Influence of pH on the Kinetics and Products of Photocatalytic Degradation of Sulfonamides in Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Photocatalytic Process
2.3. Samples Analysis
2.4. Analysis of the Results
2.5. Toxicity Prediction
3. Results
3.1. Kinetics of SN Degradation
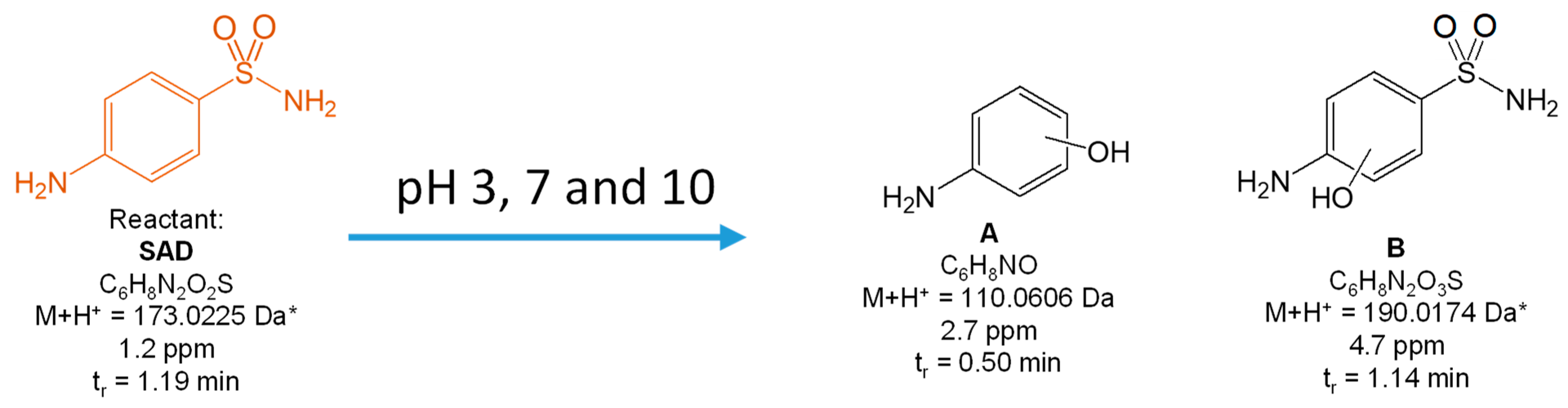
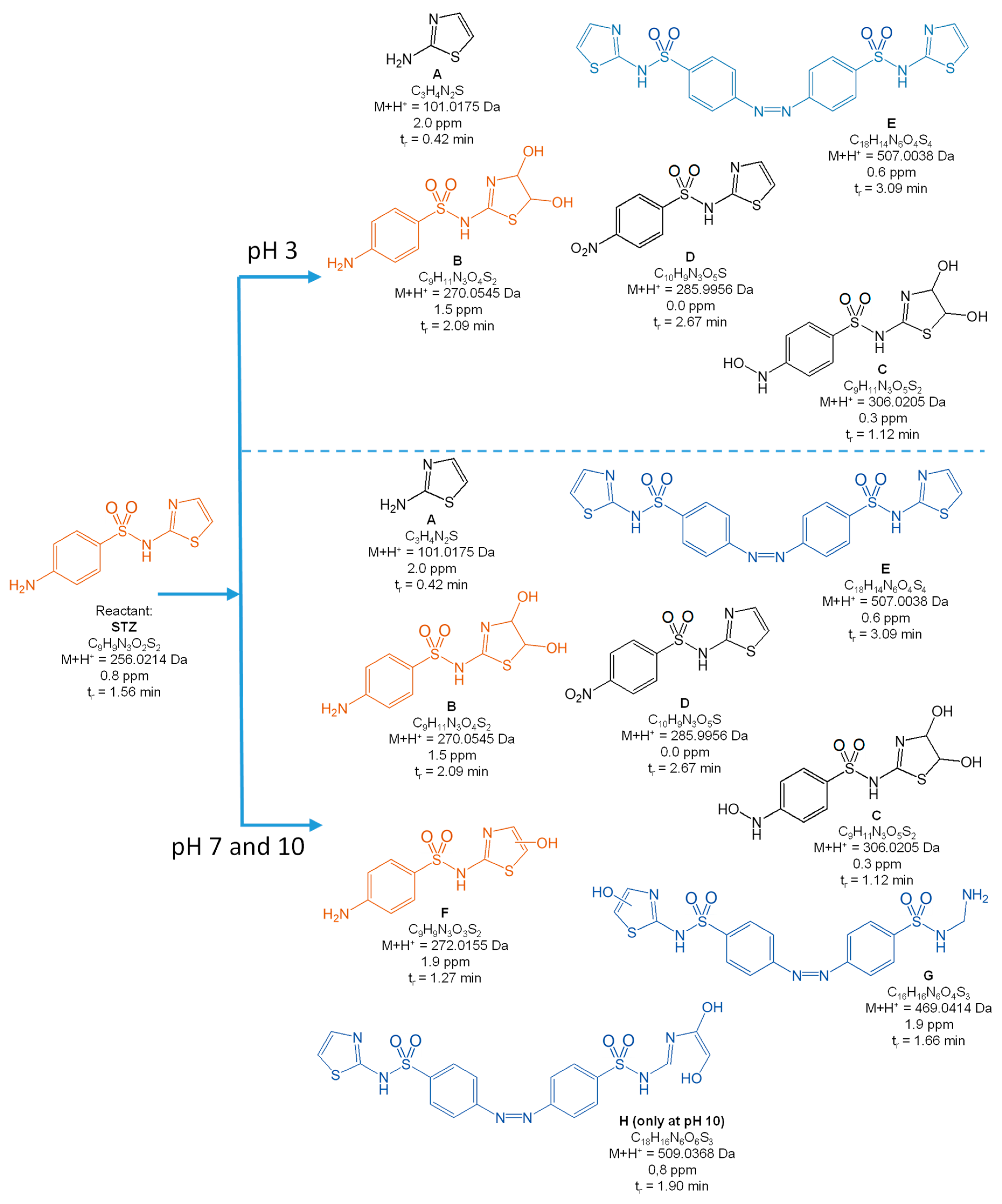
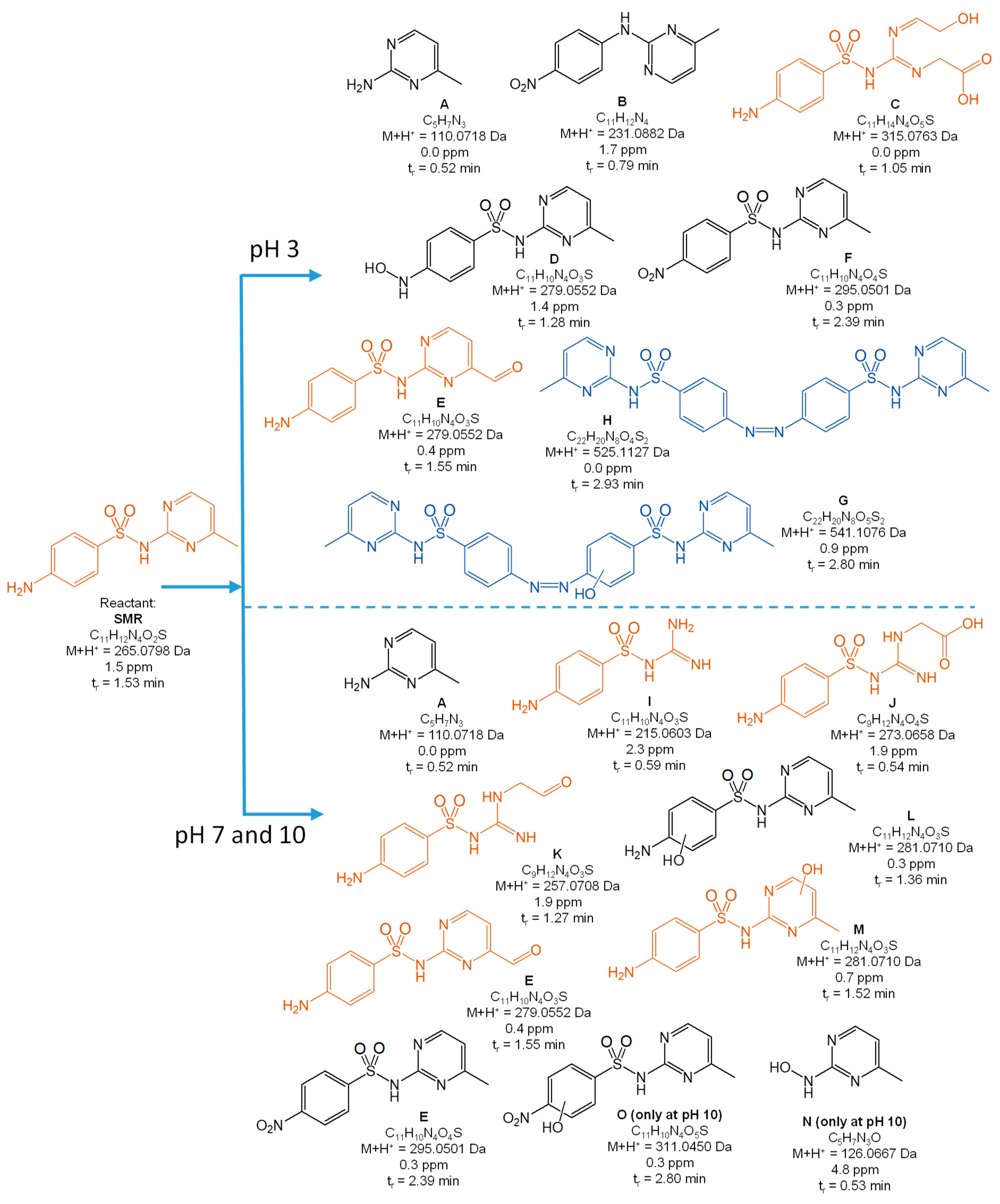
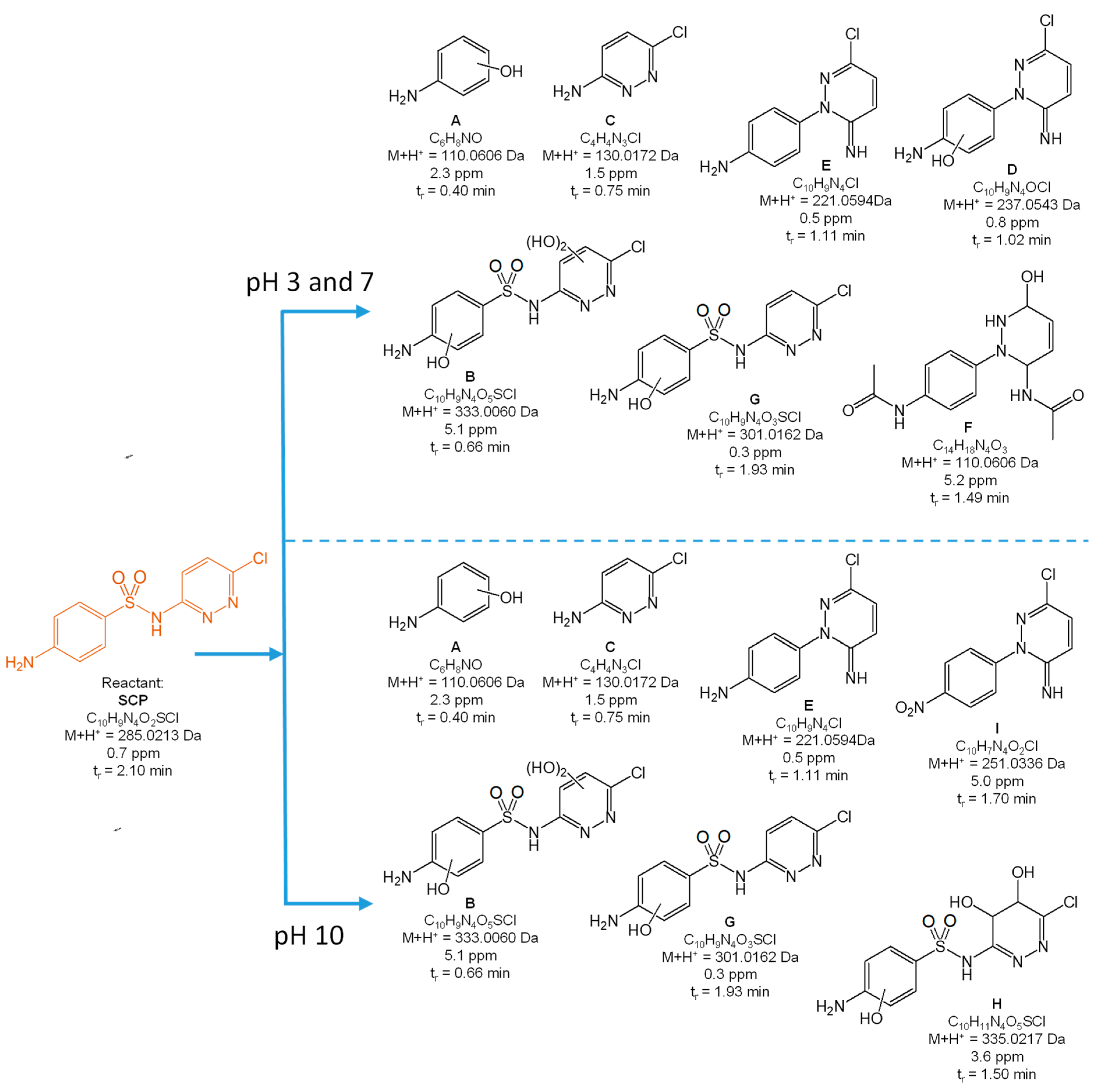
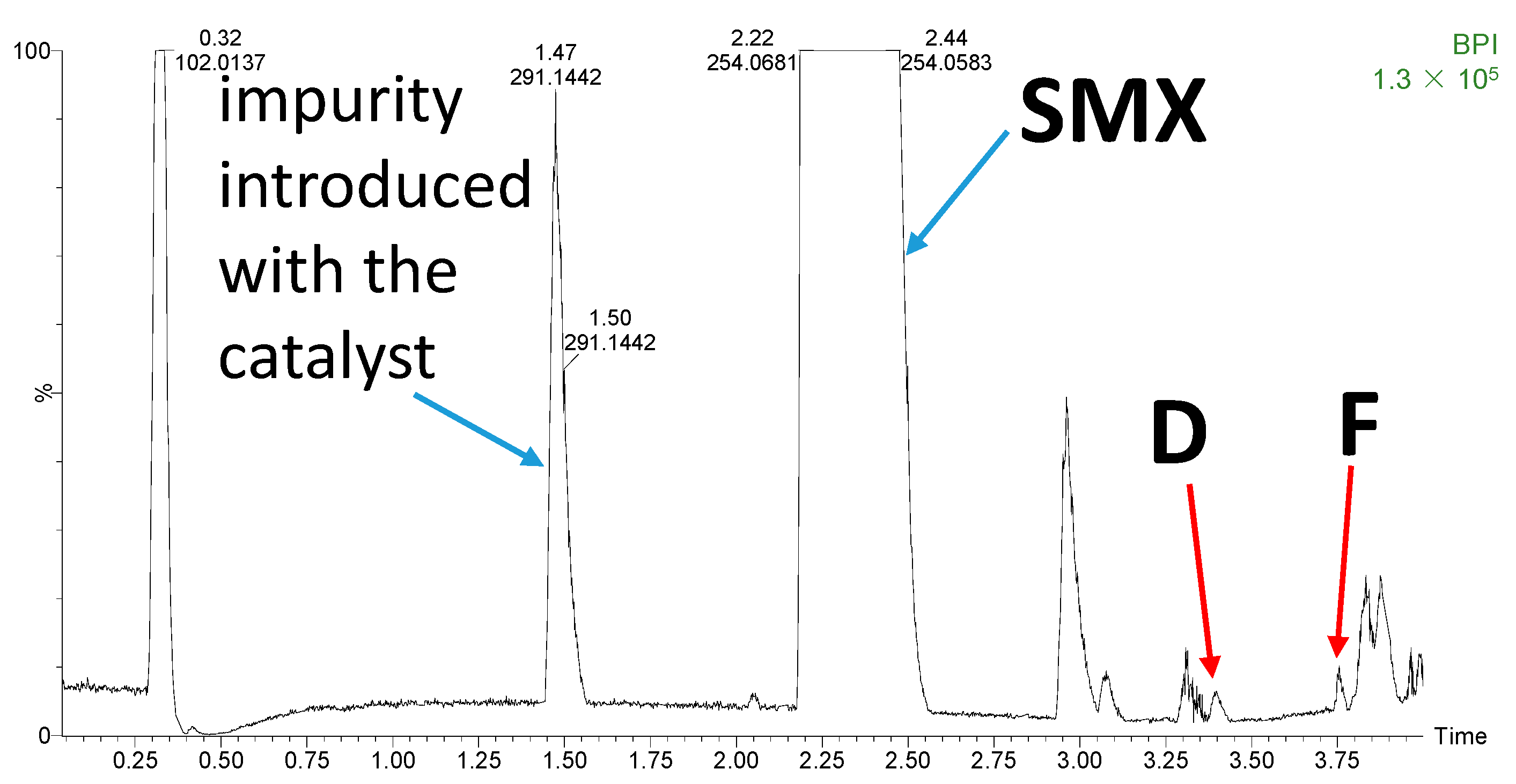
3.2. Degradation Products
3.3. Toxicity Prediction of the Degradation Products of SMX
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2019 and 2020. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2019-2020-trends-2010-2020-eleventh_en.pdf (accessed on 1 August 2022).
- FDA. Antimicrobials Sold or Distributed for Use in Food-Producing Animals. Available online: https://www.fda.gov/animal-veterinary/cvm-updates/fda-releases-annual-summary-report-antimicrobials-sold-or-distributed-2020-use-food-producing (accessed on 1 August 2022).
- Adamek, E.; Baran, W.; Sobczak, A. Assessment of the Biodegradability of Selected Sulfa Drugs in Two Polluted Rivers in Poland: Effects of Seasonal Variations, Accidental Contamination, Turbidity and Salinity. J. Hazard. Mater. 2016, 313, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chu, Y.; Fang, C. Occurrence of Veterinary Antibiotics in Swine Manure from Large-Scale Feedlots in Zhejiang Province, China. Bull. Environ. Contam Toxicol. 2017, 98, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Winfieldunited United. Asulam 3.3. Available online: https://www.winfieldunited.com/products/herbicides/asulam33/84 (accessed on 1 August 2022).
- Arena, M.; Auteri, D.; Barmaz, S.; Brancato, A.; Brocca, D.; Bura, L.; Chiusolo, A.; Court Marques, D.; Crivellente, F.; de Lentdecker, C.; et al. Peer Review of the Pesticide Risk Assessment of the Active Substance Asulam (Variant Evaluated Asulam-sodium). EFSA J. 2018, 16, e05251. [Google Scholar] [CrossRef]
- Alvarez, F.; Arena, M.; Auteri, D.; Borroto, J.; Brancato, A.; Carrasco Cabrera, L.; Castoldi, A.F.; Chiusolo, A.; Colagiorgi, A.; Colas, M.; et al. Updated Peer Review of the Pesticide Risk Assessment of the Active Substance Asulam (Variant Evaluated Asulam-sodium). EFSA J. 2021, 19, e06921. [Google Scholar] [CrossRef]
- Dekker, J.; Duke, S. Herbicide-Resistant Field Crops. Adv. Agron. 1995, 54, 69–116. [Google Scholar]
- Duke, S.O. Herbicide and Pharmaceutical Relationships. Weed Sci. 2010, 58, 334–339. [Google Scholar] [CrossRef]
- Ferrari, B.; Mons, R.; Vollat, B.; Fraysse, B.; Paxéus, N.; lo Giudice, R.; Pollio, A.; Garric, J. Environmental Risk Assessment of Six Human Pharmaceuticals: Are The Current Environmental Risk Assessment Procedures Sufficient For The Protection of The Aquatic Environment? Environ. Toxicol. Chem. 2004, 23, 1344. [Google Scholar] [CrossRef]
- Białk-Bielińska, A.; Stolte, S.; Arning, J.; Uebers, U.; Böschen, A.; Stepnowski, P.; Matzke, M. Ecotoxicity Evaluation of Selected Sulfonamides. Chemosphere 2011, 85, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, K.; Jung, J.; Park, S.; Kim, P.-G.; Park, J. Aquatic Toxicity of Acetaminophen, Carbamazepine, Cimetidine, Diltiazem and Six Major Sulfonamides, and Their Potential Ecological Risks in Korea. Environ. Int. 2007, 33, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Kim, Y.; Kim, J.; Jeong, D.-H.; Choi, K. Environmental Levels of Ultraviolet Light Potentiate the Toxicity of Sulfonamide Antibiotics in Daphnia Magna. Ecotoxicology 2008, 17, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Pascarella, L.; Parrella, A. Toxic and Genotoxic Evaluation of Six Antibiotics on Non-Target Organisms. Sci. Total Environ. 2005, 346, 87–98. [Google Scholar] [CrossRef]
- Hillis, D.G.; Antunes, P.; Sibley, P.K.; Klironomos, J.N.; Solomon, K.R. Structural Responses of Daucus Carota Root-Organ Cultures and the Arbuscular Mycorrhizal Fungus, Glomus Intraradices, to 12 Pharmaceuticals. Chemosphere 2008, 73, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Białk-Bielińska, A.; Caban, M.; Pieczyńska, A.; Stepnowski, P.; Stolte, S. Mixture Toxicity of Six Sulfonamides and Their Two Transformation Products to Green Algae Scenedesmus Vacuolatus and Duckweed Lemna Minor. Chemosphere 2017, 173, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Thiele-Bruhn, S.; Beck, I.-C. Effects of Sulfonamide and Tetracycline Antibiotics on Soil Microbial Activity and Microbial Biomass. Chemosphere 2005, 59, 457–465. [Google Scholar] [CrossRef]
- Kemper, N. Veterinary Antibiotics in the Aquatic and Terrestrial Environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Pazda, M.; Kumirska, J.; Stepnowski, P.; Mulkiewicz, E. Antibiotic Resistance Genes Identified in Wastewater Treatment Plant Systems—A Review. Sci. Total Environ. 2019, 697, 134023. [Google Scholar] [CrossRef]
- Długosz, M.; Żmudzki, P.; Kwiecień, A.; Szczubiałka, K.; Krzek, J.; Nowakowska, M. Photocatalytic Degradation of Sulfamethoxazole in Aqueous Solution Using a Floating TiO2-Expanded Perlite Photocatalyst. J. Hazard. Mater. 2015, 298, 146–153. [Google Scholar] [CrossRef]
- Biancullo, F.; Moreira, N.F.F.; Ribeiro, A.R.; Manaia, C.M.; Faria, J.L.; Nunes, O.C.; Castro-Silva, S.M.; Silva, A.M.T. Heterogeneous Photocatalysis Using UVA-LEDs for the Removal of Antibiotics and Antibiotic Resistant Bacteria from Urban Wastewater Treatment Plant Effluents. Chem. Eng. J. 2019, 367, 304–313. [Google Scholar] [CrossRef]
- Park, Y.; Kim, S.; Kim, J.; Khan, S.; Han, C. UV/TiO2 Photocatalysis as an Efficient Livestock Wastewater Quaternary Treatment for Antibiotics Removal. Water 2022, 14, 958. [Google Scholar] [CrossRef]
- Adamek, E.; Baran, W.; Ziemiańska, J.; Sobczak, A. Effect of FeCl3 on Sulfonamide Removal and Reduction of Antimicrobial Activity of Wastewater in a Photocatalytic Process with TiO2. Appl. Catal. B 2012, 126, 29–38. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, P.; Dai, L.; Li, S.; Yu, W.; Guan, J. In Situ Crystallization and Growth of TiO2 Nanospheres between MXene Layers for Improved Adsorption and Visible Light Photocatalysis. Catal. Sci. Technol. 2021, 11, 3834–3844. [Google Scholar] [CrossRef]
- Liu, X.; Wu, F.; Deng, N. Photoproduction of Hydroxyl Radicals in Aqueous Solution with Algae under High-Pressure Mercury Lamp. Environ. Sci. Technol. 2004, 38, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.M.; Varghese, R.; Aravindakumar, C.T. Photoproduction of Hydroxyl Radicals from Fe(III)-Hydroxy Complex: A Quantitative Assessment. J. Photochem. Photobiol. A Chem. 2001, 146, 67–73. [Google Scholar] [CrossRef]
- Baran, W.; Adamek, E.; Sobczak, A.; Makowski, A. Photocatalytic Degradation of Sulfa Drugs with TiO2, Fe Salts and TiO2/FeCl3 in Aquatic Environment—Kinetics and Degradation Pathway. Appl. Catal. B 2009, 90, 516–525. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, S.; Yang, J.; Wang, H.; Yu, H.; Chen, H.; Zhao, Y.; Yuan, X.; Chu, W.; Li, H. Near-Infrared Light Responsive TiO2 for Efficient Solar Energy Utilization. Adv. Funct. Mater. 2022, 32, 2108977. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, J.; Zhou, S.; Yu, H.; Liang, J.; Chu, W.; Li, H.; Wang, H.; Wu, Z.; Yuan, X. Strategies to Extend Near-Infrared Light Harvest of Polymer Carbon Nitride Photocatalysts. Coord. Chem. Rev. 2021, 439, 213947. [Google Scholar] [CrossRef]
- Sági, G.; Csay, T.; Szabó, L.; Pátzay, G.; Csonka, E.; Takács, E.; Wojnárovits, L. Analytical Approaches to the OH Radical Induced Degradation of Sulfonamide Antibiotics in Dilute Aqueous Solutions. J. Pharm. Biomed. Anal. 2015, 106, 52–60. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Wong, P.K. Quantitative Characterization of Hydroxyl Radicals Produced by Various Photocatalysts. J. Colloid. Interface Sci. 2011, 357, 163–167. [Google Scholar] [CrossRef]
- Shinde, S.S.; Bhosale, C.H.; Rajpure, K.Y. Kinetic Analysis of Heterogeneous Photocatalysis: Role of Hydroxyl Radicals. Catal. Rev. 2013, 55, 79–133. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous Photocatalysis and Its Potential Applications in Water and Wastewater Treatment: A Review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef]
- Náfrádi, M.; Veréb, G.; Firak, D.S.; Alapi, T. Photocatalysis: Introduction, Mechanism, and Effective Parameters. In Green Photocatalytic Semiconductors; Springer: Cham, Switzerland, 2022; pp. 3–31. [Google Scholar]
- Baran, W.; Cholewiński, M.; Sobczak, A.; Adamek, E. A New Mechanism of the Selective Photodegradation of Antibiotics in the Catalytic System Containing TiO2 and the Inorganic Cations. Int. J. Mol. Sci. 2021, 22, 8696. [Google Scholar] [CrossRef] [PubMed]
- Islam Molla, M.A.; Tateishi, I.; Furukawa, M.; Katsumata, H.; Suzuki, T.; Kaneco, S. Evaluation of Reaction Mechanism for Photocatalytic Degradation of Dye with Self-Sensitized TiO2 under Visible Light Irradiation. Open J. Inorg. Non-Met. Mater. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Mezyk, S.P.; Neubauer, T.J.; Cooper, W.J.; Peller, J.R. Free-Radical-Induced Oxidative and Reductive Degradation of Sulfa Drugs in Water: Absolute Kinetics and Efficiencies of Hydroxyl Radical and Hydrated Electron Reactions. J. Phys. Chem. A 2007, 111, 9019–9024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nosaka, Y. Mechanism of the OH Radical Generation in Photocatalysis with TiO2 of Different Crystalline Types. J. Phys. Chem. C 2014, 118, 10824–10832. [Google Scholar] [CrossRef]
- Guo, C.; Wang, K.; Hou, S.; Wan, L.; Lv, J.; Zhang, Y.; Qu, X.; Chen, S.; Xu, J. H2O2 and/or TiO2 Photocatalysis under UV Irradiation for the Removal of Antibiotic Resistant Bacteria and Their Antibiotic Resistance Genes. J. Hazard. Mater. 2017, 323, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Adamek, E.; Baran, W.; Sobczak, A. Photocatalytic Degradation of Veterinary Antibiotics: Biodegradability and Antimicrobial Activity of Intermediates. Process. Saf. Environ. Prot. 2016, 103, 1–9. [Google Scholar] [CrossRef]
- Yang, H.; Li, G.; An, T.; Gao, Y.; Fu, J. Photocatalytic Degradation Kinetics and Mechanism of Environmental Pharmaceuticals in Aqueous Suspension of TiO2: A Case of Sulfa Drugs. Catal. Today 2010, 153, 200–207. [Google Scholar] [CrossRef]
- Adamek, E.; Baran, W.; Sobczak, A. Effect of FeCl3 on the Photocatalytic Processes Initiated by UVa and Vis Light in the Presence of TiO2–P25. Appl. Catal. B 2015, 172–173, 136–144. [Google Scholar] [CrossRef]
- Nakabayashi, Y.; Nosaka, Y. OH Radical Formation at Distinct Faces of Rutile TiO2 Crystal in the Procedure of Photoelectrochemical Water Oxidation. J. Phys. Chem. C 2013, 117, 23832–23839. [Google Scholar] [CrossRef]
- Nakabayashi, Y.; Nosaka, Y. The PH Dependence of OH Radical Formation in Photo-Electrochemical Water Oxidation with Rutile TiO2 Single Crystals. Phys. Chem. Chem. Phys. 2015, 17, 30570–30576. [Google Scholar] [CrossRef]
- Boreen, A.L.; Arnold, W.A.; McNeill, K. Triplet-Sensitized Photodegradation of Sulfa Drugs Containing Six-Membered Heterocyclic Groups: Identification of an SO2 Extrusion Photoproduct. Environ. Sci. Technol. 2005, 39, 3630–3638. [Google Scholar] [CrossRef] [PubMed]
- Zarfl, C.; Matthies, M.; Klasmeier, J. A Mechanistical Model for the Uptake of Sulfonamides by Bacteria. Chemosphere 2008, 70, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Uhlemann, T.; Seidel, S.; Müller, C.W. Site-Specific Binding of a Water Molecule to the Sulfa Drugs Sulfamethoxazole and Sulfisoxazole: A Laser-Desorption Isomer-Specific UV and IR Study. Phys. Chem. Chem. Phys. 2018, 20, 6891–6904. [Google Scholar] [CrossRef] [PubMed]
- Tačić, A.; Nikolić, V.; Nikolić, L.; Savić, I. Antimicrobial Sulfonamide Drug. Adv. Technol. 2017, 6, 58–71. [Google Scholar] [CrossRef]
- Dong, S.; Pi, Y.; Li, Q.; Hu, L.; Li, Y.; Han, X.; Wang, J.; Sun, J. Solar Photocatalytic Degradation of Sulfanilamide by BiOCl/Reduced Graphene Oxide Nanocomposites: Mechanism and Degradation Pathways. J. Alloys Compd. 2016, 663, 1–9. [Google Scholar] [CrossRef]
- Pang, R.; Li, N.; Hou, Z.; Huang, J.; Yue, C.; Cai, Y.; Song, J. Degradation of Sulfonamide Antibiotics and a Structurally Related Compound by Chlorine Dioxide: Efficiency, Kinetics, Potential Products and Pathways. Chem. Eng. J. 2023, 451, 138502. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, Z.; Zhang, T.; Gao, N.; Ma, Y. Degradation Effect of Sulfa Antibiotics by Potassium Ferrate Combined with Ultrasound (Fe(VI)-US). Biomed Res. Int. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Calza, P.; Medana, C.; Pazzi, M.; Baiocchi, C.; Pelizzetti, E. Photocatalytic Transformations of Sulphonamides on Titanium Dioxide. Appl. Catal. B 2004, 53, 63–69. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Silvia Díaz-Cruz, M.; Barceló, D. Identification and Determination of Metabolites and Degradation Products of Sulfonamide Antibiotics. TrAC Trends Anal. Chem. 2008, 27, 1008–1022. [Google Scholar] [CrossRef]
- Bhat, A.P.; Gogate, P.R. Degradation of Nitrogen-Containing Hazardous Compounds Using Advanced Oxidation Processes: A Review on Aliphatic and Aromatic Amines, Dyes, and Pesticides. J. Hazard. Mater. 2021, 403, 123657. [Google Scholar] [CrossRef]
- Shah, S.; Hao, C. Quantum Chemical Investigation on Photodegradation Mechanisms of Sulfamethoxypyridazine with Dissolved Inorganic Matter and Hydroxyl Radical. J. Environ. Sci. 2017, 57, 85–92. [Google Scholar] [CrossRef] [PubMed]





| Name/CAS | Abbreviation | Structural Formula | Purity/Manufacturer | pKa1 a | pKa2 a |
|---|---|---|---|---|---|
| Sulfanilamide 63-74-1 | SAD | 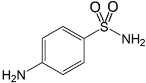 | 98.0%; Fluka | 6.92 ± 0.14 | 10.35 ± 0.21 |
| Sulfamethoxazole 723-46-6 | SMX | 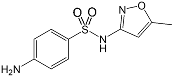 | 99.0%; Fluka | 1.86 ± 0.32 | 5.73 ± 0.20 |
| Sulfathiazole 72-14-0 | STZ |  | 99.0%; Sigma-Aldrich | 2.22 ± 0.27 | 7.15 ± 0.12 |
| Sulfisoxazole 127-69-5 | SFF | 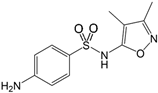 | 99.0%; Sigma-Aldrich | 1.69 ± 0.27 | 5.0 ± 0.0 |
| Sulfamerazine 127-79-7 | SMR | 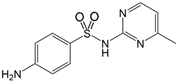 | 99.0%; Sigma-Aldrich | 2.21 ± 0.15 | 6.92 ± 0.14 |
| Sulfachloro-pyridazine 80-32-0 | SCP |  | 98.0%; Sigma-Aldrich | 2 ± 3 b | 5.90 ± 0.30 b |
| Eluent Gradient | |||
|---|---|---|---|
| Time (min) | H2O with 0.01% HCOOH | CH3CN with 0.01% HCOOH | Flow Rate (mL/min) |
| For SAD | |||
| 0 | 99.9% | 0.1% | 0.30 |
| 2 | 99.9% | 0.1% | 0.30 |
| 2.5 | 99.0% | 1.0% | 0.30 |
| 3 | 80.0% | 20% | 0.40 |
| 3.5 | 80.0% | 20% | 0.40 |
| 4 | 99.9% | 0.1% | 0.30 |
| For other SNs | |||
| 0 | 95% | 5% | 0.35 |
| 3.0 | 60% | 40% | |
| 3.3 | 20% | 80% | |
| 3.5 | 95% | 5% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapińska, D.; Adamek, E.; Masternak, E.; Zielińska-Danch, W.; Baran, W. Influence of pH on the Kinetics and Products of Photocatalytic Degradation of Sulfonamides in Aqueous Solutions. Toxics 2022, 10, 655. https://doi.org/10.3390/toxics10110655
Sapińska D, Adamek E, Masternak E, Zielińska-Danch W, Baran W. Influence of pH on the Kinetics and Products of Photocatalytic Degradation of Sulfonamides in Aqueous Solutions. Toxics. 2022; 10(11):655. https://doi.org/10.3390/toxics10110655
Chicago/Turabian StyleSapińska, Dominika, Ewa Adamek, Ewa Masternak, Wioleta Zielińska-Danch, and Wojciech Baran. 2022. "Influence of pH on the Kinetics and Products of Photocatalytic Degradation of Sulfonamides in Aqueous Solutions" Toxics 10, no. 11: 655. https://doi.org/10.3390/toxics10110655
APA StyleSapińska, D., Adamek, E., Masternak, E., Zielińska-Danch, W., & Baran, W. (2022). Influence of pH on the Kinetics and Products of Photocatalytic Degradation of Sulfonamides in Aqueous Solutions. Toxics, 10(11), 655. https://doi.org/10.3390/toxics10110655








