Effect of Cosmetics Use on the In Vitro Skin Absorption of a Biocide, 1,2-Benzisothiazolin-3-one
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Skin Preparation
2.3. Dosing Solutions
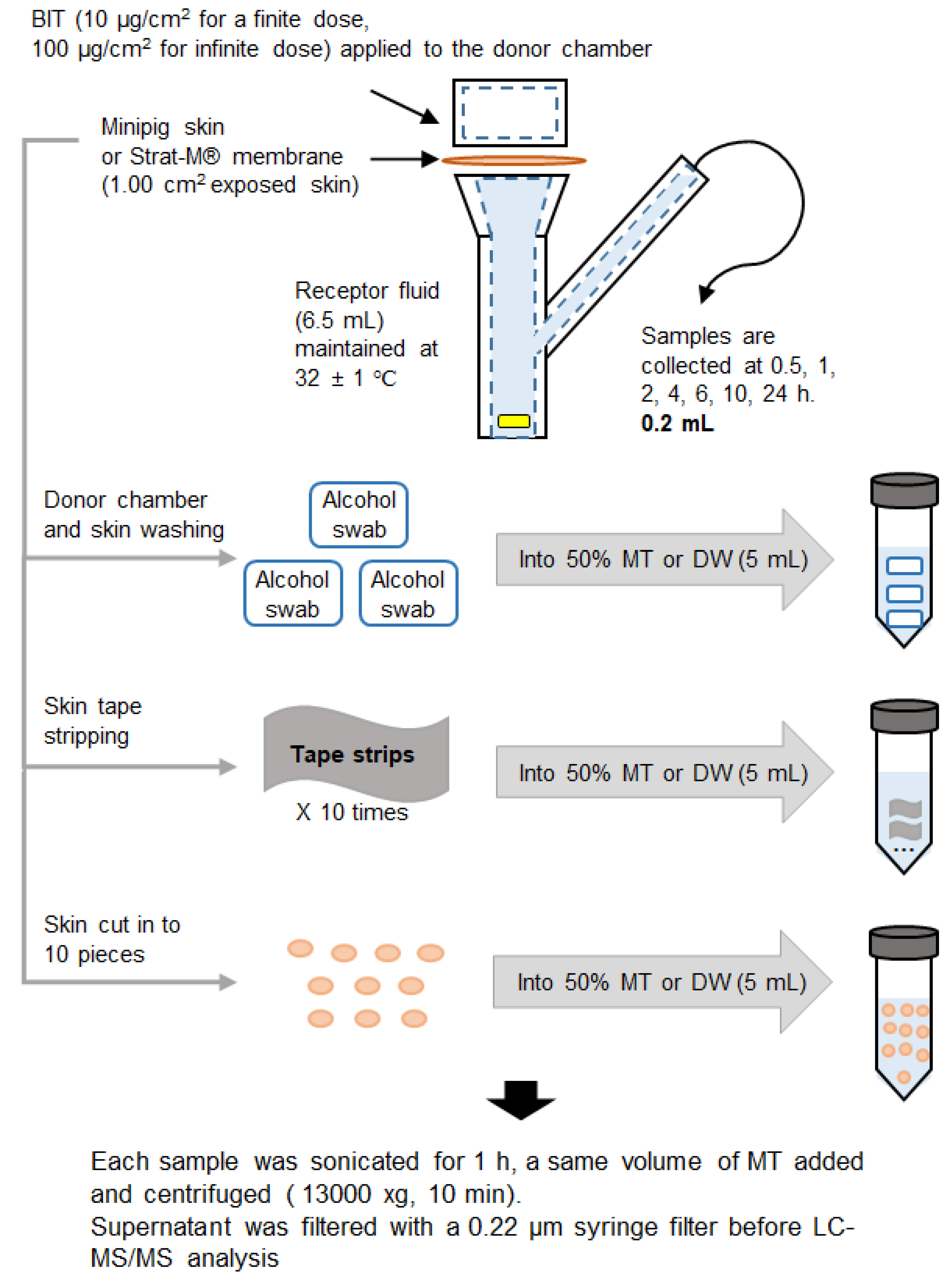
2.4. Percutaneous Absorption Assay Protocol
2.5. Sample Extraction and Calibration Sample Preparation
2.6. Liquid Chromatography Instruments and Conditions
2.7. Validation of the Analytical Method
2.8. Absorption Parameters and Statistical Analysis
2.9. Systemic Exposure Estimation and Risk Assessment
- SED: Systemic exposure dosage of BIT
- D: Amount of product used daily
- C: The maximum allowable concentration of BIT
- AP: Experimentally obtained skin absorption rate of BIT at 10 h in each case
- BW: Average human body weight, 60 kg
3. Results
3.1. Analytical Method Validation for the Receptor Fluid, Skin Wash, Tape Strip, and Skin Deposit Samples
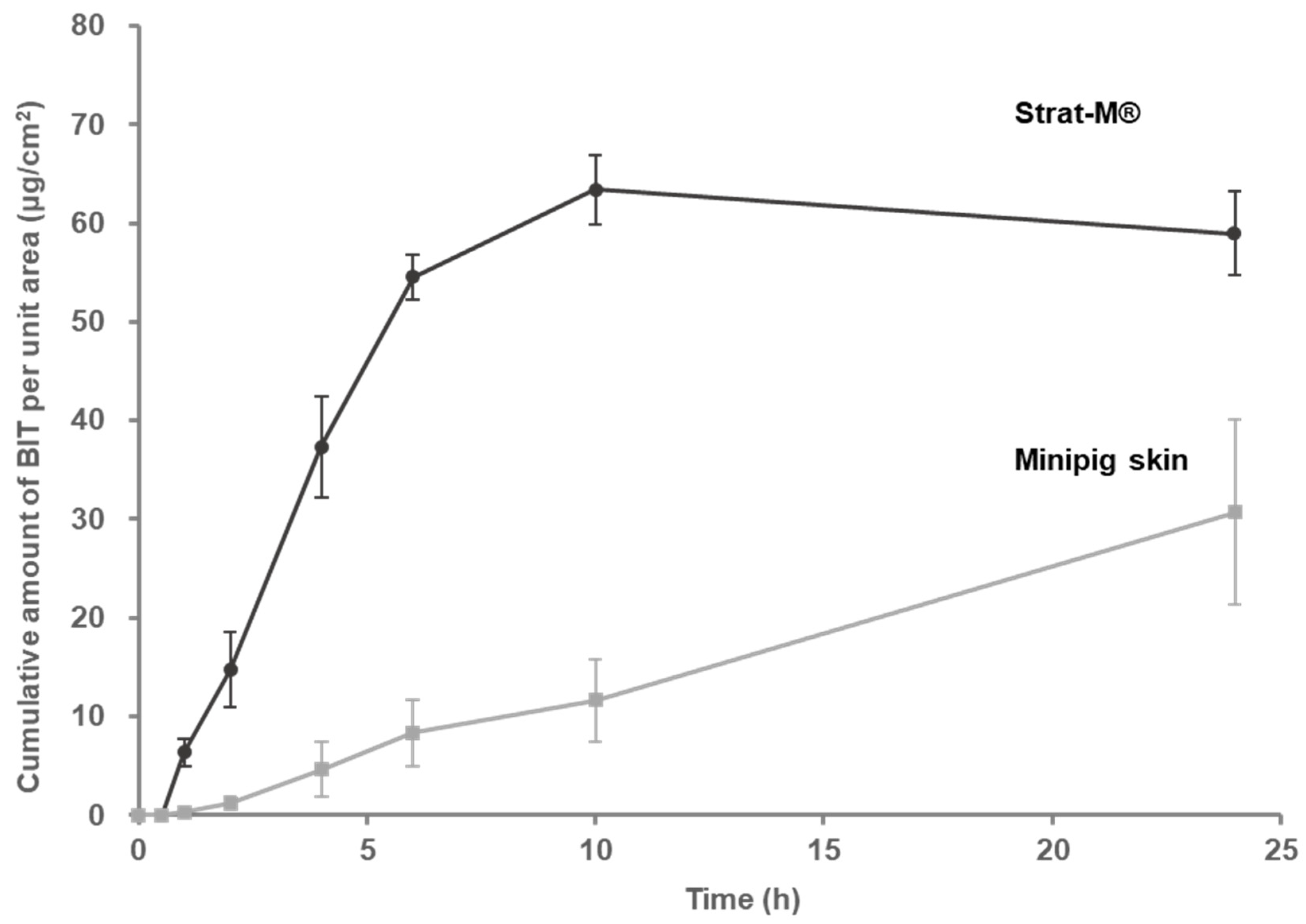
3.2. Percutaneous Absorption of BIT Applied as an Infinite Dose through Minipig Skin or STRAT-M®
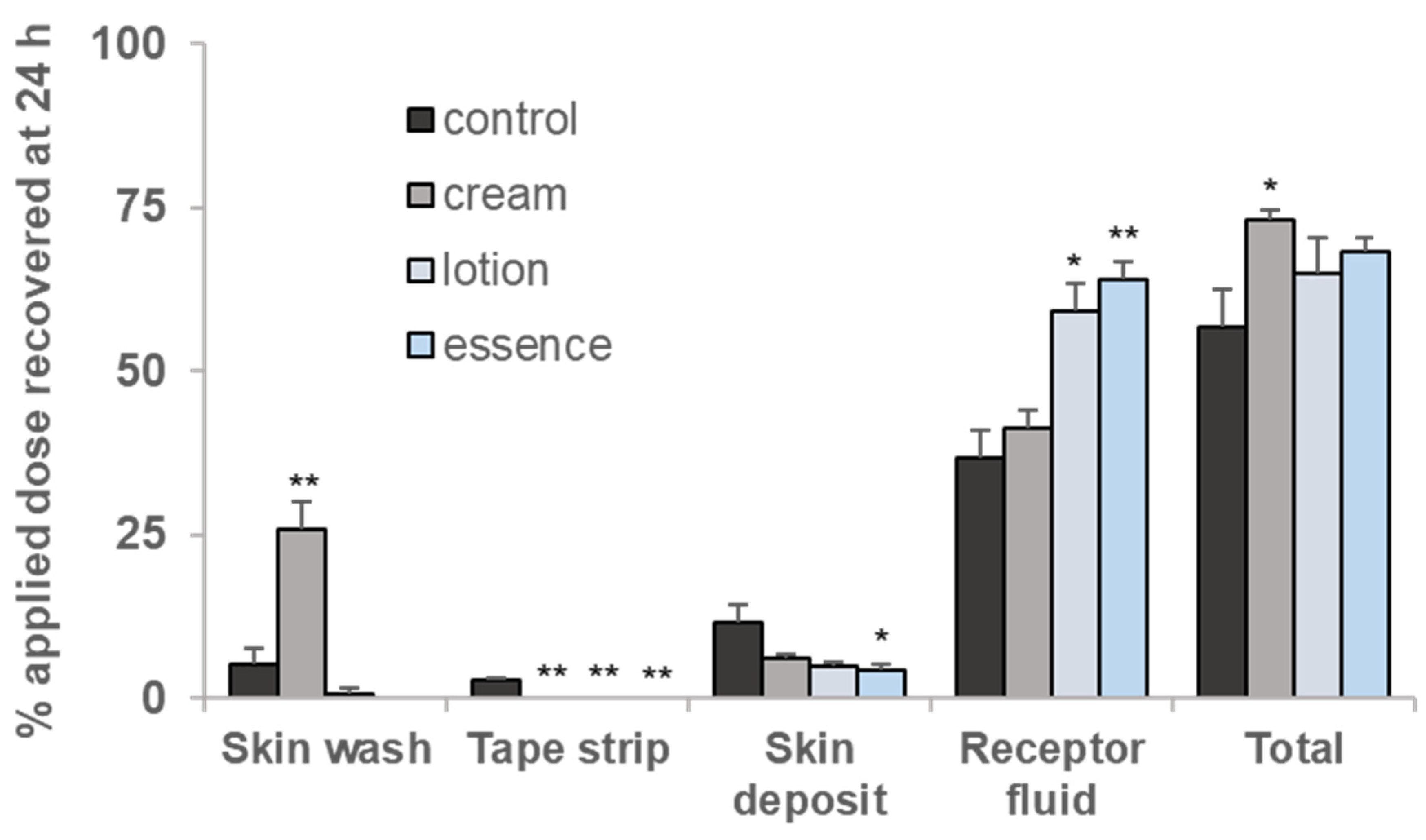
3.3. Percutaneous Absorption of BIT Applied as a Finite Dose after Pretreating Cream, Lotion, and Essence through Minipig Skin
3.4. SED and MoS Calculation of BIT after Pretreatment with Cream, Lotion, and Essence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, T.M. The mechanism of action of isothiazolone biocide. In Proceedings of the CORROSION 2006, San Diego, CA, USA, 12 March 2006. [Google Scholar]
- Berthet, A.; Spring, P.; Vernez, D.; Plateel, G.; Hopf, N.B. Ex vivo human skin permeation of methylchloroisothiazolinone (MCI) and methylisothiazolinone (MI). Arch. Toxicol. 2017, 91, 3529–3542. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Friis, U.F.; Menné, T.; Flyvholm, M.A.; Bonde, J.P.E.; Lepoittevin, J.P.; Le Coz, C.J.; Johansen, J.D. Isothiazolinones in commercial products at Danish workplaces. Contact Dermat. 2014, 71, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W. Relationship between chemical structure and activity or mode of action of microbicides. In Directory of Microbicides for the Protection of Materials: A Handbook; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005; pp. 10–12. [Google Scholar]
- Alexander, B. An assessment of the comparative sensitization potential of some common isothiazolinones. Contact Dermat. 2002, 46, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Silva, C.; Soares, P.; Garrido, E.M.; Borges, F.; Garrido, J. Isothiazolinone biocides: Chemistry, biological, and toxicity profiles. Molecules 2020, 25, 991. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L.; Aalto-Korte, K.; Alanko, K.; Hasan, T.; Jolanki, R.; Lammintausta, K.; Lauerma, A.; Laukkanen, A.; Liippo, J.; Riekki, R. Contact sensitization to methylisothiazolinone in Finland—A multicentre study. Contact Dermat. 2011, 64, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, G.; Dagnac, T.; Lores, M.; Garcia-Jares, C.; Sanchez-Prado, L.; Lamas, J.P.; Llompart, M. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2012, 1270, 41–50. [Google Scholar] [CrossRef]
- Herman, A.; Aerts, O.; de Montjoye, L.; Tromme, I.; Goossens, A.; Baeck, M. Isothiazolinone derivatives and allergic contact dermatitis: A review and update. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 267–276. [Google Scholar] [CrossRef]
- Garcia-Hidalgo, E.; Schneider, D.; von Goetz, N.; Delmaar, C.; Siegrist, M.; Hungerbühler, K. Aggregate consumer exposure to isothiazolinones via household care and personal care products: Probabilistic modelling and benzisothiazolinone risk assessment. Environ. Int. 2018, 118, 245–256. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Jeong, H.; Jung, Y.-O.; Nam, K.T.; Lim, K.-M. Skin irritation and inhalation toxicity of biocides evaluated with reconstructed human epidermis and airway models. Food Chem. Toxicol. 2021, 150, 112064. [Google Scholar] [CrossRef]
- Novick, R.M.; Nelson, M.L.; Unice, K.M.; Keenan, J.J.; Paustenbach, D.J. Estimation of the safe use concentrations of the preservative 1,2-benzisothiazolin-3-one (BIT) in consumer cleaning products and sunscreens. Food Chem. Toxicol. 2013, 56, 60–66. [Google Scholar] [CrossRef]
- Meysman, T.; Goossens, A. Occupational allergic contact dermatitis caused by benzisothiazolinone in printing ink and soap. Contact Dermat. 2017, 76, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Jang, D.Y.; Lee, D.H.; Jeong, H.; Nam, K.T.; Choi, D.W.; Lim, K.M. Local Toxicity of Biocides after Direct and Aerosol Exposure on the Human Skin Epidermis and Airway Tissue Models. Toxics 2021, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.; Schneider, K.; Gartiser, S.; Heger, W.; Mangelsdorf, I. Consumer exposure to biocides-identification of relevant sources and evaluation of possible health effects. Environ. Health 2010, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Barrias, C.C.; Granja, P.L.; Bartolo, P.J. Advanced biofabrication strategies for skin regeneration and repair. Nanomedicine 2013, 8, 603–621. [Google Scholar] [CrossRef]
- Refai, H.; Müller-Goymann, C.C. The influence of dilution of topical semisolid preparations on hydrocortisone permeation through excised human stratum corneum. Eur. J. Pharm. Biopharm. 2002, 54, 143–150. [Google Scholar] [CrossRef]
- SCCS. SCCS Notes of Guidance for the Testing of Cosmetic Substances and Their Safety Evaluation (8th Revision). 2012. Available online: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_s_006.pdf (accessed on 20 February 2022).
- Hormann, A.M.; Vom Saal, F.S.; Nagel, S.C.; Stahlhut, R.W.; Moyer, C.L.; Ellersieck, M.R.; Welshons, W.V.; Toutain, P.-L.; Taylor, J.A. Holding thermal receipt paper and eating food after using hand sanitizer results in high serum bioactive and urine total levels of bisphenol A (BPA). PLoS ONE 2014, 9, e110509. [Google Scholar] [CrossRef]
- Pawar, G.; Abdallah, M.A.-E.; de Sáa, E.V.; Harrad, S. Dermal bioaccessibility of flame retardants from indoor dust and the influence of topically applied cosmetics. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 100–105. [Google Scholar] [CrossRef]
- Karande, P.; Mitragotri, S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim. Biophys. Acta BBA-Biomembr. 2009, 1788, 2362–2373. [Google Scholar] [CrossRef]
- McCulley, L.; Cheng, C.; Mentari, E.; Diak, I.-L.; Michele, T. Alcohol-based hand sanitizer exposures and effects on young children in the US during the COVID-19 pandemic. Clin. Toxicol. 2021, 59, 355–356. [Google Scholar] [CrossRef]
- OECD. 428: Skin absorption: In vitro Method. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 23 November 2004. [Google Scholar]
- Hwang, J.H.; Jeong, H.; Lee, N.; Hur, S.; Lee, N.; Han, J.J.; Jang, H.W.; Choi, W.K.; Nam, K.T.; Lim, K.M. Ex Vivo Live Full-Thickness Porcine Skin Model as a Versatile In Vitro Testing Method for Skin Barrier Research. Int. J. Mol. Sci. 2021, 22, 657. [Google Scholar] [CrossRef]
- Bolla, P.K.; Clark, B.A.; Juluri, A.; Cheruvu, H.S.; Renukuntla, J. Evaluation of formulation parameters on permeation of ibuprofen from topical formulations using Strat-M® membrane. Pharmaceutics 2020, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Kadhum, W.R.; Kanai, S.; Todo, H.; Oshizaka, T.; Sugibayashi, K. Prediction of skin permeation by chemical compounds using the artificial membrane, Strat-M™. Eur. J. Pharm. Sci. 2015, 67, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Wilkinson, S.; Blain, P.; Dunn, M.; Aust, G.; Williams, F. Percutaneous absorption and distribution of organophosphates (chlorpyrifos and dichlorvos) following dermal exposure and decontamination scenarios using in vitro human skin model. Toxicol. Lett. 2014, 229, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Niedorf, F.; Schmidt, E.; Kietzmann, M. The automated, accurate and reproducible determination of steady-state permeation parameters from percutaneous permeation data. Altern. Lab. Anim. 2008, 36, 201–213. [Google Scholar] [CrossRef]
- Sung, C.R.; Kim, K.-B.; Lee, J.Y.; Lee, B.-M.; Kwack, S.J. Risk assessment of ethylhexyl dimethyl PABA in cosmetics. Toxicol. Res. 2019, 35, 131–136. [Google Scholar] [CrossRef]
- MFDS. Guideline on Bioanalytical Method Validation. 2014. Available online: https://www.mfds.go.kr/brd/m_210/down.do?brd_id=data0010&seq=13054&data_tp=A&file_seq=1 (accessed on 26 September 2020).
- SCCS. Opinion on Benzisothiazolinone COLIPA no P96. 2012. Available online: https://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out289_en.pdf (accessed on 15 June 2017).
- Pack, E.C.; Lee, H.G.; Jang, D.Y.; Sin, H.S.; Kim, T.Y.; Kim, H.S.; Lim, K.M.; Choi, D.W. Probabilistic risk assessment of preservatives in dishwashing detergents and wet wipes for Korean consumers. Sci. Total Environ. 2021, 782, 146829. [Google Scholar] [CrossRef]
- SCCS. SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation 11th Revision, 30–31 March 2021, SCCS/1628/21. 2021. Available online: https://ec.europa.eu/health/sites/default/files/scientific_committees/consumer_safety/docs/sccs_o_250.pdf (accessed on 20 February 2022).
- Neupane, R.; Boddu, S.H.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to biological skin in permeation studies: Current trends and possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef]
- Seo, J.-E.; Kim, S.; Kim, B.-H. In vitro skin absorption tests of three types of parabens using a Franz diffusion cell. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 320–325. [Google Scholar] [CrossRef]
- Lian, G.; Chen, L.; Han, L. An evaluation of mathematical models for predicting skin permeability. J. Pharm. Sci. 2008, 97, 584–598. [Google Scholar] [CrossRef]
- Gerstel, D.; Jacques-Jamin, C.; Schepky, A.; Cubberley, R.; Eilstein, J.; Grégoire, S.; Hewitt, N.; Klaric, M.; Rothe, H.; Duplan, H. Comparison of protocols for measuring cosmetic ingredient distribution in human and pig skin. Toxicol. In Vitro 2016, 34, 153–160. [Google Scholar] [CrossRef]
- Del Gaudio, P.; Russo, P.; Dorado, R.R.; Sansone, F.; Mencherini, T.; Gasparri, F.; Aquino, R.P. Submicrometric hypromellose acetate succinate particles as carrier for soy isoflavones extract with improved skin penetration performance. Carbohydr. Polym. 2017, 165, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Arce Jr, F.; Asano, N.; See, G.L.; Itakura, S.; Todo, H.; Sugibayashi, K. Usefulness of artificial membrane, Strat-M®, in the assessment of drug permeation from complex vehicles in finite dose conditions. Pharmaceutics 2020, 12, 173. [Google Scholar] [CrossRef]
- Ossowicz, P.; Klebeko, J.; Janus, E.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. The effect of alcohols as vehicles on the percutaneous absorption and skin retention of ibuprofen modified with l-valine alkyl esters. RSC Adv. 2020, 10, 41727–41740. [Google Scholar] [CrossRef]
- Song, J.; Jung, K.J.; Yang, M.J.; Han, S.C.; Lee, K. Assessment of acute and repeated pulmonary toxicities of oligo(2-(2-ethoxy)ethoxyethyl guanidium chloride in mice. Toxicol. Res. 2021, 37, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Watkinson, A.; Hadgraft, J.; Lane, M. Oxybutynin permeation in skin: The influence of drug and solvent activity. Int. J. Pharm. 2010, 384, 67–72. [Google Scholar] [CrossRef]
- Oliveira, G.; Hadgraft, J.; Lane, M.E. The influence of volatile solvents on transport across model membranes and human skin. Int. J. Pharm. 2012, 435, 38–49. [Google Scholar] [CrossRef]
- Barry, B.W. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 2001, 14, 101–114. [Google Scholar] [CrossRef]
- Pont, A.R.; Charron, A.R.; Brand, R.M. Active ingredients in sunscreens act as topical penetration enhancers for the herbicide 2,4-dichlorophenoxyacetic acid. Toxicol. Appl. Pharm. 2004, 195, 348–354. [Google Scholar] [CrossRef]
- Wang, T.; Gu, X. In vitro percutaneous permeation of the repellent DEET and the sunscreen oxybenzone across human skin. J. Pharm. Pharm. Sci. 2007, 10, 17–25. [Google Scholar]
- Yiin, L.M.; Tian, J.N.; Hung, C.C. Assessment of dermal absorption of DEET-containing insect repellent and oxybenzone-containing sunscreen using human urinary metabolites. Env. Sci. Pollut. Res. Int. 2015, 22, 7062–7070. [Google Scholar] [CrossRef]
- Collier, P.J.; Ramsey, A.; Waigh, R.D.; Douglas, K.T.; Austin, P.; Gilbert, P. Chemical reactivity of some isothiazolone biocides. J. Appl. Bacteriol. 1990, 69, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, M.T.; Cable, S.; Carmichael, P.L.; Cubberley, R.; Cull, T.; Delagrange, M.; Dent, M.P.; Hatherell, S.; Houghton, J.; Kukic, P. A next-generation risk assessment case study for coumarin in cosmetic products. Toxicol. Sci. 2020, 176, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Kissel, J.C. The mismeasure of dermal absorption. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 302–309. [Google Scholar] [CrossRef] [PubMed]

| Matrix | Concentration (μg/L) | Inter-Day a (n = 3) | |
|---|---|---|---|
| Recovery Rate (Mean ± SD) | Precision (% RSD) | ||
| Receptor fluid | 62.5 | 97.59 ± 13.70 | 14.04 |
| 125 | 104.79 ± 10.95 | 10.45 | |
| 250 | 100.64 ± 0.68 | 0.67 | |
| Skin Wash | 62.5 | 101.14 ± 5.33 | 5.27 |
| 125 | 98.12 ± 5.48 | 5.59 | |
| 250 | 106.14 ± 3.03 | 2.86 | |
| Tape strip | 62.5 | 98.03 ± 4.62 | 4.71 |
| 125 | 103.20 ± 4.16 | 4.03 | |
| 250 | 102.15 ± 6.16 | 6.03 | |
| Skin deposit | 62.5 | 97.46 ± 3.88 | 3.98 |
| 125 | 100.16 ± 1.17 | 1.17 | |
| 250 | 101.08 ± 1.66 | 1.64 | |
| Minipig Skin | Strat-M® | |
|---|---|---|
| Flux (Jss) (ng/cm2∙h) | 1315 | 9969.9 |
| Permeation coefficient (Kp) (cm/h) | 2.63 × 10−3 | 19.94 × 10−3 |
| Lag time (h) | 0.63 | 0.43 |
| Linear range (h) | 1–24 | 0.5–6 |
| Residual quantity in skin at 24 h (μg/cm2) | 7.87 ± 1.23 | 1.74 ± 0.51 |
| Pretreated Skin Care Product | Flux (Jss) (ng/cm2∙h) | Relative Absorption rate (Kp, cm/h) | Lag Time (h) | Linear Range (h) |
|---|---|---|---|---|
| None (control) | 293.85 | 1.47 | 0.98 | 1–10 |
| Cream | 401.91 | 2.01 | 1.90 | 2–10 |
| Lotion | 545.08 | 2.73 | 1.23 | 2–10 |
| Essence | 611.66 | 3.06 | 2.18 | 4–10 |
| Study Design | NOAEL (mg/kg bw/day) | Uncertainty Factor | Target MoS | Ref. | |||
|---|---|---|---|---|---|---|---|
| Inter-Species | Intra-Species | Exposure Duration | LOAEL to NOAEL | ||||
| Rat, diet, two-generation | 50 | 10 | 10 | 1 | 1 | 100 | [31,32] |
| Pretreated Skin Care Product | Total Amount of Cosmetic Products Used Daily (g/day) | BIT Conc. (%) | Estimated Dermal Absorption Rate after 10 h (%) | SED (mg/kg bw/day) * | MoS |
|---|---|---|---|---|---|
| None (control) | 17.4 * | 0.05 | 24.45 ± 3.39 | 35.46 × 10−3 | 1410.15 |
| Cream | 31.57 ± 2.00 | 45.77 × 10−3 | 1092.41 | ||
| Lotion | 45.21 ± 2.01 | 65.55 × 10−3 | 762.76 | ||
| Essence | 47.51 ± 2.80 | 68.89 × 10−3 | 725.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huh, Y.; Lee, D.-H.; Choi, D.; Lim, K.-M. Effect of Cosmetics Use on the In Vitro Skin Absorption of a Biocide, 1,2-Benzisothiazolin-3-one. Toxics 2022, 10, 108. https://doi.org/10.3390/toxics10030108
Huh Y, Lee D-H, Choi D, Lim K-M. Effect of Cosmetics Use on the In Vitro Skin Absorption of a Biocide, 1,2-Benzisothiazolin-3-one. Toxics. 2022; 10(3):108. https://doi.org/10.3390/toxics10030108
Chicago/Turabian StyleHuh, Yoonjung, Do-Hyeon Lee, Dalwoong Choi, and Kyung-Min Lim. 2022. "Effect of Cosmetics Use on the In Vitro Skin Absorption of a Biocide, 1,2-Benzisothiazolin-3-one" Toxics 10, no. 3: 108. https://doi.org/10.3390/toxics10030108
APA StyleHuh, Y., Lee, D.-H., Choi, D., & Lim, K.-M. (2022). Effect of Cosmetics Use on the In Vitro Skin Absorption of a Biocide, 1,2-Benzisothiazolin-3-one. Toxics, 10(3), 108. https://doi.org/10.3390/toxics10030108








