats and Their Offspring" Toxics 10, no. 3: 138. https://doi.org/10.3390/toxics10030138
APA StyleDjekkoun, N., Depeint, F., Guibourdenche, M., El Khayat El Sabbouri, H., Corona, A., Rhazi, L., Gay-Queheillard, J., Rouabah, L., Hamdad, F., Bach, V., Benkhalifa, M., & Khorsi-Cauet, H. (2022). Chronic Perigestational Exposure to Chlorpyrifos Induces Perturbations in Gut Bacteria and Glucose and Lipid Markers in Female Rats and Their Offspring. Toxics, 10(3), 138. https://doi.org/10.3390/toxics10030138