Biochemical Parameters of Female Wistar Rats and Their Offspring Exposed to Inorganic Mercury in Drinking Water during the Gestational and Lactational Periods
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Mating
2.4. Treatment
2.5. Biochemical Assays
2.5.1. Urea and Creatinine
2.5.2. Porphobilinogen Synthase (PBG-Synthase) Activity
2.5.3. Thiol Groups
2.5.4. Metallothionein (MT) Levels
2.6. Hg Determination
2.7. Statistical Analysis
3. Results
3.1. Food, Water, and Hg2+ Intake
3.2. Body and Organ Weight
3.2.1. Dams
3.2.2. Offspring
3.3. Urea and Creatinine
3.3.1. Dams
3.3.2. Offspring
3.4. PBG-Synthase Activity
3.4.1. Dams
3.4.2. Offspring
3.5. Thiol Groups
3.5.1. Dams
3.5.2. Offspring
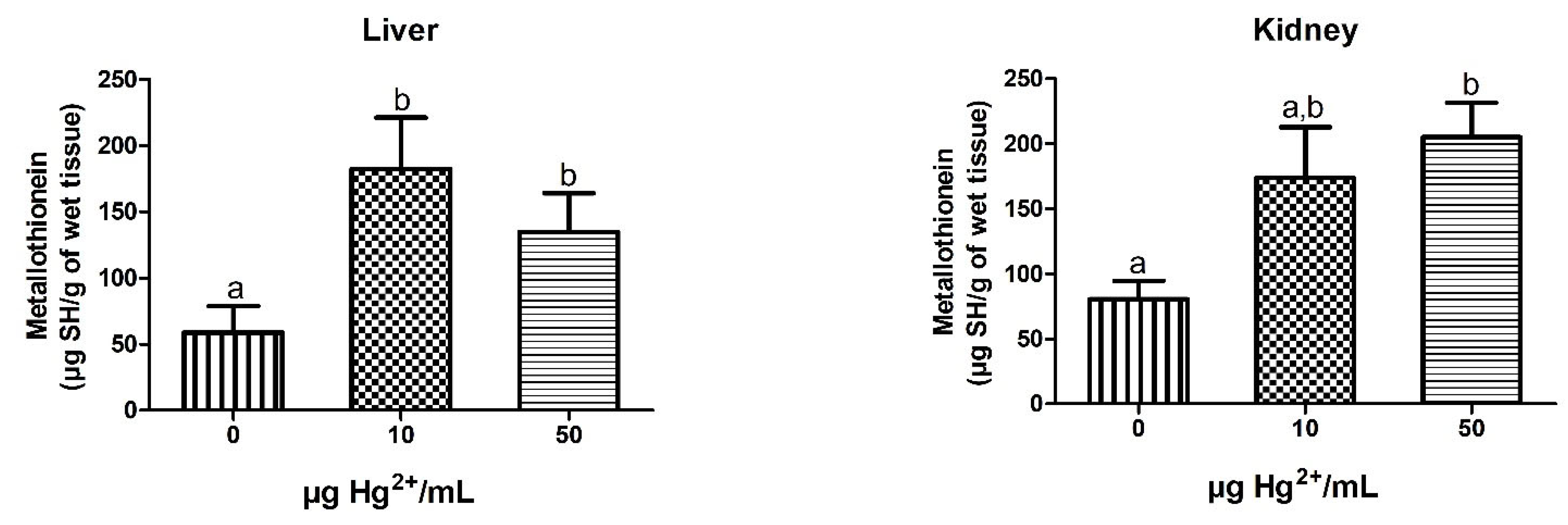
3.6. MT Levels
3.6.1. Dams
3.6.2. Offspring
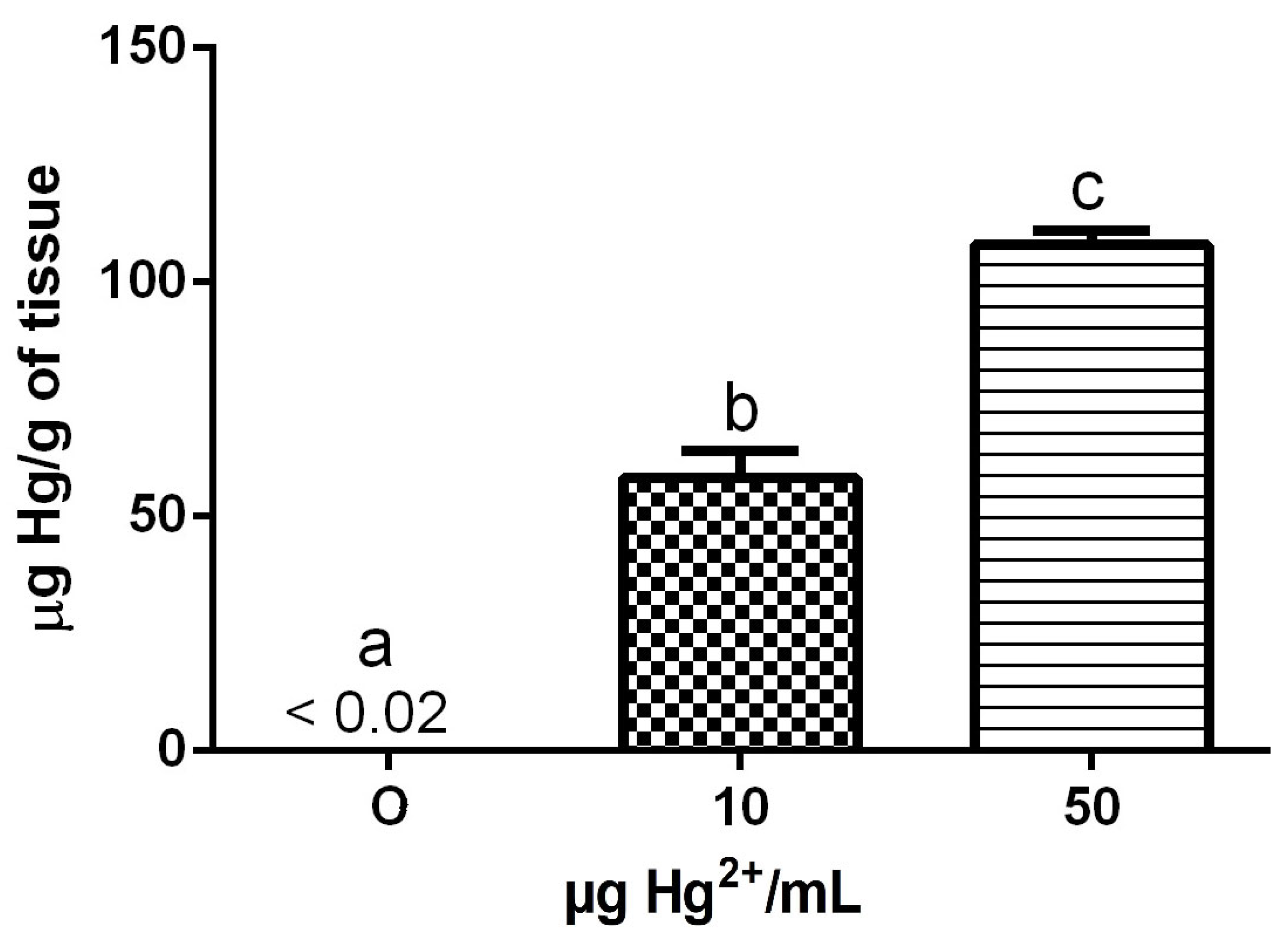
3.7. Hg Levels
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Horowitz, H.M.; Jacob, D.J.; Amos, H.M.; Streets, D.G.; Sunderland, E.M. Historical mercury releases from commercial products: Global environmental implications. Environ. Sci. Technol. 2014, 48, 10242–10250. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.S.; Nogara, P.A.; Ardisson-Araújo, D.M.P.; Aschner, M.; Rocha, J.B.T.; Dórea, J.G. Neurodevelopmental effects of mercury. In Linking Environmental Exposure to Neurodevelopmental Disorders; Aschner, M., Costa, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2, pp. 27–86. [Google Scholar] [CrossRef]
- Decharat, S.; Phethuayluk, P.; Maneelok, S.; Thepaksorn, P. Determination of mercury exposure among dental health workers in Nakhon Si Thammarat Province, Thailand. J. Toxicol. 2014, 2014, 401012. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ekinci, M.; Ceylan, E.; Keles, S.; Cagatay, H.H.; Apil, A.; Tanyildiz, B.; Uludag, G. Toxic effects of chronic mercury exposure on the retinal nerve fiber layer and macular and choroidal thickness in industrial mercury battery workers. Med. Sci. Monit. 2014, 20, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Feng, X.; Zhang, C.; Zhang, J.; Cao, Y.; You, Q.; Leung, A.O.W.; Wong, M.-H.; Wu, S.-C. Human exposure to mercury in a compact fluorescent lamp manufacturing area: By food (rice and fish) consumption and occupational exposure. Environ. Pollut. 2015, 198, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Nogara, P.A.; Oliveira, C.S.; Schmitz, G.L.; Piquini, P.C.; Farina, M.; Aschner, M.; Rocha, J.B.T. Methylmercury’s chemistry: From the environment to the mammalian brain. Biochim. Biophys. Acta—Gen. Subj. 2019, 1863, 129284. [Google Scholar] [CrossRef]
- WHO. Exposure to Mercury: A Major Public Health Concern; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Martin, S.; Griswold, W. Human health effects of heavy metals. Environ. Sci. Technol. Briefs Citiz. 2009, 15, 1–6. [Google Scholar]
- Bisen-Hersh, E.B.; Farina, M.; Barbosa, F., Jr.; Rocha, J.B.T.; Aschner, M. Behavioral effects of developmental methylmercury drinking water exposure in rodents. J. Trace Elem. Med. Biol. 2014, 28, 117–124. [Google Scholar] [CrossRef]
- Sheehan, M.C.; Burke, T.A.; Navas-Acien, A.; Breysse, P.N.; McGready, J.; Fox, M.A. Global methylmercury exposure from seafood consumption and risk of developmental neurotoxicity: A systematic review. Bull. World Health Organ. 2014, 92, 254–269. [Google Scholar] [CrossRef]
- Favero, A.M.; Oliveira, C.S.; Franciscato, C.; Oliveira, V.A.; Pereira, J.S.F.; Bertoncheli, C.M.; da Luz, S.C.A.; Dressler, V.L.; Flores, E.M.M.; Pereira, M.E. Lactating and non-lactating rats differ to renal toxicity induced by mercuric chloride: The preventive effect of zinc chloride. Cell Biochem. Func. 2014, 32, 420–428. [Google Scholar] [CrossRef]
- Mesquita, M.; Pedroso, T.F.; Oliveira, C.S.; Oliveira, V.A.; Santos, R.F.; Bizzi, C.A.; Pereira, M.E. Effects of zinc against mercury toxicity in female rats 12 and 48 hours after HgCl2 exposure. EXCLI J. 2016, 15, 256–267. [Google Scholar] [CrossRef]
- Moraes-Silva, L.; Oliveira, C.S.; Peixoto, N.C.; Pereira, M.E. Copper attenuates early and late biochemical alterations induced by inorganic mercury in young rats. J. Toxicol. Environ. Health A 2018, 81, 633–644. [Google Scholar] [CrossRef]
- Oliveira, V.A.; Favero, G.; Stacchiotti, A.; Giugno, L.; Buffoli, B.; Oliveira, C.S.; Lavazza, A.; Albanese, M.; Rodella, L.F.; Pereira, M.E.; et al. Acute mercury exposition of virgin, pregnant, and lactating rats: Histopathological kidney and liver evaluations. Environ. Toxicol. 2016, 32, 1500–1512. [Google Scholar] [CrossRef]
- Syversen, T.; Kaur, P. The toxicology of mercury and its compounds. J. Trace Elem. Med. Biol. 2012, 26, 215–226. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; Johs, A.; Zhao, L.; Wang, T.; Yang, Z.; Lin, H.; Elias, D.A.; Pierce, E.M.; Liang, L.; et al. Anaerobic mercury methylation and demethylation by Geobacter bemidjiensis Bem. Environ. Sci. Technol. 2016, 50, 4366–4373. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Kraepiel, A.M.L.; Amyot, M. The chemical cycle and bioaccumulation of mercury. Annu. Rev. Ecol. Syst. 1998, 29, 543–566. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Piccoli, B.C.; Aschner, M.; Rocha, J.B.T. Chemical speciation of selenium and mercury as determinant of their neurotoxicity. In Neurotoxicity of Metals; Aschner, M., Costa, L., Eds.; Springer: Cham, Switzerland, 2017; Volume 18, pp. 53–83. [Google Scholar] [CrossRef]
- Lorschieder, F.L.; Vimy, M.J.; Summers, A.O. Mercury exposure from “silver” tooth filling: Emerging evidence questions a traditional dental paradigm. FASEB J. 1995, 9, 504–508. [Google Scholar] [CrossRef]
- Halbach, S.; Clarkson, T.W. Enzymatic oxidation of mercury vapor by erythrocytes. Biochim. Biophys. Acta 1978, 523, 522–531. [Google Scholar] [CrossRef]
- Norseth, T.; Clarkson, T.W. Biotransformation of methylmercury salts in the rat studied by specific determination of inorganic mercury. Biochem. Pharmacol. 1970, 19, 2775–2783. [Google Scholar] [CrossRef]
- Omata, S.; Sato, M.; Sakimura, K.; Sugano, H. Time-dependent accumulation of inorganic mercury in subcellular fractions of kidney, liver, and brain of rats exposed to methylmercury. Arch. Toxicol. 1980, 44, 231–241. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Serpeloni, J.M.; Batista, B.L.; Souza, S.S.; Barbosa, F., Jr. Identification and distribution of mercury species in rat tissues following administration of thimerosal or methylmercury. Arch. Toxicol. 2010, 84, 891–896. [Google Scholar] [CrossRef]
- Yamamoto, R.; Suziki, T.; Satoh, H.; Kawais, K. Generation and dose as modifying factors of inorganic mercury accumulation in brain, liver, and kidneys of rats fed methylmercury. Environ. Res. 1986, 41, 309–318. [Google Scholar] [CrossRef]
- Axelrad, D.A.; Bellinger, D.C.; Ryan, L.M.; Woodruff, T.J. Dose-response relationship of prenatal mercury exposure and IQ: An integrative analysis of epidemiologic data. Environ. Health Perspect. 2007, 115, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Ekino, S.; Susa, M.; Ninomiya, T.; Imamura, K.; Kitamura, T. Minamata disease revisited: An update on the acute and chronic manifestations of methyl mercury poisoning. J. Neurol. Sci. 2007, 262, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.C.; Bernardi, J.V.E.; Abreu, L.; Dórea, J.G. Neurodevelopment outcomes in children exposed to organic mercury from multiple sources in a tin-ore mine environment in Brazil. Arch. Environ. Contam. Toxicol. 2015, 68, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Soresen, N.; Murata, K.; Budtz-Jorgensen, E.; Weihe, P.; Grandjean, P. Prenatal methylmercury exposure as a cardiovascular risk factor at seven years of age. Epidemiology 1999, 10, 370–375. [Google Scholar] [CrossRef]
- Nies, A.S.; Spielberg, S.P. Principles of therapeutics. In Goodman & Gilman’s the Pharmacological Basis of Therapeutics, 9th ed.; Hardman, J.G., Gilman, A.G., Limbird, L.E., Eds.; McGraw-Hill: New York, NY, USA, 1996; Chapter 3; pp. 34–63. [Google Scholar]
- Bridges, C.C.; Joshee, L.; Zalups, R.K. Effect of DMPS and DMSA on the placental and fetal disposition of methylmercury. Placenta 2009, 30, 800–805. [Google Scholar] [CrossRef]
- Bridges, C.C.; Joshee, L.; Zalups, R.K. Placental and fetal disposition of mercuric ions in rats exposed to methylmercury: Role of Mrp2. Reprod. Toxicol. 2012, 34, 628–634. [Google Scholar] [CrossRef][Green Version]
- Ilbäck, N.-G.; Sundberg, J.; Oskarsson, A. Methyl mercury exposure via placenta and milk impairs natural killer (NK) cell function in newborn rats. Toxicol. Lett. 1991, 58, 149–158. [Google Scholar] [CrossRef]
- Newland, M.C.; Paletz, E.M.; Reed, M.N. Methylmercury and nutrition: Adult effects of fetal exposure in experimental models. Neurotoxicology 2008, 29, 783–801. [Google Scholar] [CrossRef]
- Ornaghi, F.; Ferrini, S.; Prati, M.; Giavani, E. The protective effects of N-acetil-L-cysteine against methyl mercury embryotocixity in mice. Fund. Appl. Toxicol. 1993, 20, 437–445. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Joshee, L.; George, H.; Nijihara, S.; Bridges, C.C. Oral exposure of pregnant rats to toxic doses of methylmercury alters fetal accumulation. Reprod. Toxicol. 2017, 69, 65–75. [Google Scholar] [CrossRef]
- Sakamoto, M.; Kakita, A.; Wakabayaashi, K.; Takahashi, H.; Nakano, A.; Akagi, H. Evaluation of changes in methylmercury accumulation in the developing rat brain and its effects: A study with consecutive and moderate dose exposure throughout gestation and lactation periods. Brain Res. 2002, 949, 51–59. [Google Scholar] [CrossRef]
- Stringari, J.; Nunes, A.K.C.; Franco, J.L.; Bohrer, D.; Garcia, S.C.; Dafre, A.L.A.; Milatovic, D.; Souza, D.O.; Rocha, J.B.T.; Aschner, M.; et al. Prenatal methylmercury exposure hampers glutathione antioxidant system ontogenesis and causes long-lasting oxidative stress in the mouse brain. Toxicol. Appl. Pharmacol. 2008, 227, 147–154. [Google Scholar] [CrossRef]
- Vicente, E.; Boer, M.; Netto, C.; Fochesatto, C.; Dalmaz, C.; Siqueira, I.R.; Gonçalves, C.-A. Hippocampal antioxidant system in neonates from methylmercury-intoxicated rats. Neurotoxicol. Teratol. 2004, 26, 817–823. [Google Scholar] [CrossRef]
- Chehimi, L.; Roy, V.; Jeljeli, M.; Sakly, M. Chronic exposure to mercuric chloride during gestation affects sensorimotor development and later behaviour in rats. Behav. Brain Res. 2012, 234, 43–50. [Google Scholar] [CrossRef]
- Feng, W.; Wang, M.; Li, B.; Liu, J.; Chai, Z.; Zhao, J.; Deng, G. Mercury and trace element distribution in organic tissues and regional brain of fetal rat after in utero and weaning exposure to low dose of inorganic mercury. Toxicol. Lett. 2004, 152, 223–234. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Oliveira, V.A.; Ineu, R.P.; Moraes-Silva, L.; Pereira, M.E. Biochemical parameters of pregnant rats and their offspring exposed to different doses of inorganic mercury in drinking water. Food Chem. Toxicol. 2012, 50, 2382–2387. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Joshee, L.; Zalups, R.K.; Pereira, M.E.; Bridges, C.C. Disposition of inorganic mercury in pregnant rats and their offspring. Toxicology 2015, 335, 62–71. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Oliveira, V.A.; Costa, L.M.; Pedroso, T.F.; Fonseca, M.M.; Bernardi, J.S.; Fiuza, T.L.; Pereira, M.E. Inorganic mercury exposure in drinking water alters essential metal homeostasis in pregnant rats without altering rat pup behavior. Reprod. Toxicol. 2016, 65, 18–23. [Google Scholar] [CrossRef]
- Sassa, S. Delta-aminolevulinic acid dehydratase assay. Enzyme 1982, 28, 133–145. [Google Scholar] [CrossRef]
- Peixoto, N.C.; Roza, T.; Flores, E.M.; Pereira, M.E. Effects of zinc and cadmium on HgCl2-δ-ALA-D inhibition and Hg levels in tissues of suckling rats. Toxicol. Lett. 2003, 146, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Viarengo, A.; Ponzano, E.; Dondero, F.; Fabbri, R. A simple spectrophotometric method for metallothionein evaluation in marine organisms: An application to Mediterranean and antarctic molluscs. Mar. Environ. Res. 1997, 44, 69–84. [Google Scholar] [CrossRef]
- Ineu, R.P.; Oliveira, C.S.; Oliveira, V.A.; Moraes-Silva, L.; Almeida-Luz, S.C.; Pereira, M.E. Antioxidant effect of zinc chloride against ethanol-induced gastrointestinal lesions in rats. Food Chem. Toxicol. 2013, 58, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, F.M.; Caldas, E.D. Arsenic, lead, mercury and cadmium: Toxicity, levels in breast milk and the risks for breast fed infants. Environ. Res. 2016, 151, 671–688. [Google Scholar] [CrossRef]
- Sundberg, J.; Ersson, B.; Lonnerdal, B.; Oskarsson, A. Protein binding of mercury in milk and plasma from mice and man—A comparison between methylmercury and inorganic mercury. Toxicology 1999, 137, 169–184. [Google Scholar] [CrossRef]
- Bridges, C.C.; Joshee, L.; Zalups, R.K. Aging and the disposition and toxicity of mercury in rats. Exp. Gerontol. 2014, 53, 31–39. [Google Scholar] [CrossRef]
- Peixoto, N.C.; Pereira, M.E. Effectiveness of ZnCl2 in protecting against nephrotoxicity induced by HgCl2 in newborn rats. Ecotoxicol. Environ. Saf. 2007, 66, 441–446. [Google Scholar] [CrossRef]
- Franciscato, C.; Moraes-Silva, L.; Duarte, F.A.; Oliveira, C.S.; Ineu, R.P.; Flores, E.M.; Dressler, V.L.; Peixoto, N.C.; Pereira, M.E. Delayed biochemical changes induced by mercury intoxication are prevented by zinc pre-exposure. Ecotoxicol. Environ. Saf. 2011, 74, 480–486. [Google Scholar] [CrossRef]
- Oliveira, V.A.; Oliveira, C.S.; Ineu, R.P.; Moraes-Silva, L.; Siqueira, L.F.; Pereira, M.E. Lactating and non-lactating rats differ in sensitivity to HgCl2: Protective effect of ZnCl2. J. Trace Elem. Med. Biol. 2014, 28, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.S.; Joshee, L.; Zalups, R.K.; Bridges, C.C. Compensatory renal hypertrophy and the handling of an acute nephrotoxicant in a model of aging. Exp. Gerontol. 2016, 75, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Arthur, S.K.; Green, R. Renal function during lactation in the rat. J. Physiol. 1983, 334, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.; Lafayette, R.A. Renal physiology of pregnancy. Adv. Chronic Kidney Dis. 2013, 20, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, N.C.; Serafim, M.A.; Flores, E.M.M.; Bebianno, M.J.; Pereira, M.E. Metallothionein, zinc, and mercury levels in tissues of young rats exposed to zinc and subsequently to mercury. Life Sci. 2007, 81, 1264–1271. [Google Scholar] [CrossRef]
- Onosaka, S.; Cherian, M.G. The induced synthesis of metallothionein in various tissues of rats in response to metals. II. Influence of zinc status and specific effects on pancreatic metallothionein. Toxicology 1982, 23, 11–20. [Google Scholar] [CrossRef]
- Clarkson, T.W. The three modern faces of mercury. Environ. Health Perspect. 2003, 110, 11–23. [Google Scholar] [CrossRef]
- Graeme, K.A.; Pollack, C.V. Heavy metal toxicity, part I: Arsenic and mercury. J. Emerg. Med. 1998, 16, 45–56. [Google Scholar] [CrossRef]
- Moraes-Silva, L.; Bueno, T.M.; Franciscato, C.; Oliveira, C.S.; Peixoto, N.C.; Pereira, M.E. Mercury chloride increases hepatic alanine aminotransferase and glucose 6-phosphatase activities in newborn rats in vivo. Cell Biol. Int. 2012, 36, 561–566. [Google Scholar] [CrossRef]
- Fiuza, T.L.; Oliveira, C.S.; Costa, M.; Oliveira, V.A.; Zeni, G.; Pereira, M.E. Effectiveness of (PhSe)2 in protect against the HgCl2 toxicity. J. Trace Elem. Med. Biol. 2015, 29, 255–262. [Google Scholar] [CrossRef]
| 0 (n = 7) | 10 (n = 9) | 50 (n = 7) | |
|---|---|---|---|
| Food intake (g) | |||
| Gestation | |||
| Total * | 406.0 ± 48.1 | 438.4 ± 34.3 | 407.1 ± 43.3 |
| 100 g/day # | 7.6 ± 0.7 | 8.1 ± 0.4 | 7.9 ± 0.8 |
| Lactation | |||
| Total * | 945.5 ± 46.6 | 886.3 ± 41.3 | 906.3 ± 59.1 |
| 100 g/day # | 16.5 ± 0.6 | 16.4 ± 0.6 | 17.6 ± 0.9 |
| Water intake (mL) | |||
| Gestation | |||
| Total * | 1001.0 ± 62.3 a | 717.1 ± 38.7 b | 608.3 ± 46.7 b |
| 100 g/day # | 19.1 ± 1.4 a | 14.36 ± 0.5 b | 12.38 ± 0.9 b |
| Lactation | |||
| Total * | 1752.0 ± 100.9 a | 1283.0 ± 95.3 b | 1247.0 ± 145.9 b |
| 100 g/day # | 30.8 ± 1.9 a | 24.4 ± 0.9 b | 24.9 ± 1.8 b |
| Hg2+ intake (mg) | |||
| Gestation | |||
| Total * | n.d. | 7.03 ± 0.37 | 30.41 ± 2.33 $ |
| 100 g/day # | n.d. | 0.14 ± 0.00 | 0.62 ± 0.05 $ |
| Lactation | |||
| Total * | n.d. | 12.99 ± 0.87 | 62.33 ± 7.29 $ |
| 100 g/day # | n.d. | 0.24 ± 0.01 | 1.25 ± 0.09 $ |
| 0 (n = 7) | 10 (n = 9) | 50 (n = 7) | |
|---|---|---|---|
| Body weight (g) | |||
| Gestation day 0 | 221.30 ± 23.39 | 229.20 ± 12.78 | 225.50 ± 1457 |
| Last day of gestation | 318.30 ± 25.82 | 329.50 ± 19.12 | 308.50 ± 19.12 |
| Last day of lactation | 259.80 ± 21.80 | 247.30 ± 10.87 | 247.00 ± 10.68 |
| Liver | |||
| Total (g) | 12.91 ± 0.79 | 12.34 ± 0.63 | 12.97 ± 0.78 |
| Relative (%) | 4.60 ± 0.22 | 4.60 ± 0.18 | 4.72 ± 0.14 |
| Kidney | |||
| Total (g) | 2.01 ± 0.09 a | 2.20 ± 0.08 b | 2.57 ± 0.12 c |
| Relative (%) | 0.72 ± 0.03 a | 0.82 ± 0.03 b | 0.94 ± 0.03 c |
| Brain | |||
| Total (g) | 1.67 ± 0.07 | 1.69 ± 0.04 | 1.66 ± 0.02 |
| Relative (%) | 0.60 ± 0.03 | 0.63 ± 0.02 | 0.62 ± 0.03 |
| Liver | Kidney | Brain | ||||||
|---|---|---|---|---|---|---|---|---|
| Body Weight (g) | Total (g) | Relative (%) | Total (g) | Relative (%) | Total (g) | Relative (%) | ||
| 0 (n = 7) | 19.22 ± 1.33 | 0.55 ± 0.03 | 2.87 ± 0.07 | 0.24 ± 0.01 | 1.28 ± 0.03 | 0.87 ± 0.04 | 4.59 ± 0.25 | |
| PND10 | 10 (n = 9) | 17.11 ± 1.16 | 0.47 ± 0.02 | 2.81 ± 0.19 | 0.22 ± 0.01 | 1.31 ± 0.05 | 0.86 ± 0.03 | 5.16 ± 0.24 |
| 50 (n = 7) | 18.62 ± 0.78 | 0.51 ± 0.03 | 2.73 ± 0.09 | 0.25 ± 0.02 | 1.34 ± 0.04 | 0.85 ± 0.02 | 4.60 ± 0.20 | |
| 0 (n = 7) | 41.21 ± 2.11 | 1.37 ± 0.07 | 3.34 ± 0.12 | 0.63 ± 0.12 | 1.50 ± 0.24 | 1.17 ± 0.09 | 2.89 ± 0.28 | |
| PND20 | 10 (n = 9) | 35.92 ± 2.03 | 1.24 ± 0.10 | 3.43 ± 0.16 | 0.53 ± 0.11 | 1.43 ± 0.20 | 1.18 ± 0.06 | 3.37 ± 0.26 |
| 50 (n = 7) | 39.86 ± 2.35 | 1.37 ± 0.10 | 3.41 ± 0.11 | 0.74 ± 0.16 | 1.76 ± 0.29 | 1.18 ± 0.08 | 3.07 ± 0.32 | |
| 0 (n = 7) | 77.11 ± 4.22 | 3.39 ± 0.16 | 4.42 ± 0.13 | 0.87 ± 0.05 | 1.12 ± 0.02 | 1.46 ± 0.02 | 1.93 ± 0.10 | |
| PND30 | 10 (n = 9) | 61.53 ± 7.68 | 2.84 ± 0.34 | 4.67 ± 0.19 | 0.77 ± 0.06 | 1.33 ± 0.11 | 1.35 ± 0.05 | 2.40 ± 0.31 |
| 50 (n = 7) | 68.70 ± 5.50 | 3.10 ± 0.20 | 4.64 ± 0.32 | 0.90 ± 0.05 | 1.37 ± 0.11 | 1.40 ± 0.03 | 2.16 ± 0.23 | |
| 0 (n = 7) | 138.10 ± 7.23 | 5.93 ± 0.35 | 4.29 ± 0.08 a | 1.52 ± 0.05 | 0.93 ± 0.03 a | 1.52 ± 0.05 | 1.11 ± 0.05 | |
| PND40 | 10 (n = 9) | 117.20 ± 7.05 | 5.29 ± 0.49 | 4.48 ± 0.16 a,b | 1.49 ± 0.06 | 0.98 ± 0.01 a,b | 1.49 ± 0.06 | 1.28 ± 0.05 |
| 50 (n = 7) | 133.10 ± 4.96 | 6.40 ± 0.24 | 4.82 ± 0.12 b | 1.50 ± 0.03 | 1.01 ± 0.02 b | 1.50 ± 0.03 | 1.13 ± 0.04 | |
| Urea (mg/dL) | Creatinine (mg/dL) | ||
|---|---|---|---|
| Dams | |||
| 0 (n = 7) | 74.30 ± 2.33 | 0.47 ± 0.09 | |
| 10 (n = 7) | 77.01 ± 3.21 | 0.52 ± 0.11 | |
| 50 (n = 7) | 64.76 ± 3.27 | 0.53 ± 0.07 | |
| PUPS | |||
| 0 (n = 7) | 44.00 ± 2.30 | 0.46 ± 0.09 | |
| PND10 | 10 (n = 7) | 46.15 ± 5.99 | 0.30 ± 0.08 |
| 50 (n = 7) | 47.99 ± 4.34 | 0.30 ± 0.04 | |
| 0 (n = 7) | 42.99 ± 2.46 | 0.72 ± 0.26 | |
| PND20 | 10 (n = 7) | 55.90 ± 7.96 | 0.74 ± 0.27 |
| 50 (n = 7) | 57.41 ± 5.18 | 0.72 ± 0.25 | |
| 0 (n = 7) | 49.24 ± 5.74 | 0.38 ± 0.03 | |
| PND30 | 10 (n = 7) | 56.31 ± 6.64 | 0.34 ± 0.07 |
| 50 (n = 7) | 46.46 ± 4.85 | 0.39 ± 0.05 | |
| 0 (n = 7) | 47.27 ± 4.26 | 0.19 ± 0.03 | |
| PND40 | 10 (n = 7) | 57.13 ± 7.02 | 0.17 ± 0.04 |
| 50 (n = 7) | 38.66 ± 4.53 | 0.34 ± 0.06 |
| Liver | Kidney | Brain | ||
|---|---|---|---|---|
| Dams | ||||
| 0 (n = 7) | 11.16 ± 1.76 | 5.81 ± 0.93 | 1.72 ± 0.33 | |
| 10 (n = 7) | 10.74 ± 0.96 | 6.19 ± 1.32 | 1.41 ± 0.30 | |
| 50 (n = 7) | 17.21 ± 2.90 | 8.51 ± 1.24 | 1.87 ± 0.30 | |
| Pups | ||||
| 0 (n = 7) | 21.39 ± 7.97 | 9.16 ± 1.93 | 1.23 ± 0.34 | |
| PND10 | 10 (n = 7) | 20.12 ± 4.36 | 7.65 ± 1.08 | 1.76 ± 0.46 |
| 50 (n = 7) | 22.87 ± 6.49 | 9.43 ± 2.05 | 1.36 ± 0.34 | |
| 0 (n = 7) | 19.78 ± 2.77 | 7.04 ± 0.87 | 1.64 ± 0.29 | |
| PND20 | 10 (n = 7) | 22.40 ± 2.80 | 6.59 ± 1.00 | 1.35 ± 0.21 |
| 50 (n = 7) | 24.18 ± 3.56 | 10.37 ± 1.48 | 1.67 ± 0.25 | |
| 0 (n = 7) | 21.75 ± 3.95 | 9.77 ± 0.90 | 1.13 ± 0.23 | |
| PND30 | 10 (n = 7) | 17.73 ± 2.45 | 9.42 ± 1.87 | 0.95 ± 0.24 |
| 50 (n = 7) | 21.54 ± 2.67 | 9.73 ± 1.34 | 1.20 ± 0.21 | |
| 0 (n = 7) | 16.16 ± 1.77 | 8.03 ± 0.46 | 1.15 ± 0.15 | |
| PND40 | 10 (n = 7) | 23.20 ± 2.39 | 8.64 ± 0.28 | 1.13 ± 0.11 |
| 50 (n = 7) | 16.84 ± 1.45 | 8.15 ± 0.59 | 1.04 ± 0.10 |
| Liver | Kidney | ||
|---|---|---|---|
| PUPS | |||
| 0 (n = 5) | 96.76 ± 24.33 | 117.60 ± 21.31 | |
| PND 10 | 10 (n = 4) | 147.80 ± 37.15 | 217.00 ± 58.50 |
| 50 (n = 5) | 95.20 ± 16.92 | 113.10 ± 31.08 | |
| 0 (n = 5) | 90.49 ± 24.70 | 84.02 ± 33.51 | |
| PND 20 | 10 (n = 4) | 110.30 ± 29.88 | 122.00 ± 34.83 |
| 50 (n = 5) | 120.20 ± 27.05 | 138.10 ± 22.69 | |
| 0 (n = 5) | 62.65 ± 18.91 | 66.24 ± 18.91 | |
| PND 30 | 10 (n = 4) | 88.86 ± 20.55 | 95.44 ± 33.31 |
| 50 (n = 5) | 56.16 ± 19.30 | 61.83 ± 23.57 | |
| 0 (n = 5) | 89.55 ± 24.70 | 85.72 ± 27.94 | |
| PND 40 | 10 (n = 4) | 81.36 ± 26.62 | 92.07 ± 21.36 |
| 50 (n = 5) | 58.40 ± 20.45 | 63.39 ± 18.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galiciolli, M.E.A.; Pedroso, T.F.; Mesquita, M.; Oliveira, V.A.; Pereira, M.E.; Oliveira, C.S. Biochemical Parameters of Female Wistar Rats and Their Offspring Exposed to Inorganic Mercury in Drinking Water during the Gestational and Lactational Periods. Toxics 2022, 10, 664. https://doi.org/10.3390/toxics10110664
Galiciolli MEA, Pedroso TF, Mesquita M, Oliveira VA, Pereira ME, Oliveira CS. Biochemical Parameters of Female Wistar Rats and Their Offspring Exposed to Inorganic Mercury in Drinking Water during the Gestational and Lactational Periods. Toxics. 2022; 10(11):664. https://doi.org/10.3390/toxics10110664
Chicago/Turabian StyleGaliciolli, Maria Eduarda A., Taíse F. Pedroso, Mariana Mesquita, Vitor A. Oliveira, Maria E. Pereira, and Cláudia S. Oliveira. 2022. "Biochemical Parameters of Female Wistar Rats and Their Offspring Exposed to Inorganic Mercury in Drinking Water during the Gestational and Lactational Periods" Toxics 10, no. 11: 664. https://doi.org/10.3390/toxics10110664
APA StyleGaliciolli, M. E. A., Pedroso, T. F., Mesquita, M., Oliveira, V. A., Pereira, M. E., & Oliveira, C. S. (2022). Biochemical Parameters of Female Wistar Rats and Their Offspring Exposed to Inorganic Mercury in Drinking Water during the Gestational and Lactational Periods. Toxics, 10(11), 664. https://doi.org/10.3390/toxics10110664








