Exposure to Microplastics during Early Developmental Stage: Review of Current Evidence
Abstract
:1. Introduction
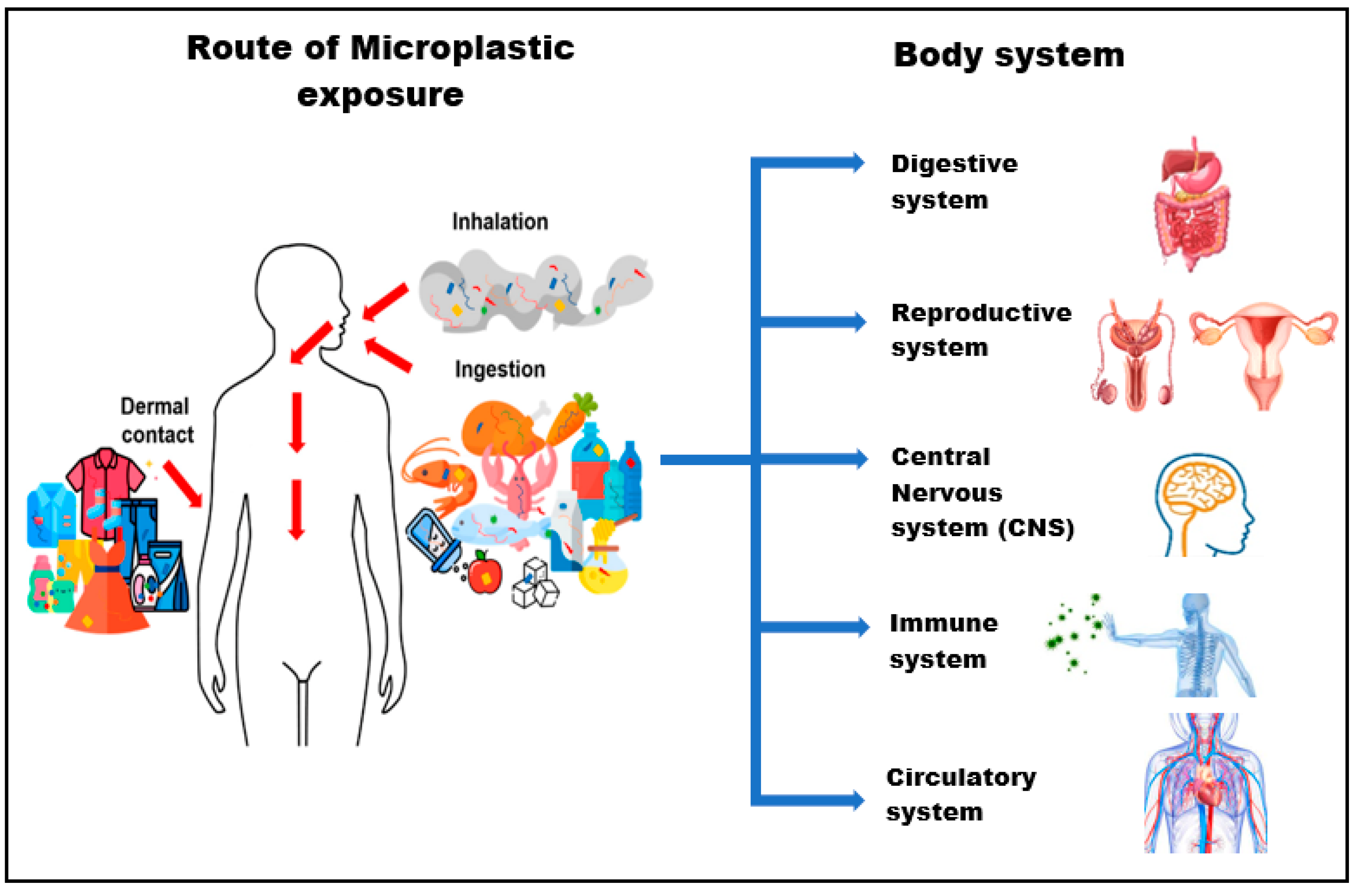
2. Sources and Modes of MP Transmission
3. Potential Risks of MPs Exposure during the Early Developmental Stage
3.1. Digestive System
3.2. Reproductive System
3.3. Central Nervous System
3.4. Immune System
3.5. Circulatory System
4. Limitations
5. Future Recommendations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, A.; Sarkar, A.; Yadav, O.P.; Achari, G.; Slobodnik, J. Potential human health risks due to environmental exposure to nano- and microplastics and knowledge gaps: A scoping review. Sci. Total Environ. 2021, 757, 143872. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Cheng, Q.; Ding, T.; Yiguang, Q.; Yuechao, Z.; Sun, H.; Peng, C.; Naz, I.; Li, J.; Liu, J. Micro- and nanoplastics in the environment: Occurrence, detection, characterization and toxicity—A critical review. J. Clean. Prod. 2021, 313, 127863. [Google Scholar] [CrossRef]
- O’Connor, J.; Mahon, A.M.; Ramsperger, A.; Trotter, B.; Redondo-Hasselerharm, P.; Koelmans, A.; Lally, H.; Murphy, S. Microplastics in Freshwater Biota: A Critical Review of Isolation, Characterization, and Assessment Methods. Glob. Chall. 2019, 4, 1800118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Chernick, M.; Rittschof, D.; Hinton, D.E. Chronic dietary exposure to polystyrene microplastics in maturing Japanese medaka (Oryzias latipes). Aquat. Toxicol. 2020, 220, 105396. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Venâncio, C.; Lopes, I.; Oliveira, M. Nanoplastics and marine organisms: What has been studied? Environ. Toxicol. Pharmacol. 2019, 67, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shi, Y.; Yang, L.; Xiao, L.; Kehoe, D.K.; Gun’ko, Y.K.; Boland, J.J.; Wang, J.J. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat. Food 2020, 1, 746–754. [Google Scholar] [CrossRef]
- Visalli, G.; Facciolà, A.; Pruiti Ciarello, M.; De Marco, G.; Maisano, M.; Di Pietro, A. Acute and Sub-Chronic Effects of Microplastics (3 and 10 µm) on the Human Intestinal Cells HT-29. Int. J. Environ. Res. Public Health 2021, 18, 5833. [Google Scholar] [CrossRef] [PubMed]
- Street, M.E.; Bernasconi, S. Microplastics, environment and child health. Ital. J. Pediatr. 2021, 47, 75. [Google Scholar] [CrossRef]
- Lucaccioni, L.; Trevisani, V.; Marrozzini, L.; Bertoncelli, N.; Predieri, B.; Lugli, L.; Berardi, A.; Iughetti, L. Endocrine-Disrupting Chemicals and Their Effects during Female Puberty: A Review of Current Evidence. Int. J. Mol. Sci. 2020, 21, 2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroquino, M.J.; Posada, M.; Landrigan, P.J. Environmental Toxicology: Children at Risk. In Environmental Toxicology: Selected Entries from the Encyclopedia of Sustainability Science and Technology; Laws, E.A., Ed.; Springer: New York, NY, USA, 2013; pp. 239–291. [Google Scholar] [CrossRef]
- Solleiro-Villavicencio, H.; Gomez-De León, C.T.; Del Río-Araiza, V.H.; Morales-Montor, J. The detrimental effect of microplastics on critical periods of development in the neuroendocrine system. Birth Defects Res. 2020, 112, 1326–1340. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sripada, K.; Wierzbicka, A.; Abass, K.; Grimalt, J.O.; Erbe, A.; Röllin, H.B.; Weihe, P.; Díaz, G.J.; Singh, R.R.; Visnes, T.; et al. A Children’s Health Perspective on Nano- and Microplastics. Environ. Health Perspect. 2022, 130, 15001. [Google Scholar] [CrossRef] [PubMed]
- Yee, M.S.; Hii, L.W.; Looi, C.K.; Lim, W.M.; Wong, S.F.; Kok, Y.Y.; Tan, B.K.; Wong, C.Y.; Leong, C.O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Santillo, D.; Miller, K.; Johnston, P. Microplastics as contaminants in commercially important seafood species. Integr. Environ. Assess. Manag. 2017, 13, 516–521. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Keong Choo, C.; Larat, V.; Galloway, T.S.; Salamatinia, B. The presence of microplastics in commercial salts from different countries. Sci. Rep. 2017, 7, 46173. [Google Scholar] [CrossRef] [Green Version]
- Weaver, J. Microplastics Found in Human Placentas. Available online: https://designedconscious.com/plastics-in-the-ocean/sustainability-news-stories/microplastics-found-in-human-placentas/ (accessed on 24 December 2020).
- Akhbarizadeh, R.; Moore, F.; Keshavarzi, B. Investigating microplastics bioaccumulation and biomagnification in seafood from the Persian Gulf: A threat to human health? Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2019, 36, 1696–1708. [Google Scholar] [CrossRef]
- Aurisano, N.; Huang, L.; Milà i Canals, L.; Jolliet, O.; Fantke, P. Chemicals of concern in plastic toys. Environ. Int. 2021, 146, 106194. [Google Scholar] [CrossRef]
- Luo, T.; Zhang, Y.; Wang, C.; Wang, X.; Zhou, J.; Shen, M.; Zhao, Y.; Fu, Z.; Jin, Y. Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ. Pollut. 2019, 255, 113122. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef]
- Hathaway, Q.A.; Nichols, C.E.; Shepherd, D.L.; Stapleton, P.A.; McLaughlin, S.L.; Stricker, J.C.; Rellick, S.L.; Pinti, M.V.; Abukabda, A.B.; McBride, C.R.; et al. Maternal-engineered nanomaterial exposure disrupts progeny cardiac function and bioenergetics. Am. J. Physiol.-Heart Circ. Physiol. 2017, 312, H446–H458. [Google Scholar] [CrossRef] [Green Version]
- Amato-Lourenço, L.F.; Dos Santos Galvão, L.; de Weger, L.A.; Hiemstra, P.S.; Vijver, M.G.; Mauad, T. An emerging class of air pollutants: Potential effects of microplastics to respiratory human health? Sci. Total Environ. 2020, 749, 141676. [Google Scholar] [CrossRef] [PubMed]
- Brasche, S.; Bischof, W. Daily time spent indoors in German homes—Baseline data for the assessment of indoor exposure of German occupants. Int. J. Hyg. Environ. Health 2005, 208, 247–253. [Google Scholar] [CrossRef]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of Nanoplastic in the Environment and Possible Impact on Human Health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Román, R.; Naik, A.; Kalia, Y.N.; Guy, R.H.; Fessi, H. Skin penetration and distribution of polymeric nanoparticles. J. Control. Release 2004, 99, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Biagini Myers, J.M.; Khurana Hershey, G.K. Eczema in early life: Genetics, the skin barrier, and lessons learned from birth cohort studies. J. Pediatr. 2010, 157, 704–714. [Google Scholar] [CrossRef] [Green Version]
- Senathirajah, K.; Attwood, S.; Bhagwat, G.; Carbery, M.; Wilson, S.; Palanisami, T. Estimation of the mass of microplastics ingested—A pivotal first step towards human health risk assessment. J. Hazard. Mater. 2021, 404, 124004. [Google Scholar] [CrossRef] [PubMed]
- Moya, J.; Bearer, C.F.; Etzel, R.A. Children’s behavior and physiology and how it affects exposure to environmental contaminants. Pediatrics 2004, 113, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Mohamed Nor, N.H.; Kooi, M.; Diepens, N.J.; Koelmans, A.A. Lifetime Accumulation of Microplastic in Children and Adults. Environ. Sci. Technol. 2021, 55, 5084–5096. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.T.; Rangel-Buitrago, N. The past, present, and future of plastic pollution. Mar. Pollut. Bull. 2022, 176, 113429. [Google Scholar] [CrossRef] [PubMed]
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Environment: Intake through the Food Web, Human Exposure and Toxicological Effects. Toxics 2021, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Wang, Y.; Zhao, H.; Wang, D.; Guo, M.; Mu, M.; Liu, Y.; Nie, X.; Li, B.; Li, J.; et al. A comparative review of microplastics and nanoplastics: Toxicity hazards on digestive, reproductive and nervous system. Sci. Total Environ. 2021, 774, 145758. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Yang, Y.F.; Chen, C.Y.; Lu, T.H.; Liao, C.M. Toxicity-based toxicokinetic/toxicodynamic assessment for bioaccumulation of polystyrene microplastics in mice. J. Hazard. Mater. 2019, 366, 703–713. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuan, Q.; Zhang, L.; Meng, L.; Fu, X.; Hou, Y. Polystyrene microplastics induced female reproductive toxicity in mice. J. Hazard. Mater. 2022, 424, 127629. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Qin, X.; Zhang, J.; Zhu, Y.; Zeng, W.; Lin, Y.; Liu, X. Polystyrene microplastics disturb maternal-fetal immune balance and cause reproductive toxicity in pregnant mice. Reprod. Toxicol. 2021, 106, 42–50. [Google Scholar] [CrossRef]
- Leonardi, A.; Cofini, M.; Rigante, D.; Lucchetti, L.; Cipolla, C.; Penta, L.; Esposito, S. The Effect of Bisphenol A on Puberty: A Critical Review of the Medical Literature. Int. J. Environ. Res. Public Health 2017, 14, 1044. [Google Scholar] [CrossRef] [Green Version]
- Ilekis, J.V.; Tsilou, E.; Fisher, S.; Abrahams, V.M.; Soares, M.J.; Cross, J.C.; Zamudio, S.; Illsley, N.P.; Myatt, L.; Colvis, C.; et al. Placental origins of adverse pregnancy outcomes: Potential molecular targets: An Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am. J. Obstet. Gynecol. 2016, 215, S1–S46. [Google Scholar] [CrossRef] [Green Version]
- Aneman, I.; Pienaar, D.; Suvakov, S.; Simic, T.P.; Garovic, V.D.; McClements, L. Mechanisms of Key Innate Immune Cells in Early- and Late-Onset Preeclampsia. Front. Immunol. 2020, 11, 1864. [Google Scholar] [CrossRef]
- Ticconi, C.; Pietropolli, A.; Di Simone, N.; Piccione, E.; Fazleabas, A. Endometrial Immune Dysfunction in Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2019, 20, 5332. [Google Scholar] [CrossRef] [Green Version]
- Park, E.J.; Han, J.S.; Park, E.J.; Seong, E.; Lee, G.H.; Kim, D.W.; Son, H.Y.; Han, H.Y.; Lee, B.S. Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation. Toxicol. Lett. 2020, 324, 75–85. [Google Scholar] [CrossRef]
- Newbold, R.R.; Jefferson, W.N.; Padilla-Banks, E. Prenatal exposure to bisphenol a at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ. Health Perspect. 2009, 117, 879–885. [Google Scholar] [CrossRef] [Green Version]
- Barakat, R.; Lin, P.P.; Rattan, S.; Brehm, E.; Canisso, I.F.; Abosalum, M.E.; Flaws, J.A.; Hess, R.; Ko, C. Prenatal Exposure to DEHP Induces Premature Reproductive Senescence in Male Mice. Toxicol. Sci. 2017, 156, 96–108. [Google Scholar] [CrossRef]
- Ma, T.; Yin, X.; Han, R.; Ding, J.; Zhang, H.; Han, X.; Li, D. Effects of In Utero Exposure to Di-n-Butyl Phthalate on Testicular Development in Rat. Int. J. Environ. Res. Public Health 2017, 14, 1284. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Lei, Z.; Cui, L.; Hou, Y.; Yang, L.; An, R.; Wang, Q.; Li, S.; Zhang, H.; Zhang, L. Polystyrene microplastics lead to pyroptosis and apoptosis of ovarian granulosa cells via NLRP3/Caspase-1 signaling pathway in rats. Ecotoxicol. Environ. Saf. 2021, 212, 112012. [Google Scholar] [CrossRef]
- Rachoń, D. Endocrine disrupting chemicals (EDCs) and female cancer: Informing the patients. Rev. Endocr. Metab. Disord. 2015, 16, 359–364. [Google Scholar] [CrossRef] [Green Version]
- DeBartolo, D.; Jayatilaka, S.; Yan Siu, N.; Rose, M.; Ramos, R.L.; Betz, A.J. Perinatal exposure to benzyl butyl phthalate induces alterations in neuronal development/maturation protein expression, estrogen responses, and fear conditioning in rodents. Behav. Pharmacol. 2016, 27, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Ejaredar, M.; Lee, Y.; Roberts, D.J.; Sauve, R.; Dewey, D. Bisphenol A exposure and children’s behavior: A systematic review. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 175–183. [Google Scholar] [CrossRef]
- Xu, X.H.; Zhang, J.; Wang, Y.M.; Ye, Y.P.; Luo, Q.Q. Perinatal exposure to bisphenol-A impairs learning-memory by concomitant down-regulation of N-methyl-D-aspartate receptors of hippocampus in male offspring mice. Horm. Behav. 2010, 58, 326–333. [Google Scholar] [CrossRef]
- Chang, H.; Wang, M.; Xia, W.; Chen, T.; Huo, W.; Mao, Z.; Zhu, Y.; Li, Y.; Xu, S. Perinatal exposure to low-dose bisphenol A disrupts learning/memory and DNA methylation of estrogen receptor alpha in the hippocampus. Toxicol. Res. 2016, 5, 828–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.M.; Jeon, S.; Jeong, H.J.; Kim, B.N.; Kim, Y. Dibutyl phthalate exposure during gestation and lactation in C57BL/6 mice: Maternal behavior and neurodevelopment in pups. Environ. Res. 2020, 182, 109025. [Google Scholar] [CrossRef]
- Kumar, D.; Thakur, M.K. Anxiety like behavior due to perinatal exposure to Bisphenol-A is associated with decrease in excitatory to inhibitory synaptic density of male mouse brain. Toxicology 2017, 378, 107–113. [Google Scholar] [CrossRef]
- Metwally, F.M.; Rashad, H.; Zeidan, H.M.; Kilany, A.; Abdol Raouf, E.R. Study of the Effect of Bisphenol A on Oxidative Stress in Children with Autism Spectrum Disorders. Indian J. Clin. Biochem. 2018, 33, 196–201. [Google Scholar] [CrossRef]
- Piler, M.B.; Radha, M.J. Gestational and lactational exposition to di-n-butyl phthalate increases neurobehavioral perturbations in rats: A three generational comparative study. Toxicol. Rep. 2020, 7, 480–491. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Saravia, J.; You, D.; Thevenot, P.; Lee, G.I.; Shrestha, B.; Lomnicki, S.; Cormier, S.A. Early-life exposure to combustion-derived particulate matter causes pulmonary immunosuppression. Mucosal Immunol. 2014, 7, 694–704. [Google Scholar] [CrossRef] [Green Version]
- Del Río-Araiza, V.H.; Palacios-Arreola, M.I.; Nava-Castro, K.E.; Pérez-Sánchez, N.Y.; Ruíz-Manzano, R.; Segovia-Mendoza, M.; Girón-Pérez, M.I.; Navidad-Murrieta, M.S.; Morales-Montor, J. Perinatal exposure to bisphenol A increases in the adulthood of the offspring the susceptibility to the human parasite Toxocara canis. Environ. Res. 2020, 184, 109381. [Google Scholar] [CrossRef] [PubMed]
- Lett, Z.; Hall, A.; Skidmore, S.; Alves, N.J. Environmental microplastic and nanoplastic: Exposure routes and effects on coagulation and the cardiovascular system. Environ. Pollut. 2021, 291, 118190. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.M.; Saranya, V.; Vijayakumar, S.; Mythili Meera, M.; Ruprekha, S.; Kunal, R.; Pranay, A.; Thomas, J.; Mukherjee, A.; Chandrasekaran, N. Assessment on interactive prospectives of nanoplastics with plasma proteins and the toxicological impacts of virgin, coronated and environmentally released-nanoplastics. Sci. Rep. 2019, 9, 8860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.; Choi, D.; Han, S.; Jung, S.Y.; Choi, J.; Hong, J. Potential toxicity of polystyrene microplastic particles. Sci. Rep. 2020, 10, 7391. [Google Scholar] [CrossRef]
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef] [Green Version]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.-A.; Cedervall, T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef] [Green Version]
- Segovia-Mendoza, M.; Nava-Castro, K.E.; Palacios-Arreola, M.I.; Garay-Canales, C.; Morales-Montor, J. How microplastic components influence the immune system and impact on children health: Focus on cancer. Birth Defects Res. 2020, 112, 1341–1361. [Google Scholar] [CrossRef]
- Ichimura, M.; Kato, S.; Tsuneyama, K.; Matsutake, S.; Kamogawa, M.; Hirao, E.; Miyata, A.; Mori, S.; Yamaguchi, N.; Suruga, K.; et al. Phycocyanin prevents hypertension and low serum adiponectin level in a rat model of metabolic syndrome. Nutr. Res. 2013, 33, 397–405. [Google Scholar] [CrossRef]
- Waring, R.H.; Harris, R.M.; Mitchell, S.C. Plastic contamination of the food chain: A threat to human health? Maturitas 2018, 115, 64–68. [Google Scholar] [CrossRef]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef]
- Sun, R.; Xu, K.; Yu, L.; Pu, Y.; Xiong, F.; He, Y.; Huang, Q.; Tang, M.; Chen, M.; Yin, L.; et al. Preliminary study on impacts of polystyrene microplastics on the hematological system and gene expression in bone marrow cells of mice. Ecotoxicol. Environ. Saf. 2021, 218, 112296. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Vargas-Morales, O.; Zern, B.; Anselmo, A.C.; Gupta, V.; Zakrewsky, M.; Mitragotri, S.; Muzykantov, V. The Effect of Polymeric Nanoparticles on Biocompatibility of Carrier Red Blood Cells. PLoS ONE 2016, 11, e0152074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapalamadugu, K.C.; Vandevoort, C.A.; Settles, M.L.; Robison, B.D.; Murdoch, G.K. Maternal bisphenol a exposure impacts the fetal heart transcriptome. PLoS ONE 2014, 9, e89096. [Google Scholar] [CrossRef] [PubMed]
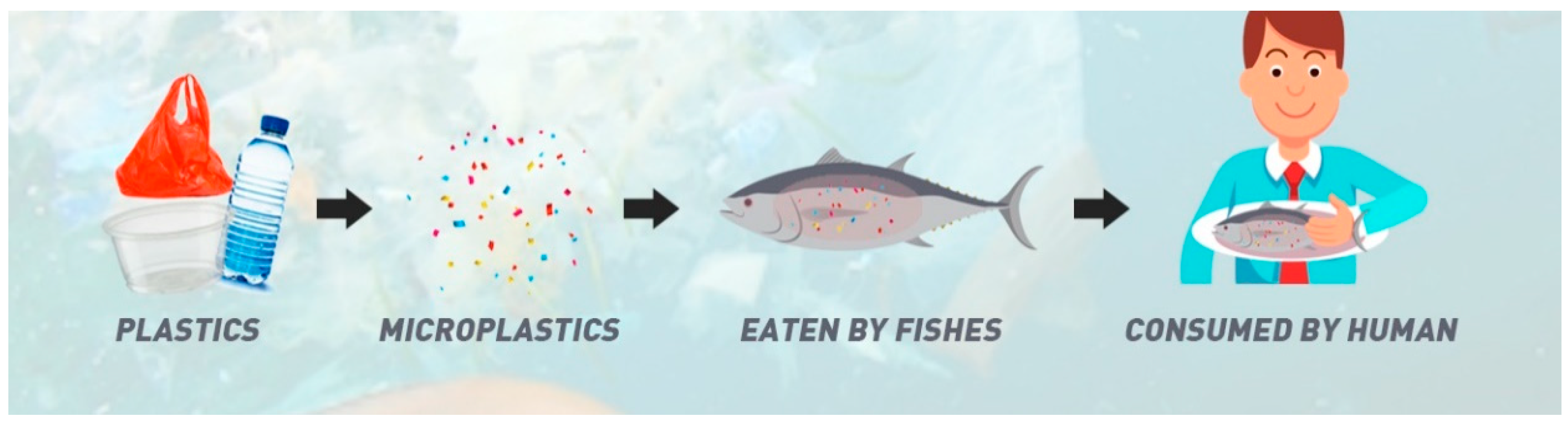
| Organisms | Types of MPs | Size (µm) and Concentration | Age and Duration of Exposure | Consequences to Early Life Stages | References |
|---|---|---|---|---|---|
| ICR mice | PS-MPs | Size: 5 µm and 20 µm | Age: 5 weeks Exposure: 28 days | -MPs accumulate in kidneys, liver, and gut -Induce disturbance of energy -Induce disturbance of lipid metabolism -Oxidative stress | [37] |
| Mice | Fluorescent PS-MPs | Size: 5 µm and 20 µm Concentration: 0.2 mg/mL | Age: 5 weeks Exposure: 28 days | -Decrease lipid metabolism-associated biomarkers of TG and total cholesterol (TCH) level -Change in oxidative stress markers -Change in energy and lipid metabolism | [39] |
| ICR Mice | PS-MPs | Size: 0.5 µm and 5 µm Concentration: 100 µg/L and 1000 µg/L | Age: offspring 1 day Exposure: from gestational day 1 to birthday 1 | -Decrease amino acid in female mouse offspring but increase it in male mouse offspring -Change in acyl-carnitine and free carnitine -Fatty acid metabolism disorder -Induce metabolic disorders in offspring | [21] |
| Mice (C57BL/6) | PE-MPs | Size: — Concentration: 6, 60, and 600 µg/day | Age: early exposure Exposure: 5 weeks | -Inflammation development -Decrease the percentage of Th17 and Trey -Inflammation to the intestine (duodenum and colon) -Induce intestinal dysbacteriosis | [22] |
| ICR mice | Pristine and fluorescent PS-MPs | Size: <5 µm Concentration: — | Age: 5 weeks Exposure: 6 weeks | -Reduce intestinal mucus secretion -Cause damage to the intestinal barrier function -Decrease actinobacteria content -Cause metabolic disorder -Alter the structure of gut microbiota in cecal contents -Impair intestinal barrier function -Increase TBA in the liver -Effect on feeding behavior and growth rate | [38] |
| Organisms | Types of MPs | Size (µm) and Concentration | Age and Duration of Exposure | Summary of Findings | References |
|---|---|---|---|---|---|
| ICR mice | PE-MPs | Size: — Concentration: 0.125, 0.5, and 2 mg/day/mouse | Age: 6 days (male and female) Exposure: 90 days | -Alter the number of live births -Decrease the sex ratio of pups -Induce damage to various tissues -Induce clinical and pathological changes -Alter growth and reproduction and increase the number of abnormal neonates | [47] |
| Mice | BPA (plastic additives) | Size: — Concentration: 0.1, 1, 10, 100, and 1000 µg/kg/day | Age: mice offspring 2 days after delivery Exposure: from Day 0 (pregnancy) to delivery | -Ovarian cyst -Structural (prenatal exposure) and cellular (neonatal exposure) alterations -Altered HOX gene expression during the differentiation of the reproductive tract -Oviductal alterations -Weak Erα binding -Uterus: Increase in stromal polyps -Transform SHE cells and induce aneuploidy | [48] |
| Male mice | DEHP | Size: 20, 200, and 500 µm Concentration: 750 mg/kg/day | Age: day 1 after birth Exposure: gestation day 11 until birth | -Gonadal dysfunction in offspring -Reduce fertility -Low serum testosterone, high estradiol, and high LH level -Epididymal abnormalities -Induce premature reproductive senescence | [49] |
| Rats | DBP (plastic additives) | Size: — Concentration: 50, 250, and 500 mg/kg/day | Age: 21 days Exposure: gestation day 14 until day 18 after birth | -Male development and reproduction toxicity -Decrease in AGD -Histological damage of the testis -Apoptosis of seminiferous tubule cells -Disruption of the expression of Rasd1 and MEKY2 and the Bcl-2/Bax ratio | [50] |
| Mice (57BL/6) | Saline of PS-MPs | Size: 5.0–5.9 µm Concentration: 0.1 mg/day | Age: 5 weeks Exposure: 30–40 days | -Change in the sex ratio of offspring -NLRP3/Caspase-1 signaling pathway -Decrease ovarian reserve -Decrease the number of total follicles and ovary size | [51] |
| Organisms | Types of MPs | Size (µm) and Concentration | Age and Duration of Exposure | Consequences to Early Life Stages | References |
|---|---|---|---|---|---|
| Mice | DBP | Size: — Concentration: 0, 50, and 100 mg/kg/day | Age: offspring Exposure: gestation day 13 to postnatal day 15 | -Reduction in the protein expression levels of Nr4a3, Egr1, Arc, and BDNF, and the phosphorylation of AKT -Decrease scores in negative geotaxis at PND 7 and swimming scores and olfactory orientation tests at PND 14 -Increase dark neurons -Delay pup development | [57] |
| Inbred Swiss albino mice | BPA (plastic additives) | Size: — Concentration: 50 µg/kg/day | Age: 21 days Exposure: 3 weeks and 8 weeks | -Anxiety-like behavior -Alterations in the ratio of excitatory–inhibitory proteins -Inhibited PSD95 expression in the cerebral cortex and hippocampus -Reduce morphological changes, spine stability, and blocked LTP induction | [58] |
| Mice | Pristine PS-MPs | Size: 5 µm and 20 µm Concentration: - | Age: 5 weeks Exposure: 28 days | -Increase activity of AChE: reduction in cholinergic neurotransmission efficiency -Increase threonine, aspartate, and taurine in serum (neurotransmitter substance) -Reduce phenylalanine | [37] |
| Human (infants) | BPA (plastic additives) | No data | Age: 6 years Exposure: no data | -Increase oxidative stress -Cause mitochondrial dysfunction that will disturb the function and behavior of children with ASD | [59] |
| Winstar stain rats | DBP (plastic additives) | Size: — Concentration: 500 mg/kg BW | Age: no data Exposure: gestation days 6 to 21 (3 weeks lactation) | -Changes in sensory motor development reflex response -Low memory retention -Alteration of cytoarchitecture in the hippocampus -Disrupt neural and endocrine functions | [60] |
| Organisms | Types of MPs | Size (µm) and Concentration | Age and Duration of Exposure | Consequences to Early Life Stages | References |
|---|---|---|---|---|---|
| ICR mice | PS-MPs | Size: 40–48 µm Concentration: 10 mg/kg/day | Age: 6 weeks Exposure: 90 days | -Induce immune response -Physical stress on stomach walls -Disrupt metabolic homeostasis -Alter composition of lymphocytes -Increase IgA concentration -Accumulation of damaged organelles | [47] |
| Mice (BALB/C and C57BL/6) | PS-MPs | Size: 10 µm Concentration: — | Age: 8–10 weeks (old mice) Exposure: during peri-implantation period | -Decrease percentage of decidual natural killer cells -Cytokine secretion shifts toward an immunosuppressive state -Decrease NK cells in the decidua -Disturb pro-inflammatory cells (T8 cells and M1-subtype macrophage) -Disturb pro-inflammatory cytokinase (IL-2, IL-6, TNF-α, and IFN-γ) -Oxidative stress to immune cells | [42] |
| C57BL/6 mice | PE-MPs | Size: No data Concentration: 6, 60, and 600 µg/day | Age: early exposure Exposure: 5 weeks | -Decrease the percentage of Th17 and Treg cells among CD4+ cells -Induce inflammation and higher TLR4, AP-1, and IRF5 expression -Induce intestinal dysbacteriosis and inflammation | [22] |
| Mice (Mus musculus) | PE-MPs | Size: 45–53 µm Concentration: – | Age: 5 weeks Exposure: 30 days | -Dysfunction of the intestinal epithelial barrier -Alteration of gut microbiota, which leads to abnormal immune response -Change in genera of important microbes that are essential for energy metabolism and immune function (e.g., Butyricimonas, Lactobacillus, and Ruminococcus) | [37] |
| Wistar rats | BPA (plastic additives) | Size: 45–53 µm Concentration: 250 µg/kg/day | Age: postnatal Exposure: day 5 of pregnancy to day 21 postnatal | -Decrease in the production of specific antibodies -Downregulate Th2 cytokines (IL-4, IL-5, and IL-13), and upregulate Th1 cytokines (IFN-γ and TNF-α) -Affect the performance of immune response during adult life -Abnormal cytokine and antibody production | [63] |
| Organisms | Types of MPs | Size (µm) and Concentration | Age and Duration of Exposure | Consequences to Early Life Stages | References |
|---|---|---|---|---|---|
| C57BL/6 mice | PS-MPs | Size: 5 µm Concentration: 0.1 mg and 0.5 mg | Age: 5 weeks Exposure: 28 days | -Decrease white blood cell count -Increase pit count -Induce oxidative stress -Inhibit the colony-forming ability of bone marrow cells -Damage blood system | [73] |
| Male Wistar rats | PS-MPs | Size: 0.5 µm Concentration: 0.5, 5, and 50 mg/L | Age: offspring Exposure: 90 days | -Lead to collagen proliferation of the heart -Induce oxidative stress -Activate the cardiac fibrosis-related Wnt/β-catenin signaling pathway -Lead to cardiovascular toxicity -Damage structure | [22] |
| Mice | PE | Size: 200 nm Concentration: — | Age: offspring | -Increase lipid peroxidation and alter membrane structure -Contribute to RBC aging and removal from circulation -Impair RBC for oxygen transport -Ability of PE particles to circulate naturally for long times in the bloodstream -Transfer PE reversibly to the pulmonary vasculature via RBC carriage | [74] |
| Monkey | BPA | Size: — Concentration: 400 µg/kg bw | -Effect of cardiovascular fitness -Alter transcription of genes that are reorganized for their role in cardiac pathophysiology -Upregulate ventricles and the right atrium of the heart | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amran, N.H.; Zaid, S.S.M.; Mokhtar, M.H.; Manaf, L.A.; Othman, S. Exposure to Microplastics during Early Developmental Stage: Review of Current Evidence. Toxics 2022, 10, 597. https://doi.org/10.3390/toxics10100597
Amran NH, Zaid SSM, Mokhtar MH, Manaf LA, Othman S. Exposure to Microplastics during Early Developmental Stage: Review of Current Evidence. Toxics. 2022; 10(10):597. https://doi.org/10.3390/toxics10100597
Chicago/Turabian StyleAmran, Nur Hanisah, Siti Sarah Mohamad Zaid, Mohd Helmy Mokhtar, Latifah Abd Manaf, and Shatrah Othman. 2022. "Exposure to Microplastics during Early Developmental Stage: Review of Current Evidence" Toxics 10, no. 10: 597. https://doi.org/10.3390/toxics10100597








