Critical Review of the Recent Literature on Organic Byproducts in E-Cigarette Aerosol Emissions
Abstract
1. Introduction
2. Foreword: The Optimal Regime of Vaping
- Overheating conditions in which e-liquids undergo temperatures above the boiling temperature of glycerol (VG) leads to significant increase in e-liquid degradation reactions, wick pyrolysis and wire oxidation, leading to a hotter aerosol than that in optimal conditions.
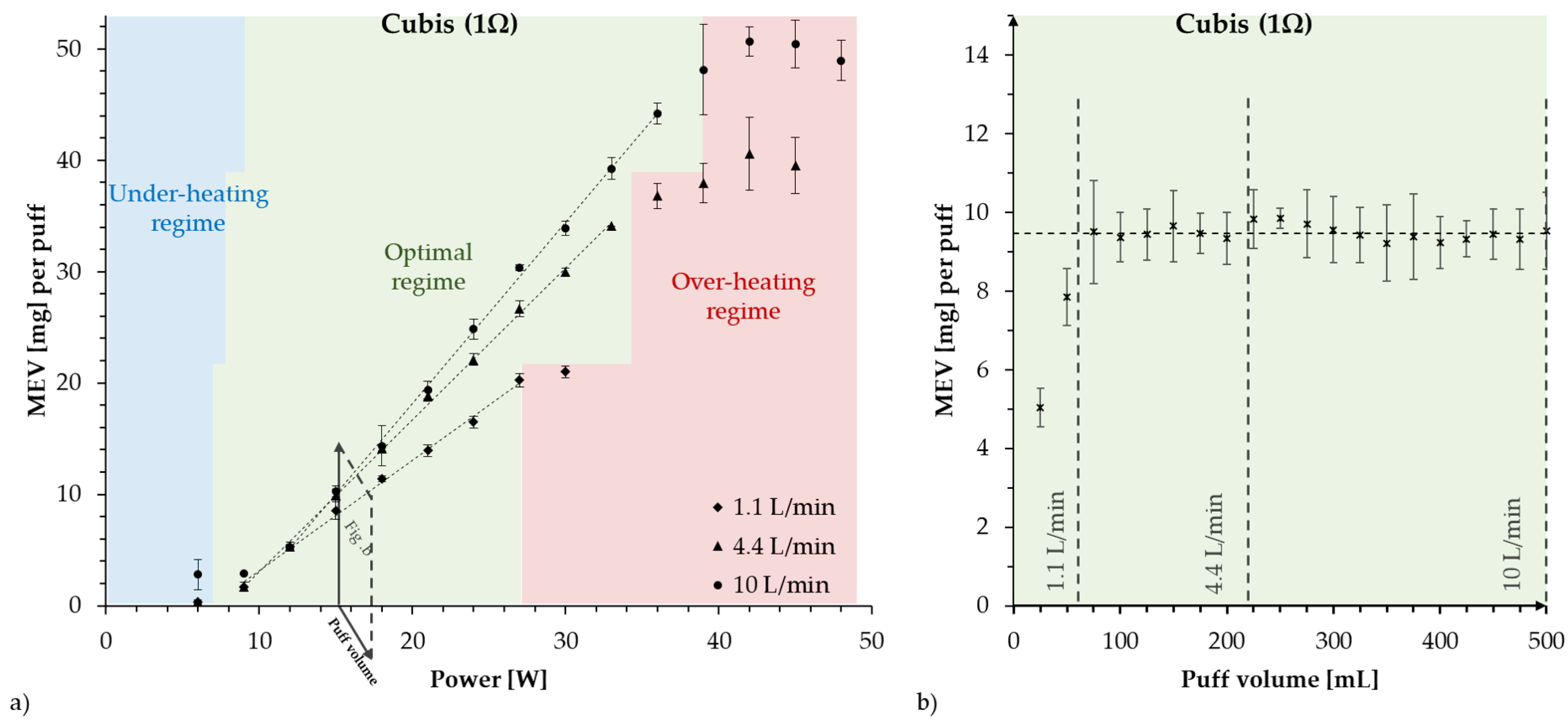
- Overheating conditions are not restricted to high-powered devices, they can also affect low-powered devices. This is illustrated in Figure 1, showing the optimal regime for low- and high-powered devices: a linear relation between mass of e-liquid vaporized (MEV) and supplied power W. The difference is that the optimal regime extends for a wider range of power settings in high-powered devices, while it is restricted to narrow power ranges in low-powered devices. Therefore, it is relatively easy in these devices to enter an overheating condition with little extra supplied power, while for a high-powered device, the deviation from the optimal regime can be more gradual. Several studies have shown that overheating and dry puffs occur in low-powered devices [51].
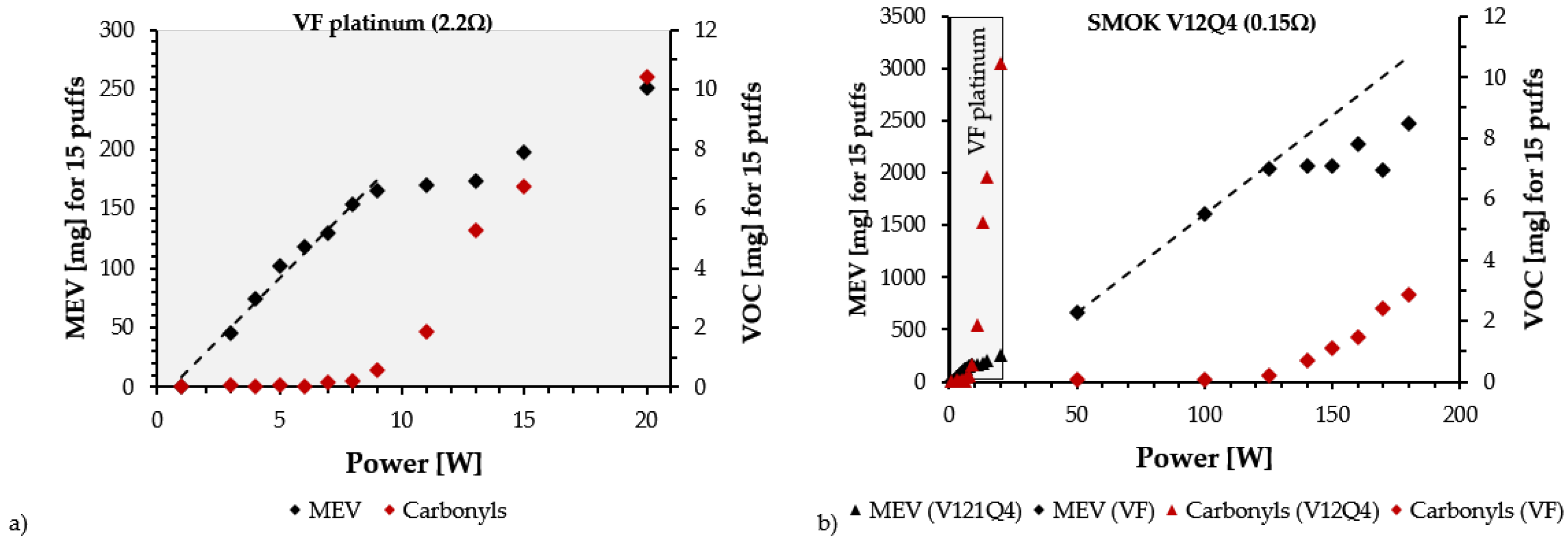
- The use of a CORESTA regime on devices intended for DTL vaping leads to the narrowing of the power range of the optimal regime range by decreasing its maximal power. This has been confirmed by experimental results reported by Soulet et al. [8] (see Figure 2a below) and Floyd et al. [52]. Therefore, experiments of this type can lead to overheating conditions, even under the power range required by the manufacturer.
- The manufacturer-recommended power range is evaluated before releasing a device into the market, with users identifying a dry puff by perceptions if used above these ranges. Since the boiling point of pure VG (288 °C) and autoignition temperature of an organic material like cotton (350–400 °C) are close, the onset of overheating will initiate cotton degradation, leading to a “dry puff”. In a recent study, which we review in Section 6, Visser et al. [34] matched the chemical characterizations of carbonyls in emissions and the human perceptions of dry puffs in the generated aerosol. Their findings support the claim that dry puff conditions are perceived as a repellent sensation that degrades the pleasant taste of vaping aerosol that prevails under normal conditions [34,53].
3. Realistic Testing vs. Realistic Vaping
3.1. Vaping Styles: MTL and DTL vs. Device Characteristics
- Low powered devices (ciga-likes, second generation clearomizers, cartridge and refillable pods, tank stating kits) typically operate at powers well below 20–25 W, have narrow mouthpieces and thus involve lower puffing volumes under high air resistance, similar to smoking. Beginner vapers (many of whom are still current smokers) tend to adopt the MTL style that is close to the puffing habits and puff volumes of cigarette smoking [57,58]. Typically, vapers using low powered devices for MTL style use PG dominated e-liquid solutions with higher nicotine concentrations.
- High powered sub-ohm devices operating at W > 40–50 W, use external batteries, often allow users to set up power/temperature, are more bulky and expensive than low powered devices, thus requiring higher level of maintenance and expertise. Their mouthpieces are wide and thus their low air resistance facilitates drawing large puffing volumes that need not be retained in the reduced volume of the oropharyngeal cavity. Therefore, they are not likely preferred by beginners or vapers simply trying to quit and remain smoke-free, but by veteran vapers who have quit smoking long ago and thus tend to enjoy the recreational hobby-like aspect of vaping, often puffing large clouds, using low nicotine concentrations and e-liquids with predominantly VG content.
3.2. Inhalation Behavior
3.3. Organoleptic Perceptions
- Below 100 °C, cellulose loses water that is contained in its fibers.
- Between 150 °C and 290 °C, dehydration reactions occur resulting in a small weight lost.
- Between 290 °C and 380 °C, fast depolymerisation of cellulose happens releasing close to 80% of volatile condensable compounds (boil-oil) as levoglucosan reaching 60%, furans as 5-hydroxymethylfurfural (5-HMF), 5-methylfurfural (5-MF), furfural, furfuryl alcohol and gaseous compounds as CO, CO and small chain compounds (glycolaldehyde, acetaldehyde, acetol).
- Between 380 °C and 800 °C, boil-oil also contains phenols and ketones compounds formed by the charring process of the remaining solid structure, with an important release of methane and CO resulting in a carbon mass at 800 °C.
3.4. Puffing Frequency and Duration
4. Methodology: Towards a Consistency Standard of Laboratory Testing
- electronic cigarette(s) OR e-cigarette(s) OR vaping product(s)
- AND aerosol(s) OR emission(s)
- AND (aldehyde(s) OR carbonyl(s) OR formaldehyde OR acetaldehyde OR acrolein OR acetone OR crotonaldehyde) OR (carbon monoxide) OR (free radical(s))
- Experimental consistency. The consistency between the experimental procedure (puffing parameters, devices, analytic methods) and the best approximation available to user behavior. Experimental inconsistencies occur mainly between the type of device tested and (i) the puffing protocol, (ii) a supplied of power higher than the limits recommended by the manufacturer as inferred from the optimal regime.
- Reproducibility of the experiments. The articles under revision must provide sufficient information that allows, in principle, a possible replication of the experiments. Vaping aerosol requires for its generation the usage of: a device (mod and atomizer), an e-liquid and a vaping regime (puffing). The authors must also provide the commercial name of the devices, as well as the technical information on the coil used (if it is a removable part), the commercial name of the e-liquid with as full information as possible, including the e-liquid composition if it is an in-lab production, all this together with the vaping regime: puff duration, the airflow rate, puff frequency, number of puffs/series. Experiments conducted with rebuilt devices (“Do It Yourself” devices) cannot be considered relevant to approximate real usage, as they are handmade coils.
- Toxicological confidence. The authors must provide detailed account of the experimental outcomes to correctly compute daily exposure (with the right time frame and air dilution volume) and compare it with toxicological threshold limits published by official organizations. The utility and relevance of this comparison is closely tied to how well the study complies with the criteria of Experimental Consistency and Reproducibility, otherwise the risk assessment is either an over (or under) estimation, speculative, irrelevant to end users or only applicable/relevant to special minority niches.
- Old and/or used devices. Authors testing such devices must communicate their storage conditions and current state, as well as justify the reason why such devices are tested. This is important, as there is evidence that devices older than 2–3 years (used or new) may degrade and undergo leaking corrosion (see full discussion of this issue in [79]).

5. Previously Published Review Articles
5.1. A Groundbreaking Review
- The experiment by Jensen et al. [81], published as a letter to the editor, examined the emissions of a top coil C4 device (now obsolete), detecting extremely high levels of formaldehyde hemiacetals, not formaldehyde, yet using the outcomes of their tests they estimated a lifetime cancer risk for formaldehyde inhalation in vaping that was 15 times higher than that from estimates from cigarette smoking. The replication in [83] showed using the same device and e-liquid that such levels only occur for abnormal usage under overheating and human identifiable dry puffs. Given the implications and the widespread diffusion of [81], there were calls for its retraction [84]. The team of Jensen et al. responded to this criticism in this communication [85], but as stated in [86], they could not deny that these worrying levels of byproducts did happen under abnormal usage conditions.
- Sleiman et al. [82] tested two devices: a top-coil and a bottom-coil, both with a silica wick atomizer filled with a commercial tobacco-flavored liquid. High levels of carbonyls were found (in ng/mg): formaldehyde 1300–48,200, acetaldehyde 260–19,080, acrolein 120–10,060, acetone 70–1410 and crotonaldehyde 10–720, with levels in the upper end of the ranges far exceeding the respective emissions from tobacco cigarettes, leading to warnings about serious risks from vaping. For the replication in [87] Farsalinos and colleagues used the same devices and aided by volunteering vapers showed that such high emissions happened under clear dry puff conditions, with usage in the normal ranges producing outcomes closer to the low end of the ng/mg ranges well below respective levels in cigarette smoke. They also tested a more recent device mentioned in [82] finding even lower aldehyde levels.
5.2. A Recent Descriptive Review
6. Studies Focused on Quantifying Carbonyls and Other Byproducts
6.1. Studies Published in 2018
6.2. Studies Published in 2019
6.3. Studies Published in 2020
6.4. Studies Published in 2021
- The temperature dependence of carbonyl production is very sensitive to the coil metal alloy (the authors’ Figure 2), an expected result given the different heat conducting coefficients of these metals.
- The mass yields (in g/puff) displayed in their Figure 3 show formaldehyde, hydroxyacetone, acetaldehyde, acrolein, and propionaldehyde, characterized by an exponential dependence on temperature that is seen to become steep at the upper temperature range (266 °C), while the dependence is linear for acetone, dihydroxyacetone, and glyceraldehyde.
- The PG/VG and nicotine ratios and nicotine proportion in the particle phase closely mirrors the PG/VG e-liquid ratio, with the 30/70 mixture (at 191 °C) leading to roughly 3/4 VG and 1/4 PG with 0.3% nicotine.
- As expected the mass concentration of 7 carbonyls increase with an increasing puff duration at same temperature (191 °C) and with fixed airflow (which makes these values unrealistic).
6.5. Studies Published in 2022
7. Carbon Monoxide (CO)
8. Reactive Oxygen Species (ROS)
- close to 2-fold between 100 and 300 °C under constant-temperature regime.
- at even steeper rate from 10 to 50 W under constant wattage, with coil temperatures higher than those of the constant-temperature regime.
- close to 3-fold in e-liquid mixtures with higher PG content in comparison with ratios of PG/VG 0:100 and PG/VG 100:0. This was associated with an increases in aerosol-induced oxidation of biologically relevant lipids.
9. Chemical Pathways of Solvents Degradation
10. Carbonyls and Nicotine Compensatory Behavior
11. Summary and Evaluation
| Toxicological Confidence | |||
|---|---|---|---|
| ● | ● | ● | ● |
| Fully Reliable | Restricted Reliability | Completely Unreliable | Missing Information |
| Experimental Consistency | |||
| ✔ | +/− | ✖ | ? |
| Fully Consistent | Restricted Consistency | Completely Inconsistent | Missing Information |
- 10 testing them at too high power levels, thus almost certain overheating and most likely irrelevant to all end users (graded as ✖)
- 8 under recommended power levels, thus with likelihood of overheating with very restricted relevance limited to a small minority of users, see Section 2, Section 3.1 and Section 12.1, (graded as +/−).
| Experimental Consistency | Reproducibility | Toxicological Confidence | Comments | |||
|---|---|---|---|---|---|---|
| First Author & Hyperlink | Vaping Regime | Power Range | Emissions Generation | Trapping & Analysis | ||
| CARBONYLS | ||||||
| 2018 | ||||||
| Vreeke [13] | +/− | +/− | ✔ | ✔ | ● | (1) |
| Korzun [14] | ✔ | ✔ | ✔ | ✔ | ● | (4) |
| El Hellani [15] | +/− | ✔ | ✔ | ✔ | ● | (4)(5)(8) |
| 2019 | ||||||
| Beauval [16] | +/− | +/− | ✔ | ✔ | ● | (2) |
| Ooi [17] | ✖ | ✖ | ? | ? | ● | (1)(3)(4)(5)(6) |
| Balushkin [18] | +/− | +/− | ✔ | ✔ | ● | (2)(10) |
| Reilly [19] | ✔ | ✔ | ✔ | ✔ | ● | |
| 2020 | ||||||
| Son [21] | ✔ | +/− | ✔ | ✔ | ● | (5) |
| Talih [22] | ✖ | +/− | ✔ | ✔ | ● | (1)(5) |
| Zelinkova [23] | ✖ | ✔ | ✔ | ✔ | ● | (2) |
| Uchiyama [24] | ✖ | ✖ | ✔ | ✖ | ● | (1)(2)(6) |
| Cunningham [25] | ✔ | ✔ | ✔ | ✔ | ● | |
| Noël [26] | ✖ | ✖ | ? | ✔ | ● | (1)(4)(6) |
| Mallock [27] | ✔ | ✔ | ✔ | ✔ | ● | |
| 2021 | ||||||
| Chen [28] | ✔ | ✔ | ✔ | ✔ | ● | |
| Crosswhite [29] | ✔ | ✔ | ✔ | ✔ | ● | |
| Li [30] | +/− | ✔ | ✔ | ✔ | ● | (2)(3)(4) |
| Yan [31] | ✖ | ✖ | ✔ | ✔ | ● | (1)(3)(4) |
| Tehrani [32] | ✖ | ✖ | ✔ | ✔ | ● | (1)(3)(4)(7)(8) |
| Cancelada [20] | +/− | +/− | ✔ | ✔ | ● | (2)(3) |
| 2022 | ||||||
| Xu [33] | ✔ | ✔ | ✔ | ✔ | ● | |
| Visser [34] | ✔ | ✔ | ✔ | ✔ | ● | |
| Experimental Consistency | Reproducibility | Toxicological Confidence | Comments | |||
|---|---|---|---|---|---|---|
| First Author & Hyperlink | Vaping Regime | Power Range | Emissions Generation | Trapping & Analysis | ||
| CO | ||||||
| Casebolt [35] | ✖ | ✖ | +/− | ✔ | ● | (1)(3) |
| El Hellani [36] | ✖ | ✖ | +/1 | ✔ | ● | (1)(3)(4) |
| Son [37] | +/− | ✔ | ✔ | ✔ | ● | (2)(3) |
| ROS | ||||||
| Bitzer (a) [38] | ✖ | +/− | ✔ | ✔ | ● | (2)(3) |
| Bitzer (b) [39] | ✖ | ✖ | ✔ | ✔ | ● | (2)(3) |
| Son [40] | ✔ | +/− | ✔ | ✔ | ● | (3)(4) |
| Haddad [41] | ✖ | ✖ | +/− | ✔ | ● | (1)(3)(9) |
| Degradation reactions & carbonyl formation | ||||||
| Jensen [42] | ✔ | ✔ | ✔ | ✔ | ● | (9) |
| Wang [43] | (NA) | +/− | +/− | ✔ | ● | (3) |
| Li [44] | ✔ | ✔ | ✔ | ✔ | ● | (8)(9) |
| Melvin [45] | ✔ | ✔ | +/− | ✔ | ● | (6)(9)(10) |
| Jaegers [46] | (NA) | (NA) | ✔ | ✔ | (NA) | |
| Nicotine compensation vs. carbonyls | ||||||
| Dawkins [47] | ✔ | ✔ | ✔ | ✔ | ● | |
| Kosmider [48] | ✔ | ✔ | ✔ | ✔ | ● | |
12. Discussion
12.1. Testing Sub-Ohm Devices with Insufficient Airflow
12.2. Arbitrarily Fixing Power, Temperature and Puff Duration
12.3. Physical Limitations on Chemical Processes
13. Assessment of Risk Communication
- questionable and unrealistic for all users if coming from the outcomes of 10 studies that used high power settings with these protocols to test sub-ohm devices [17,22,24,26,31,32,35,36,41]. The 10 studies are marked with red traffic light ● in Table 3 and Table 4. It is practically certain that these testing conditions lead to overheating and possibly dry puffs, likely producing aerosols that could be repellent to end users (see Section 3.3).
- of very limited validity if coming from 8 studies that used these protocols to test sub-ohm devices, but at recommended power settings [13,16,18,20,23,24,30,37,38,39]. These assessments must be taken with skepticism, as they come from testing conditions that might involve overheating as a likelihood, but not as a near certainty (the corresponding traffic light is ●). As we argue in Section 3.1 and Section 12.1, risk assessments from these testing conditions with recommended power settings are only relevant to a minority of consumers using these devices with an MTL vaping style, likely ∼20% of users of these devices and ∼5% of all users (since users of sub-ohm devices are very likely a minority of vapers).
14. Conclusions
- Cohort longitudinal studies for updating the scientific knowledge on the puffing behavior of EC users for all currently available devices.
- The development of inbuilt safety features, as user alerting systems, on EC devices that will at least notify users that the device is operating beyond its optimal regime with thermodynamic efficiency.
- Guidelines to inform and regulate the market of old age EC devices and their maintenance and storage conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EC | Electronic Cigarette |
| HPHC | Harmful and Potentially Harmful Compounds |
| CO | Carbon Monoxide |
| ROS | Reactive Oxygen Species |
| CORESTA | Cooperation Centre for Scientific Research Relative to Tobacco |
| CORESTA-like | Minor modifications of CORESTA protocols |
| MTL | Mouth to Lung |
| DTL | Direct to Lung |
| MEV | Mass of E-liquid Vaporized |
| PG | Propylene glycol |
| VG | Vegetable glycerine (glycerol) |
| DNPH | 2,4-dinitrophenyl hydrazine (2,4-DNPH) |
| LC | Liquid Chromatography |
| GC | Gas Chromatography |
| MS | Mass Spectrometry |
| HP | High Performance |
| HR | High Resolution |
| UV | Ultraviolet |
| EPR | Electron Paramagnetic Resonance |
| NMR | Nuclear Magnetic Resonance |
| ICP | Inductively Coupled Plasma |
| C | degrees centigrades |
| pg | picogram |
| ng | nanogram |
| g | microgram |
| g | gram |
| mg | milligram |
| mL | milliliter |
| L | Litter |
| cm | centimeter |
| m | meter |
| s | secondsr |
| min | minutes |
| h | hour |
| W | watts |
| V | Volts |
| ohms |
References
- Amos, A.; Arnott, D.; Aveyard, P.; Bauld, L.; Bogdanovica, I.; Britton, J.; Chenoweth, M.; Collin, J.; Dockrell, M.; Hajek, P.; et al. Nicotine without Smoke: Tobacco Harm Reduction; Royal College of Physicians: London, UK, 2016. [Google Scholar]
- Daynard, R. Public health consequences of e-cigarettes: A consensus study report of the National Academies of Sciences, Engineering, and Medicine. J. Public Health Policy 2018, 39, 379–381. [Google Scholar] [CrossRef]
- McNeill, A.; Brose, L.S.; Calder, R.; Bauld, L.; Robson, D. Evidence Review of E-Cigarettes and Heated Tobacco Products 2018; A Report Commissioned by Public Health England; Public Health England: London, UK, 2018.
- Pisinger, C.; Døssing, M. A systematic review of health effects of electronic cigarettes. Prev. Med. 2014, 69, 248–260. [Google Scholar] [CrossRef]
- Soulet, S.; Sussman, R.A. A Critical Review of Recent Literature on Metal Contents in E-Cigarette Aerosol. Toxics 2022, 10, 510. [Google Scholar] [CrossRef]
- CORESTA Recommended Method No 81, 2017. Collaborative Study for Determination of Glycerin, Propylene Glycol, Water and Nicotine in Collected Aerosol of E-Cigarettes. Available online: https://www.coresta.org/2015-collaborative-study-determination-glycerin-propylene-glycol-water-and-nicotine-collected-30486 (accessed on 14 October 2022).
- EVAP. Available online: https://www.coresta.org/groups/e-vapour (accessed on 14 October 2022).
- Soulet, S.; Duquesne, M.; Toutain, J.; Pairaud, C.; Mercury, M. Impact of vaping regimens on electronic cigarette efficiency. Int. J. Environ. Res. Public Health 2019, 16, 4753. [Google Scholar] [CrossRef] [PubMed]
- Soulet, S.; Duquesne, M.; Toutain, J.; Pairaud, C.; Lalo, H. Influence of coil power ranges on the e-liquid consumption in vaping devices. Int. J. Environ. Res. Public Health 2018, 15, 1853. [Google Scholar] [CrossRef]
- Wadkin, R.; Allen, C.; Fearon, I.M. E-Cigarette Puffing Topography: The Importance of Assessing User Behaviour to Inform Emissions Testing. Drug Test. Anal. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Gillman, G. Carbonyl emissions in e-cigarette aerosol: A systematic review and methodological considerations. Front. Physiol. 2018, 8, 1119. [Google Scholar] [CrossRef]
- Ward, A.M.; Yaman, R.; Ebbert, J.O. Electronic nicotine delivery system design and aerosol toxicants: A systematic review. PLoS ONE 2020, 15, e0234189. [Google Scholar] [CrossRef]
- Vreeke, S.; Peyton, D.H.; Strongin, R.M. Triacetin enhances levels of acrolein, formaldehyde hemiacetals, and acetaldehyde in electronic cigarette aerosols. ACS Omega 2018, 3, 7165–7170. [Google Scholar] [CrossRef]
- Korzun, T.; Lazurko, M.; Munhenzva, I.; Barsanti, K.C.; Huang, Y.; Jensen, R.P.; Escobedo, J.O.; Luo, W.; Peyton, D.H.; Strongin, R.M. E-cigarette airflow rate modulates toxicant profiles and can lead to concerning levels of solvent consumption. ACS Omega 2018, 3, 30–36. [Google Scholar] [CrossRef]
- El-Hellani, A.; Salman, R.; El-Hage, R.; Talih, S.; Malek, N.; Baalbaki, R.; Karaoghlanian, N.; Nakkash, R.; Shihadeh, A.; Saliba, N.A. Nicotine and carbonyl emissions from popular electronic cigarette products: Correlation to liquid composition and design characteristics. Nicotine Tob. Res. 2018, 20, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Beauval, N.; Verriele, M.; Garat, A.; Fronval, I.; Dusautoir, R.; Anthérieu, S.; Garçon, G.; Lo-Guidice, J.M.; Allorge, D.; Locoge, N. Influence of puffing conditions on the carbonyl composition of e-cigarette aerosols. Int. J. Hyg. Environ. Health 2019, 222, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Ooi, B.G.; Dutta, D.; Kazipeta, K.; Chong, N.S. Influence of the e-cigarette emission profile by the ratio of glycerol to propylene glycol in e-liquid composition. ACS Omega 2019, 4, 13338–13348. [Google Scholar] [CrossRef]
- Belushkin, M.; Tafin Djoko, D.; Esposito, M.; Korneliou, A.; Jeannet, C.; Lazzerini, M.; Jaccard, G. Selected harmful and potentially harmful constituents levels in commercial e-cigarettes. Chem. Res. Toxicol. 2019, 33, 657–668. [Google Scholar] [CrossRef]
- Reilly, S.M.; Bitzer, Z.T.; Goel, R.; Trushin, N.; Richie, J.P., Jr. Free radical, carbonyl, and nicotine levels produced by Juul electronic cigarettes. Nicotine Tob. Res. 2019, 21, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Cancelada, L.; Tang, X.; Russell, M.L.; Maddalena, R.L.; Litter, M.I.; Gundel, L.A.; Destaillats, H. Volatile aldehyde emissions from “sub-ohm” vaping devices. Environ. Res. 2021, 197, 111188. [Google Scholar] [CrossRef]
- Son, Y.; Weisel, C.; Wackowski, O.; Schwander, S.; Delnevo, C.; Meng, Q. The impact of device settings, use patterns, and flavorings on carbonyl emissions from electronic cigarettes. Int. J. Environ. Res. Public Health 2020, 17, 5650. [Google Scholar] [CrossRef]
- Talih, S.; Salman, R.; Karam, E.; El-Hourani, M.; El-Hage, R.; Karaoghlanian, N.; El-Hellani, A.; Saliba, N.; Shihadeh, A. Hot Wires and Film Boiling: Another Look at Carbonyl Formation in Electronic Cigarettes. Chem. Res. Toxicol. 2020, 33, 2172–2180. [Google Scholar] [CrossRef]
- Zelinkova, Z.; Wenzl, T. Influence of battery power setting on carbonyl emissions from electronic cigarettes. Tob. Induc. Dis. 2020, 18, 77. [Google Scholar] [CrossRef]
- Uchiyama, S.; Noguchi, M.; Sato, A.; Ishitsuka, M.; Inaba, Y.; Kunugita, N. Determination of thermal decomposition products generated from E-cigarettes. Chem. Res. Toxicol. 2020, 33, 576–583. [Google Scholar] [CrossRef]
- Cunningham, A.; McAdam, K.; Thissen, J.; Digard, H. The evolving e-cigarette: Comparative chemical analyses of e-cigarette vapor and cigarette smoke. Front. Toxicol. 2020, 2, 586674. [Google Scholar] [CrossRef] [PubMed]
- Noël, A.; Hossain, E.; Perveen, Z.; Zaman, H.; Penn, A.L. Sub-ohm vaping increases the levels of carbonyls, is cytotoxic, and alters gene expression in human bronchial epithelial cells exposed at the air–liquid interface. Respir. Res. 2020, 21, 305. [Google Scholar] [CrossRef]
- Mallock, N.; Trieu, H.L.; Macziol, M.; Malke, S.; Katz, A.; Laux, P.; Henkler-Stephani, F.; Hahn, J.; Hutzler, C.; Luch, A. Trendy e-cigarettes enter Europe: Chemical characterization of JUUL pods and its aerosols. Arch. Toxicol. 2020, 94, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Bailey, P.C.; Yang, C.; Hiraki, B.; Oldham, M.J.; Gillman, I.G. Targeted characterization of the chemical composition of juul systems aerosol and comparison with 3r4f reference cigarettes and iqos heat sticks. Separations 2021, 8, 168. [Google Scholar] [CrossRef]
- Crosswhite, M.R.; Bailey, P.C.; Jeong, L.N.; Lioubomirov, A.; Yang, C.; Ozvald, A.; Jameson, J.B.; Gillman, I.G. Non-targeted chemical characterization of Juul Virginia tobacco flavored aerosols using liquid and gas chromatography. Separations 2021, 8, 130. [Google Scholar] [CrossRef]
- Li, Y.; Burns, A.E.; Tran, L.N.; Abellar, K.A.; Poindexter, M.; Li, X.; Madl, A.K.; Pinkerton, K.E.; Nguyen, T.B. Impact of e-Liquid Composition, Coil Temperature, and Puff Topography on the Aerosol Chemistry of Electronic Cigarettes. Chem. Res. Toxicol. 2021, 34, 1640–1654. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zagorevski, D.; Ilievski, V.; Kleiman, N.J.; Re, D.B.; Navas-Acien, A.; Hilpert, M. Identification of newly formed toxic chemicals in E-cigarette aerosols with Orbitrap mass spectrometry and implications on E-cigarette control. Eur. J. Mass Spectrom. 2021, 27, 141–148. [Google Scholar] [CrossRef]
- Tehrani, M.W.; Newmeyer, M.N.; Rule, A.M.; Prasse, C. Characterizing the chemical landscape in commercial e-cigarette liquids and aerosols by liquid chromatography–high-resolution mass spectrometry. Chem. Res. Toxicol. 2021, 34, 2216–2226. [Google Scholar] [CrossRef]
- Xu, T.; Niu, Z.Y.; Xu, J.; Li, X.D.; Luo, Q.; Luo, A.; Huang, Y.L.; Jiang, X.T.; Wu, Z.H. Chemical analysis of selected harmful and potentially harmful constituents and in vitro toxicological evaluation of leading flavoured e-cigarette aerosols in the Chinese market. Drug Test. Anal. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Visser, W.F.; Krüsemann, E.J.; Klerx, W.N.; Boer, K.; Weibolt, N.; Talhout, R. Improving the analysis of e-cigarette emissions: Detecting human “dry puff” conditions in a laboratory as validated by a panel of experienced vapers. Int. J. Environ. Res. Public Health 2021, 18, 11520. [Google Scholar] [CrossRef]
- Casebolt, R.; Cook, S.J.; Islas, A.; Brown, A.; Castle, K.; Dutcher, D.D. Carbon monoxide concentration in mainstream E-cigarette emissions measured with diode laser spectroscopy. Tob. Control 2022, 29, 652–655. [Google Scholar] [CrossRef]
- El-Hellani, A.; Al-Moussawi, S.; El-Hage, R.; Talih, S.; Salman, R.; Shihadeh, A.; Saliba, N.A. Carbon Monoxide and Small Hydrocarbon Emissions from Sub-ohm Electronic Cigarettes. Chem. Res. Toxicol. 2019, 32, 312. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Bhattarai, C.; Samburova, V.; Khlystov, A. Carbonyls and carbon monoxide emissions from electronic cigarettes affected by device type and use patterns. Int. J. Environ. Res. Public Health 2020, 17, 2767. [Google Scholar] [CrossRef]
- Bitzer, Z.T.; Goel, R.; Reilly, S.M.; Foulds, J.; Muscat, J.; Elias, R.J.; Richie, J.P., Jr. Effects of solvent and temperature on free radical formation in electronic cigarette aerosols. Chem. Res. Toxicol. 2018, 31, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, Z.T.; Goel, R.; Reilly, S.M.; Elias, R.J.; Silakov, A.; Foulds, J.; Muscat, J.; Richie, J.P., Jr. Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. Free Radic. Biol. Med. 2018, 120, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Mishin, V.; Laskin, J.D.; Mainelis, G.; Wackowski, O.A.; Delnevo, C.; Schwander, S.; Khlystov, A.; Samburova, V.; Meng, Q. Hydroxyl radicals in e-cigarette vapor and e-vapor oxidative potentials under different vaping patterns. Chem. Res. Toxicol. 2019, 32, 1087–1095. [Google Scholar] [CrossRef]
- Haddad, C.; Salman, R.; El-Hellani, A.; Talih, S.; Shihadeh, A.; Saliba, N.A. Reactive oxygen species emissions from supra-and sub-ohm electronic cigarettes. J. Anal. Toxicol. 2019, 43, 45–50. [Google Scholar] [CrossRef]
- Jensen, R.P.; Strongin, R.M.; Peyton, D.H. Solvent chemistry in the electronic cigarette reaction vessel. Sci. Rep. 2017, 7, 42549. [Google Scholar] [CrossRef]
- Wang, P.; Chen, W.; Liao, J.; Matsuo, T.; Ito, K.; Fowles, J.; Shusterman, D.; Mendell, M.; Kumagai, K. A device-independent evaluation of carbonyl emissions from heated electronic cigarette solvents. PLoS ONE 2017, 12, e0169811. [Google Scholar] [CrossRef]
- Li, Y.; Burns, A.E.; Burke, G.J.; Poindexter, M.E.; Madl, A.K.; Pinkerton, K.E.; Nguyen, T.B. Application of high-resolution mass spectrometry and a theoretical model to the quantification of multifunctional carbonyls and organic acids in e-cigarette aerosol. Environ. Sci. Technol. 2020, 54, 5640–5650. [Google Scholar] [CrossRef]
- Melvin, M.S.; Avery, K.C.; Ballentine, R.M.; Flora, J.W.; Gardner, W.; Karles, G.D.; Pithawalla, Y.B.; Smith, D.C.; Ehman, K.D.; Wagner, K.A. Formation of Diacetyl and Other α-Dicarbonyl Compounds during the Generation of E-Vapor Product Aerosols. ACS Omega 2020, 5, 17565–17575. [Google Scholar] [CrossRef] [PubMed]
- Jaegers, N.R.; Hu, W.; Weber, T.J.; Hu, J.Z. Low-temperature (<200 C) degradation of electronic nicotine delivery system liquids generates toxic aldehydes. Sci. Rep. 2021, 11, 7800. [Google Scholar]
- Dawkins, L.; Cox, S.; Goniewicz, M.; McRobbie, H.; Kimber, C.; Doig, M.; Kośmider, L. ‘Real-world’ compensatory behaviour with low nicotine concentration e-liquid: Subjective effects and nicotine, acrolein and formaldehyde exposure. Addiction 2018, 113, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Kosmider, L.; Cox, S.; Zaciera, M.; Kurek, J.; Goniewicz, M.L.; McRobbie, H.; Kimber, C.; Dawkins, L. Daily exposure to formaldehyde and acetaldehyde and potential health risk associated with use of high and low nicotine e-liquid concentrations. Sci. Rep. 2020, 10, 6546. [Google Scholar] [CrossRef] [PubMed]
- Soulet, S.; Duquesne, M.; Pairaud, C.; Toutain, J. Highlighting Specific Features to Reduce Chemical and Thermal Risks of Electronic Cigarette Use through a Technical Classification of Devices. Appl. Sci. 2021, 11, 5254. [Google Scholar] [CrossRef]
- Soulet, S.; Casile, C. Thermodynamic behaviour of an e-cigarette: Investigation of nicotine delivery consistency using nicotine yield. Therm. Sci. Eng. Prog. 2022, 35, 101452. [Google Scholar] [CrossRef]
- Gillman, I.; Kistler, K.; Stewart, E.; Paolantonio, A. Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regul. Toxicol. Pharmacol. 2016, 75, 58–65. [Google Scholar] [CrossRef]
- Floyd, E.; Greenlee, S.; Oni, T.; Sadhasivam, B.; Queimado, L. The Effect of Flow Rate on a Third-Generation Sub-Ohm Tank Electronic Nicotine Delivery System, Comparison of CORESTA Flow Rates to More Realistic Flow Rates. Int. J. Environ. Res. Public Health 2021, 18, 7535. [Google Scholar] [CrossRef]
- DiPiazza, J.; Caponnetto, P.; Askin, G.; Christos, P.; Maglia, M.L.P.; Gautam, R.; Roche, S.; Polosa, R. Sensory experiences and cues among E-cigarette users. Harm Reduct. J. 2020, 17, 75. [Google Scholar] [CrossRef]
- McAdam, K.; Warrington, A.; Hughes, A.; Adams, D.; Margham, J.; Vas, C.; Davis, P.; Costigan, S.; Proctor, C. Use of social media to establish vapers puffing behaviour: Findings and implications for laboratory evaluation of e-cigarette emissions. Regul. Toxicol. Pharmacol. 2019, 107, 104423. [Google Scholar] [CrossRef]
- Ecigclick. Mouth to Lung (MTL) VS Direct to Lung (DTL) Vaping: What’s the Difference? 2022. Available online: https://www.ecigclick.co.uk/mouth-to-lung-vs-direct-to-lung/ (accessed on 8 November 2022).
- Vaping 360. Mouth to Lung (MTL) VS Direct to Lung (DTL) Vaping: What’s the Difference? 2022. Available online: https://vaping360.com/learn/what-is-vaping-how-to-vape/ (accessed on 8 November 2022).
- Tobin, M.J.; Schneider, A.W.; Sackner, M.A. Breathing pattern during and after smoking cigarettes. Clin. Sci. 1982, 63, 473. [Google Scholar] [CrossRef]
- Marian, C.; O’Connor, R.J.; Djordjevic, M.V.; Rees, V.W.; Hatrukami, D.K.; Shields, P.G. Reconciling human smoking behavior and machine smoking patterns: Implications for understanding smoking behavior and the impact on laboratory studies. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 3305. [Google Scholar] [CrossRef] [PubMed]
- Gravely, S.; Meng, G.; Hammond, D.; Reid, J.; Seo, Y.; Hyland, A.; Cummings, M.; Rivard, C.; Fong, G.; Kasza, K. Electronic nicotine delivery systems (ENDS) flavours and devices used by adults before and after the 2020 US FDA ENDS enforcement priority: Findings from the 2018 and 2020 US ITC Smoking and Vaping Surveys. Tob. Control 2022, 31 (Suppl. S3), s167. [Google Scholar]
- Action on Smoking and Health (ASH). Fact Sheet: Use of E-Cigarettes (Vapes) among Adults in Great Britain. 2022. Available online: https://ash.org.uk/resources/view/use-of-e-cigarettes-among-adults-in-great-britain-2021 (accessed on 8 November 2022).
- Kotz, D.; Böckmann, M.; Kastaun, S. The use of tobacco, e-cigarettes, and methods to quit smoking in Germany: A representative study using 6 waves of data over 12 months (the DEBRA study). Dtsch. Ärzteblatt Int. 2018, 115, 235. [Google Scholar]
- Leventhal, A.M.; Dai, H.; Barrington-Trimis, J.L.; Tackett, A.; Pedersen, E.R.; Tran, E.D. Use of social media to establish vapers puffing behaviour: Disposable e-cigarette use prevalence, correlates, and associations with previous tobacco product use in young adults. Nicotine Tob. Res. 2022, 24, 372. [Google Scholar] [CrossRef] [PubMed]
- Tattan-Birch, H.; Jackson, S.E.; Kock, L.; Dockrell, M.; Brown, J. Rapid growth in disposable e-cigarette vaping among young adults in Great Britain from 2021 to 2022: A repeat cross-sectional survey. medRxiv, 2022; online ahead of print. [Google Scholar] [CrossRef]
- Rana, S.S.; Kharabanda, O.P.; Agarwal, B. Influence of tongue volume, oral cavity volume and their ratio on upper airway: A cone beam computed tomography study. J. Oral Biol. Craniofacial Res. 2020, 10, 110. [Google Scholar] [CrossRef]
- Havermans, A.; Krüsemann, E.J.Z.; Pennings, J.; Graaf, K.D.; Boesveldt, S.; Talhout, R. Nearly 20,000 e-liquids and 250 unique flavour descriptions: An overview of the Dutch market based on information from manufacturers. Tob. Control 2021, 30, 57–62. [Google Scholar] [CrossRef]
- Zhang, C.; Chao, L.; Zhang, Z.; Zhang, L.; Li, Q.; Fan, H.; Zhang, S.; Liu, Q.; Qiao, Y.; Tian, Y.; et al. Pyrolysis of cellulose: Evolution of functionalities and structure of bio-char versus temperature. Renew. Sustain. Energy Rev. 2021, 135, 110416. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, E.; Chen, A. Volatile production from pyrolysis of cellulose, hemicellulose and lignin. J. Energy Inst. 2017, 90, 902–913. [Google Scholar] [CrossRef]
- Collard, F.X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Bouzalakou-Butel, L.A.; Provatidis, P.; Sturrock, K.; Fiore, A. Primary Investigation into the Occurrence of Hydroxymethylfurfural (HMF) in a Range of Smoked Products. J. Chem. 2018, 2018, e5942081. [Google Scholar] [CrossRef]
- Cadwallader, K.R. Wood Smoke Flavor. In Handbook of Meat, Poultry and Seafood Quality; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 201–210. [Google Scholar] [CrossRef]
- McGrath, T.E.; Chan, W.G.; Hajaligol, M.R. Low temperature mechanism for the formation of polycyclic aromatic hydrocarbons from the pyrolysis of cellulose. J. Anal. Appl. Pyrolysis 2003, 66, 51–70. [Google Scholar] [CrossRef]
- Prasad, K. A review of electronic cigarette use behaviour studies. Beitr. Tab. Int. Contrib. Tob. Res. 2018, 28, 81–92. [Google Scholar]
- Robinson, R.; Hensel, E.; Morabito, P.; Roundtree, K. Electronic cigarette topography in the natural environment. PLoS ONE 2015, 10, e0129296. [Google Scholar] [CrossRef]
- Dautzenberg, B.; Bricard, D. Real-time characterization of e-cigarettes use: The 1 million puffs study. J. Addict. Res. Ther 2015, 6, 4172. [Google Scholar] [CrossRef]
- Robinson, R.; Hensel, E.; Roundtree, K.; Difrancesco, A.; Nonnemaker, J.; Lee, Y. Week long topography study of young adults using electronic cigarettes in their natural environment. PLoS ONE 2016, 11, e0164038. [Google Scholar] [CrossRef]
- Cox, S.; Kośmider, L.; McRobbie, H.; Goniewicz, M.; Kimber, C.; Doig, M.; Dawkins, L. E-cigarette puffing patterns associated with high and low nicotine e-liquid strength: Effects on toxicant and carcinogen exposure. BMC Public Health 2016, 16, 999. [Google Scholar] [CrossRef]
- Cox, S.; Goniewicz, M.L.; Kosmider, L.; McRobbie, H.; Kimber, C.; Dawkins, L. The time course of compensatory puffing with an electronic cigarette: Secondary analysis of real-world puffing data with high and low nicotine concentration under fixed and adjustable power settings. Nicotine Tob. Res. 2021, 23, 1153–1159. [Google Scholar] [CrossRef]
- Kośmider, L.; Jackson, A.; Leigh, N.; O’connor, R.; Goniewicz, M.L. Circadian puffing behavior and topography among e-cigarette users. Tob. Regul. Sci. 2018, 4, 41–49. [Google Scholar] [CrossRef]
- Williams, M.; Luo, W.; McWhirter, K.; Ikegbu, O.; Talbot, P. Chemical Elements, Flavor Chemicals, and Nicotine in Unused and Used Electronic Cigarettes Aged 5–10 Years and Effects of pH. ChemRxiv 2022. Available online: https://chemrxiv.org/engage/chemrxiv/article-details/635cb36faca19892ffe9ebc4 (accessed on 8 November 2022).
- Farsalinos, K.E.; Voudris, V.; Poulas, K. E-cigarettes generate high levels of aldehydes only in ‘dry puff’ conditions. Addiction 2015, 110, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.P.; Wental, L.; Pankow, J.F.; Strongin, R.M.; Peyton, D.H. Hidden Formaldehyde in E-Cigarette Aerosols. N. Engl. J. Med. 2015, 372, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, M.; Logue, J.M.; Montesinos, V.N.; Russell, M.L.; Litter, M.I.; Gundel, L.A.; Destaillats, H. Emissions from electronic cigarettes: Key parameters affecting the release of harmful chemicals. Environ. Sci. Technol. 2016, 50, 9644–9651. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.E.; Voudris, V.; Spyrou, A.; Poulas, K. E-cigarettes emit very high formaldehyde levels only in conditions that are aversive to users: A replication study under verified realistic use conditions. Food Chem. Toxicol. 2017, 109, 90–94. [Google Scholar] [CrossRef]
- Bates, C.D.; Farsalinos, K.E. Research letter on e-cigarette cancer risk was so misleading it should be retracted. Addiction 2015, 110, 1686–1687. [Google Scholar] [CrossRef][Green Version]
- Pankow, J.F.; Strongin, R.M.; Peyton, D.H. Formaldehyde From E-Cigarettes-It’s Not as Simple as Some Suggest. Addiction 2015, 110, 1687–1688. [Google Scholar] [CrossRef][Green Version]
- Bates, C.D.; Farsalinos, K.E. E-cigarettes need to be tested for safety under realistic conditions. Addiction 2015, 110, 1688–1689. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Kistler, K.A.; Pennington, A.; Spyrou, A.; Kouretas, D.; Gillman, G. Aldehyde levels in e-cigarette aerosol: Findings from a replication study and from use of a new-generation device. Food Chem. Toxicol. 2018, 111, 64–70. [Google Scholar] [CrossRef]
- Khlystov, A.; Samburova, V. Flavoring compounds dominate toxic aldehyde production during e-cigarette vaping. Environ. Sci. Technol. 2016, 50, 13080–13085. [Google Scholar] [CrossRef]
- Farsalinos, K.; Gillman, G.; Kistler, K.; Yannovits, N. Comment on “flavoring compounds dominate toxic aldehyde Production during E Cigarette vaping”. Environ. Sci. Technol. 2017, 51, 2491–2492. [Google Scholar] [CrossRef]
- Khlystov, A.; Samburova, V. Response to Comment on “Flavoring Compounds Dominate Toxic Aldehyde Production during E Cigarette Vaping”. Environ. Sci. Technol. 2017, 51, 2493–2494. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K. Measuring aldehyde emissions in e-cigarettes and the contribution of flavors: A response to Khlystov and Samburova. Food Chem. Toxicol. 2018, 120, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Gillman, I.G.; Pennington, A.S.; Humphries, K.E.; Oldham, M.J. Determining the impact of flavored e-liquids on aldehyde production during Vaping. Regul. Toxicol. Pharmacol. 2020, 112, 104588. [Google Scholar] [CrossRef] [PubMed]
- Ting, C.Y.; Ahmad Sabri, N.A.; Tiong, L.L.; Zailani, H.; Wong, L.P.; Agha Mohammadi, N.; Anchah, L. Heavy metals (Cr, Pb, Cd, Ni) in aerosols emitted from electronic cigarettes sold in Malaysia. J. Environ. Sci. Health Part A 2020, 55, 55–62. [Google Scholar] [CrossRef]
- Dalton, P.; Soreth, B.; Maute, C.; Novaleski, C.; Banton, M. Lack of respiratory and ocular effects following acute propylene glycol exposure in healthy humans. Inhal. Toxicol. 2018, 30, 124–132. [Google Scholar] [CrossRef]
- Son, Y.; Wackowski, O.; Weisel, C.; Schwander, S.; Mainelis, G.; Delnevo, C.; Meng, Q. Evaluation of e-vapor nicotine and nicotyrine concentrations under various e-liquid compositions, device settings, and vaping topographies. Chem. Res. Toxicol. 2018, 31, 861–868. [Google Scholar] [CrossRef]
- Zhao, D.; Navas-Acien, A.; Ilievski, V.; Slavkovich, V.; Olmedo, P.; Adria-Mora, B.; Domingo-Relloso, A.; Aherrera, A.; Kleiman, N.J.; Rule, A.M.; et al. Metal concentrations in electronic cigarette aerosol: Effect of open-system and closed-system devices and power settings. Environ. Res. 2019, 174, 125–134. [Google Scholar] [CrossRef]
- Stein, Y.S.; Antal, M.J.; Jones, M. A study of the gas-phase pyrolysis of glycerol. J. Anal. Appl. Pyrolysis 1983, 4, 283–296. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S. The mechanism for thermal decomposition of cellulose and its main products. Bioresour. Technol. 2009, 100, 6496–6504. [Google Scholar] [CrossRef]
- Wang, Q.; Song, H.; Pan, S.; Dong, N.; Wang, X.; Sun, S. Initial pyrolysis mechanism and product formation of cellulose: An Experimental and Density functional theory(DFT) study. Sci. Rep. 2020, 10, 3626. [Google Scholar] [CrossRef]
- Goel, R.; Durand, E.; Trushin, N.; Prokopczyk, B.; Foulds, J.; Elias, R.J.; Richie, J.P., Jr. Highly reactive free radicals in electronic cigarette aerosols. Chem. Res. Toxicol. 2015, 28, 1675–1677. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Y.; Sisler, J.D.; Shaffer, J.; Leonard, S.S.; Morris, A.M.; Qian, Y.; Bello, D.; Demokritou, P. Assessment of reactive oxygen species generated by electronic cigarettes using acellular and cellular approaches. J. Hazard. Mater. 2018, 344, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.H.; Kuang, X.M.; Scott, J.A.; Rocha, G.O.; Paulson, S.E. Terephthalate Probe for Hydroxyl Radicals: Yield of 2-Hydroxyterephthalic Acid and Transition Metal Interference. Anal. Lett. 2018, 51, 2488–2497. [Google Scholar] [CrossRef]
- Talih, S.; Balhas, Z.; Eissenberg, T.; Salman, R.; Karaoghlanian, N.; El Hellani, A.; Baalbaki, R.; Saliba, N.; Shihadeh, A. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: Measurements and model predictions. Nicotine Tob. Res. 2015, 17, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Saliba, N.A.; El Hellani, A.; Honein, E.; Salman, R.; Talih, S.; Zeaiter, J.; Shihadeh, A. Surface chemistry of electronic cigarette electrical heating coils: Effects of metal type on propylene glycol thermal decomposition. J. Anal. Appl. Pyrolysis 2018, 134, 520. [Google Scholar] [CrossRef] [PubMed]
| Reviewed Studies | Section | References |
|---|---|---|
| 2 previous review articles | Section 5 | [11,12] |
| 22 studies on carbonyls and byproducts | Section 6 | [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34] |
| 3 studies on CO | Section 7 | [35,36,37] |
| 4 studies on ROS | Section 8 | [38,39,40,41] |
| 5 studies on byproduct formation | Section 9 | [42,43,44,45,46] |
| 2 studies on carbonyls vs. nicotine | Section 10 | [47,48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soulet, S.; Sussman, R.A. Critical Review of the Recent Literature on Organic Byproducts in E-Cigarette Aerosol Emissions. Toxics 2022, 10, 714. https://doi.org/10.3390/toxics10120714
Soulet S, Sussman RA. Critical Review of the Recent Literature on Organic Byproducts in E-Cigarette Aerosol Emissions. Toxics. 2022; 10(12):714. https://doi.org/10.3390/toxics10120714
Chicago/Turabian StyleSoulet, Sebastien, and Roberto A. Sussman. 2022. "Critical Review of the Recent Literature on Organic Byproducts in E-Cigarette Aerosol Emissions" Toxics 10, no. 12: 714. https://doi.org/10.3390/toxics10120714
APA StyleSoulet, S., & Sussman, R. A. (2022). Critical Review of the Recent Literature on Organic Byproducts in E-Cigarette Aerosol Emissions. Toxics, 10(12), 714. https://doi.org/10.3390/toxics10120714







