Nanoplastics and Microplastics May Be Damaging Our Livers
Abstract
1. Introduction
2. Methods
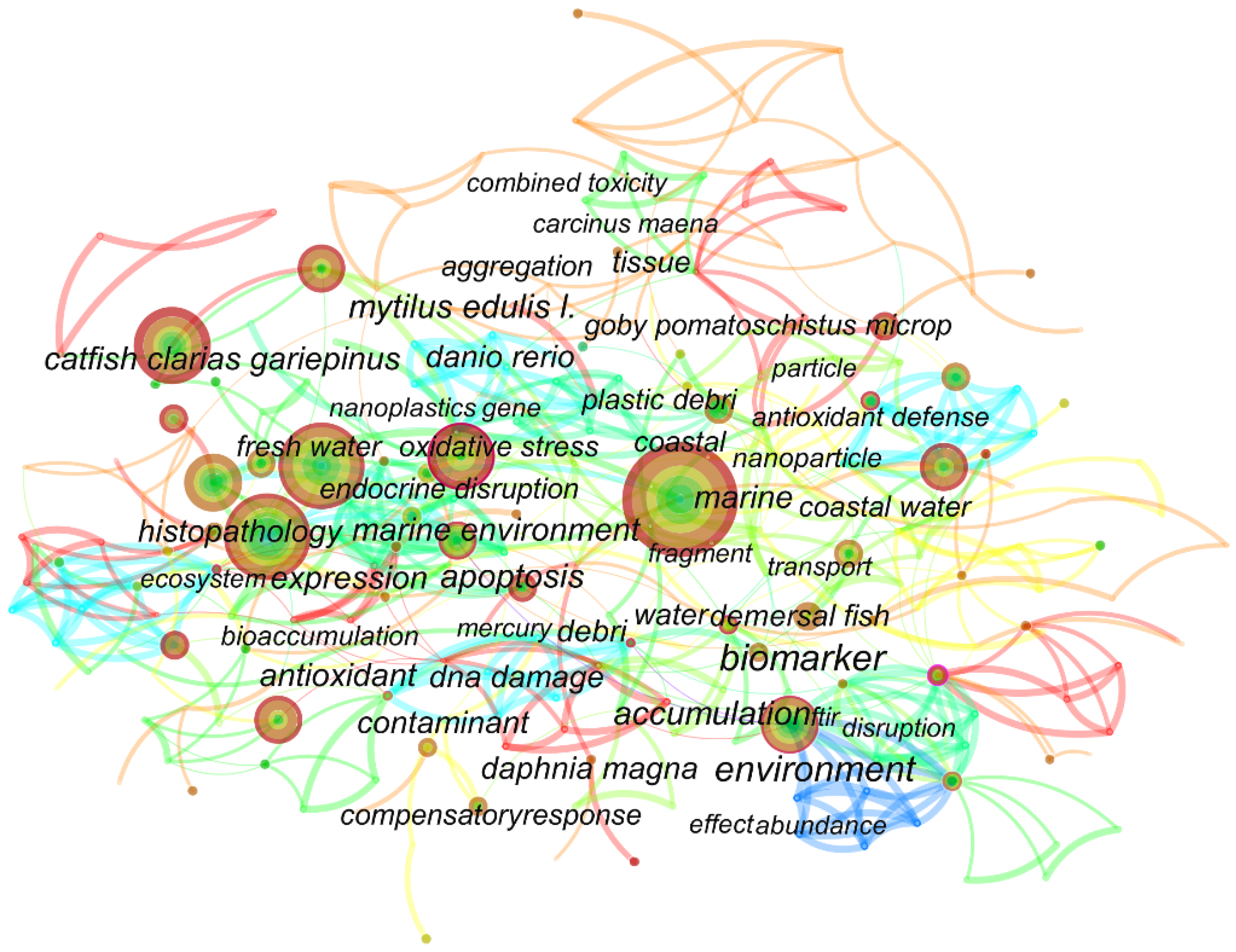
3. Keyword Co-Occurrence Analysis
4. Toxicity of MPs/NPs on the Liver
4.1. Internalization of MPs and NPs in Different Organisms
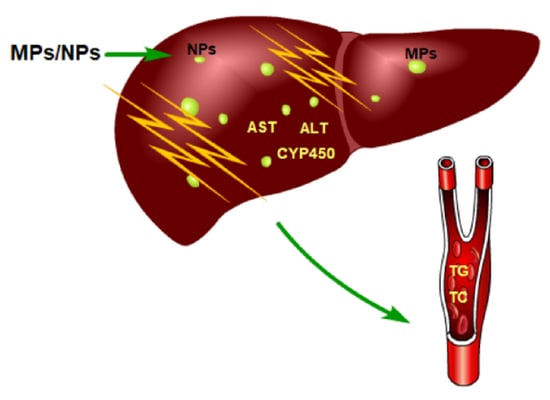
4.2. Accumulation of MPs and NPs in the Liver
4.3. Liver Morphological Changes Caused by MPs and NPs
4.4. Changes in Liver Function Caused by MPs and NPs
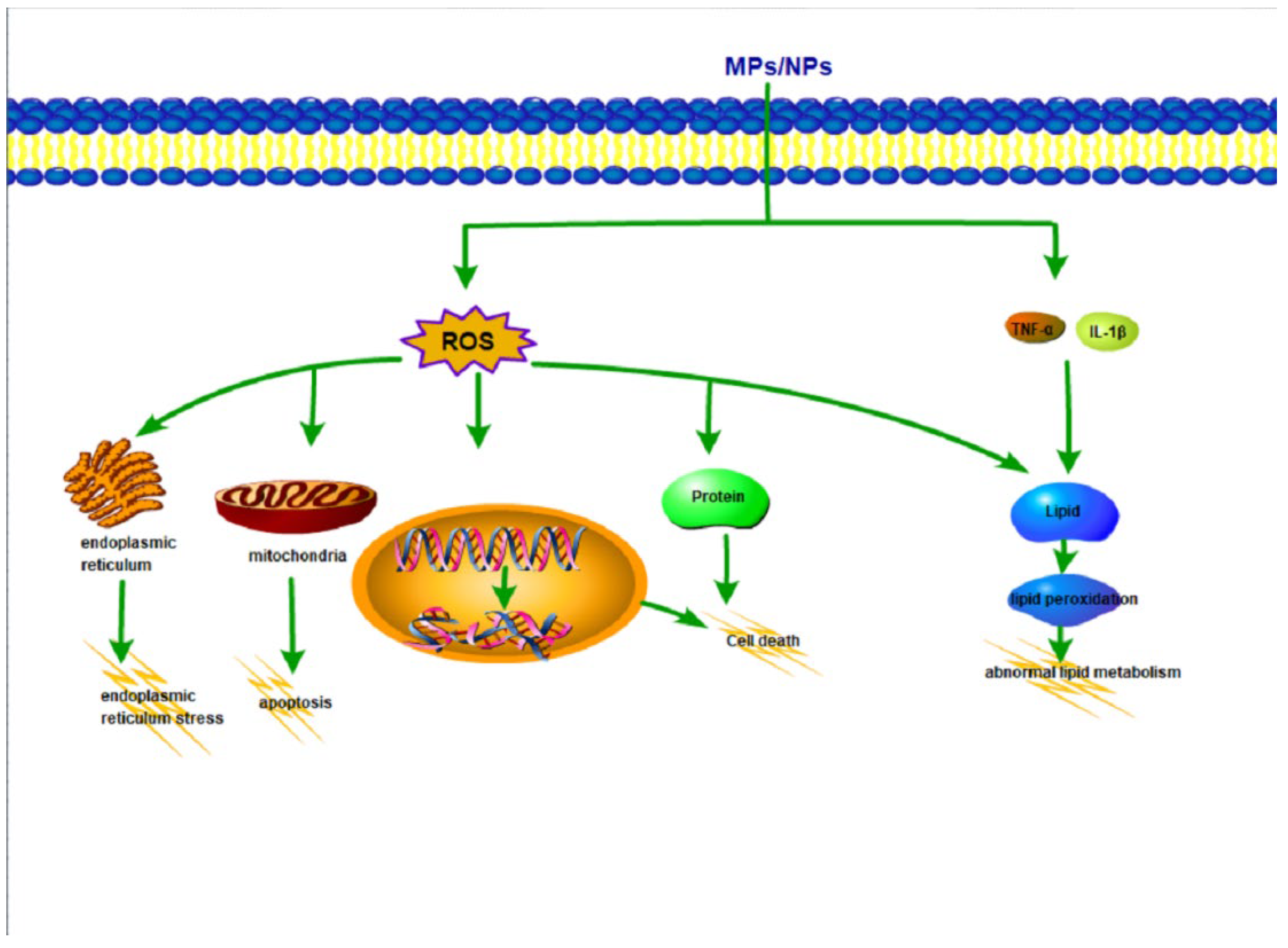
5. Potential Mechanisms of MPs/NPs Toxicity on the Liver
5.1. Oxidative Stress
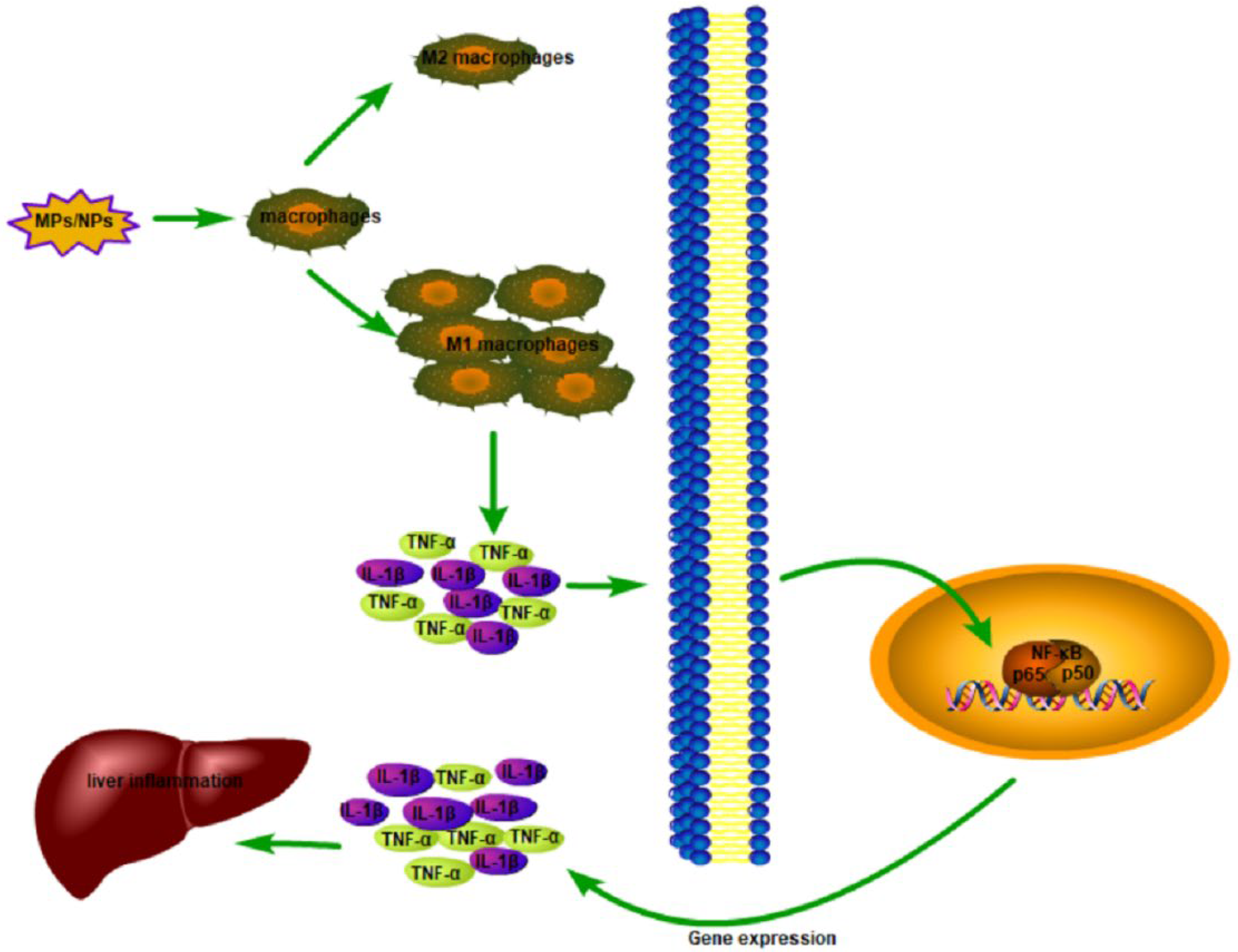
5.2. Inflammation
5.3. Lipid Metabolism
5.4. Energy Metabolism
5.5. Programmed Cell Death
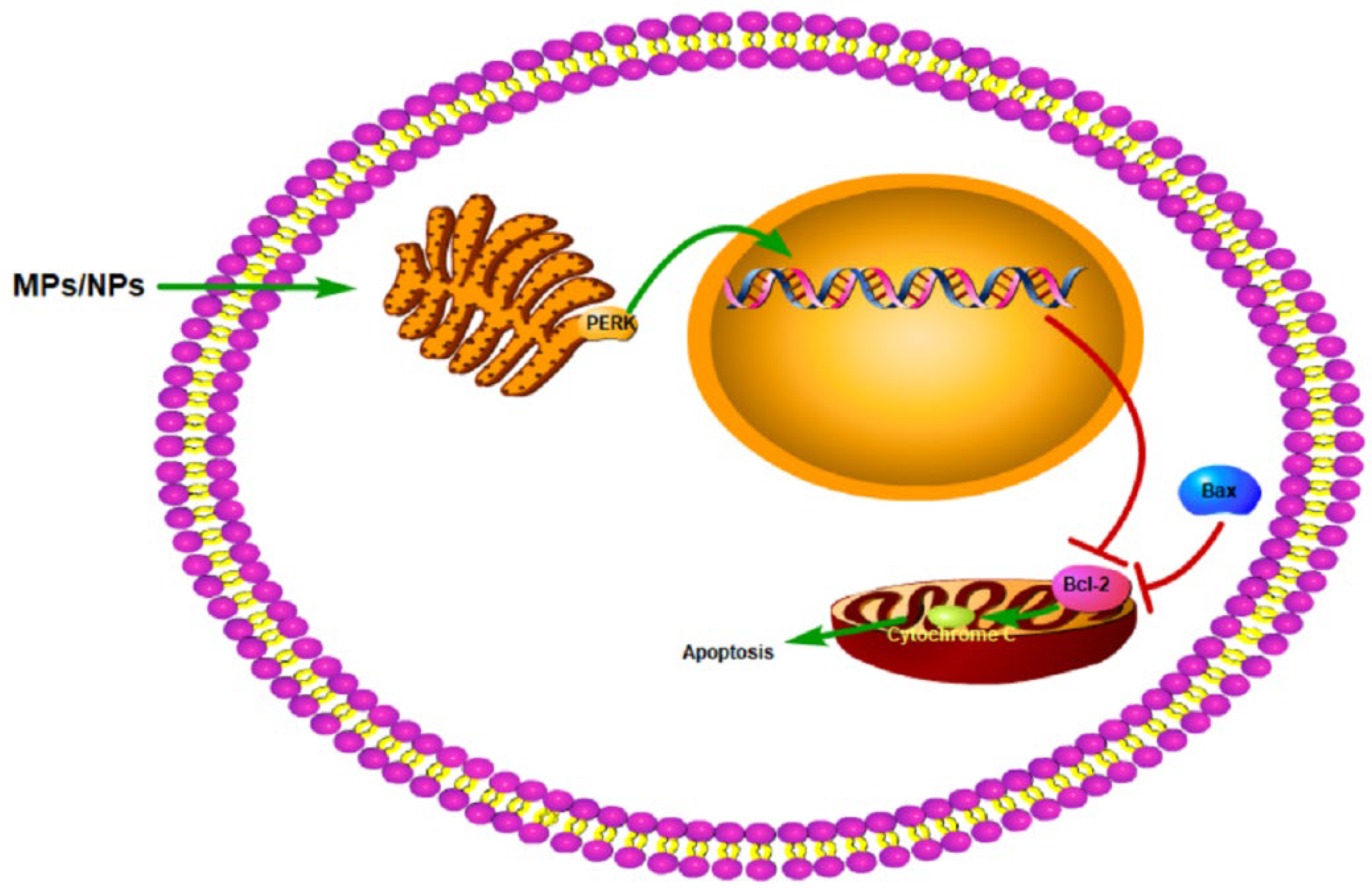
5.5.1. Apoptosis
5.5.2. Pyroptosis and Ferroptosis
5.6. Other Mechanisms
5.6.1. Mitochondrial Damage
5.6.2. Autophagy
5.6.3. Endoplasmic Reticulum Stress
6. Conclusions
6.1. Animal Health
6.2. Human Health
6.3. What Can We Do in the Future?
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.J. Solving the plastic problem: From cradle to grave, to reincarnation. Sci. Prog. 2019, 102, 218–248. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Dawson, A.L.; Kawaguchi, S.; King, C.; Townsend, K.; King, R.; Huston, W.; Nash, S.B. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 2018, 9, 1001. [Google Scholar] [CrossRef]
- Chae, Y.; Kim, D.; Kim, S.W.; An, Y.-J. Trophic transfer and individual impact of nano-sized polystyrene in a four-species freshwater food chain. Sci. Rep. 2018, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, Á.T.; Navarro, S.; García-De-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Scheurer, M.; Bigalke, M. Microplastics in Swiss Floodplain Soils. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Lwanga, E.H.; Mendoza-Vega, J.; Quej, V.K.; Chi, J.D.L.A.; Del Cid, L.S.; Chi, C.; Escalona-Segura, G.; Gertsen, H.; Salánki, T.; Van Der Ploeg, M.; et al. Field evidence for transfer of plastic debris along a terrestrial food chain. Sci. Rep. 2017, 7, 14071. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Welden, N.A.; Cowie, P.R. Long-term microplastic retention causes reduced body condition in the langoustine, Nephrops norvegicus. Environ. Pollut. 2016, 218, 895–900. [Google Scholar] [CrossRef]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.-A.; Cedervall, T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Janssen, C. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Non-pollen particulates in honey and sugar. Food Addit. Contam. Part A 2013, 30, 2136–2140. [Google Scholar] [CrossRef]
- Gautam, R.; Jo, J.; Acharya, M.; Maharjan, A.; Lee, D.; K.C., P.B.; Kim, C.; Kim, K.; Kim, H.; Heo, Y. Evaluation of potential toxicity of polyethylene microplastics on human derived cell lines. Sci. Total Environ. 2022, 838, 156089. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Yousefi, N.; Tufenkji, N. Are There Nanoplastics in Your Personal Care Products? Environ. Sci. Technol. Lett. 2017, 4, 280–285. [Google Scholar] [CrossRef]
- Pauly, J.L.; Stegmeier, S.J.; A Allaart, H.; Cheney, R.T.; Zhang, P.J.; Mayer, A.G.; Streck, R.J. Inhaled cellulosic and plastic fibers found in human lung tissue. Cancer Epidemiol. Biomark. Prev. 1998, 7, 419–428. [Google Scholar]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2020, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Nor, N.H.M.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H.-Q. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Babaei, A.A.; Rafiee, M.; Khodagholi, F.; Ahmadpour, E.; Amereh, F. Nanoplastics-induced oxidative stress, antioxidant defense, and physiological response in exposed Wistar albino rats. Environ. Sci. Pollut. Res. 2021, 29, 11332–11344. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, srep46687. [Google Scholar] [CrossRef]
- Jin, H.; Yan, M.; Pan, C.; Liu, Z.; Sha, X.; Jiang, C.; Li, L.; Pan, M.; Li, D.; Han, X.; et al. Chronic exposure to polystyrene microplastics induced male reproductive toxicity and decreased testosterone levels via the LH-mediated LHR/cAMP/PKA/StAR pathway. Part. Fibre Toxicol. 2022, 19, 13. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Li, R.; Zhou, Q.; Peijnenburg, W.J.G.M.; Yin, N.; Yang, J.; Tu, C.; Zhang, Y. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustain. 2020, 3, 929–937. [Google Scholar] [CrossRef]
- Gambardella, C.; Morgana, S.; Bramini, M.; Rotini, A.; Manfra, L.; Migliore, L.; Piazza, V.; Garaventa, F.; Faimali, M. Ecotoxicological effects of polystyrene microbeads in a battery of marine organisms belonging to different trophic levels. Mar. Environ. Res. 2018, 141, 313–321. [Google Scholar] [CrossRef]
- Chen, Y.; Ling, Y.; Li, X.; Hu, J.; Cao, C.; He, D. Size-dependent cellular internalization and effects of polystyrene microplastics in microalgae P. helgolandica var. tsingtaoensis and S. quadricauda. J. Hazard. Mater. 2020, 399, 123092. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y.; Liang, Y.; Cao, W.; Sun, C.; Ju, P.; Zheng, L. The interactions between microplastic polyvinyl chloride and marine diatoms: Physiological, morphological, and growth effects. Ecotoxicol. Environ. Saf. 2020, 203, 111000. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Coppock, R.; Lindeque, P.K.; Altin, D.; Reed, S.; Pond, D.W.; Sørensen, L.; Galloway, T.S.; Booth, A.M. Effects of Nylon Microplastic on Feeding, Lipid Accumulation, and Moulting in a Coldwater Copepod. Environ. Sci. Technol. 2019, 53, 7075–7082. [Google Scholar] [CrossRef]
- Ma, C.; Chen, Q.; Li, J.; Li, B.; Liang, W.; Su, L.; Shi, H. Distribution and translocation of micro- and nanoplastics in fish. Crit. Rev. Toxicol. 2021, 51, 740–753. [Google Scholar] [CrossRef]
- Forte, M.; Iachetta, G.; Tussellino, M.; Carotenuto, R.; Prisco, M.; De Falco, M.; Laforgia, V.; Valiante, S. Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells. Toxicol. In Vitro 2016, 31, 126–136. [Google Scholar] [CrossRef]
- Yan, Y.; Such, G.; Johnston, A.; Best, J.; Caruso, F. Engineering Particles for Therapeutic Delivery: Prospects and Challenges. ACS Nano 2012, 6, 3663–3669. [Google Scholar] [CrossRef]
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.; Farokhzad, O.C. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control. Release 2014, 190, 485–499. [Google Scholar] [CrossRef]
- Dausend, J.; Musyanovych, A.; Dass, M.; Walther, P.; Schrezenmeier, H.; Landfester, K.; Mailänder, V. Uptake Mechanism of Oppositely Charged Fluorescent Nanoparticles in HeLa Cells. Macromol. Biosci. 2008, 8, 1135–1143. [Google Scholar] [CrossRef]
- Dos Santos, T.; Varela, J.; Lynch, I.; Salvati, A.; Dawson, K.A. Effects of transport inhibitors on the cellular uptake of carboxylated polystyrene nanoparticles in different cell lines. PLoS ONE 2011, 6, e24438. [Google Scholar] [CrossRef] [PubMed]
- Brandts, I.; Garcia-Ordoñez, M.; Tort, L.; Teles, M.; Roher, N. Polystyrene nanoplastics accumulate in ZFL cell lysosomes and in zebrafish larvae after acute exposure, inducing a synergistic immune response in vitro without affecting larval survival in vivo. Environ. Sci. Nano 2020, 7, 2410–2422. [Google Scholar] [CrossRef]
- Fiorentino, I.; Gualtieri, R.; Barbato, V.; Mollo, V.; Braun, S.; Angrisani, A.; Turano, M.; Furia, M.; Netti, P.; Guarnieri, D.; et al. Energy independent uptake and release of polystyrene nanoparticles in primary mammalian cell cultures. Exp. Cell Res. 2015, 330, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Huang, Y.; Liu, S.; Zhang, S.; Zou, H.; Wang, Z.; Zhu, W.; Geng, J. Toxicological effects of nano- and micro-polystyrene plastics on red tilapia: Are larger plastic particles more harmless? J. Hazard. Mater. 2020, 396, 122693. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.A.; Trevisan, R.; Massarsky, A.; Kozal, J.S.; Levin, E.D.; Di Giulio, R.T. Maternal transfer of nanoplastics to offspring in zebrafish (Danio rerio): A case study with nanopolystyrene. Sci. Total Environ. 2018, 643, 324–334. [Google Scholar] [CrossRef]
- Shen, R.; Yang, K.; Cheng, X.; Guo, C.; Xing, X.; Sun, H.; Liu, D.; Liu, X.; Wang, D. Accumulation of polystyrene microplastics induces liver fibrosis by activating cGAS/STING pathway. Environ. Pollut. 2022, 300, 118986. [Google Scholar] [CrossRef] [PubMed]
- Abarghouei, S.; Hedayati, A.; Raeisi, M.; Hadavand, B.S.; Rezaei, H.; Abed-Elmdoust, A. Size-dependent effects of microplastic on uptake, immune system, related gene expression and histopathology of goldfish (Carassius auratus). Chemosphere 2021, 276, 129977. [Google Scholar] [CrossRef]
- Ye, G.; Zhang, X.; Liu, X.; Liao, X.; Zhang, H.; Yan, C.; Lin, Y.; Huang, Q. Polystyrene microplastics induce metabolic disturbances in marine medaka (Oryzias melastigmas) liver. Sci. Total Environ. 2021, 782, 146885. [Google Scholar] [CrossRef]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, H.; Zhao, J.; Luo, X.; Wang, Z.; Xing, B. Photodegradation Elevated the Toxicity of Polystyrene Microplastics to Grouper (Epinephelus moara) through Disrupting Hepatic Lipid Homeostasis. Environ. Sci. Technol. 2020, 54, 6202–6212. [Google Scholar] [CrossRef]
- Sarasamma, S.; Audira, G.; Siregar, P.; Malhotra, N.; Lai, Y.-H.; Liang, S.-T.; Chen, J.-R.; Chen, K.H.-C.; Hsiao, C.-D. Nanoplastics Cause Neurobehavioral Impairments, Reproductive and Oxidative Damages, and Biomarker Responses in Zebrafish: Throwing up Alarms of Wide Spread Health Risk of Exposure. Int. J. Mol. Sci. 2020, 21, 1410. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Xu, D.; Li, J.; Wang, Z.; Ding, Y.; Wang, X.; Li, X.; Xu, N.; Mai, K.; Ai, Q. Dietary polystyrene nanoplastics exposure alters liver lipid metabolism and muscle nutritional quality in carnivorous marine fish large yellow croaker (Larimichthys crocea). J. Hazard. Mater. 2021, 419, 126454. [Google Scholar] [CrossRef] [PubMed]
- Im, C.; Kim, H.; Zaheer, J.; Kim, J.Y.; Lee, Y.-J.; Kang, C.M.; Kim, J.S. PET Tracing of Biodistribution for Orally Administered 64Cu-Labeled Polystyrene in Mice. J. Nucl. Med. 2021, 63, 461–467. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuan, Q.; Zhang, L.; Meng, L.; Fu, X.; Hou, Y. Polystyrene microplastics induced female reproductive toxicity in mice. J. Hazard. Mater. 2021, 424, 127629. [Google Scholar] [CrossRef] [PubMed]
- Chagas, T.Q.; Freitas, Í.N.; Montalvão, M.F.; Nobrega, R.H.; Machado, M.R.F.; Charlie-Silva, I.; Araújo, A.P.D.C.; Guimarães, A.T.B.; Alvarez, T.G.D.S.; Malafaia, G. Multiple endpoints of polylactic acid biomicroplastic toxicity in adult zebrafish (Danio rerio). Chemosphere 2021, 277, 130279. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, M.; Xu, G.; Shi, H.; Leung, J.Y.; Wang, Y. Microplastic accumulation via trophic transfer: Can a predatory crab counter the adverse effects of microplastics by body defence? Sci. Total Environ. 2020, 754, 142099. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Lu, L.; Zheng, M.; Zhang, X.; Tian, H.; Wang, W.; Ru, S. Polystyrene microplastics cause tissue damages, sex-specific reproductive disruption and transgenerational effects in marine medaka (Oryzias melastigma). Environ. Pollut. 2019, 254, 113024. [Google Scholar] [CrossRef]
- Araújo, A.P.D.C.; Malafaia, G. Microplastic ingestion induces behavioral disorders in mice: A preliminary study on the trophic transfer effects via tadpoles and fish. J. Hazard. Mater. 2020, 401, 123263. [Google Scholar] [CrossRef]
- Yin, L.; Chen, B.; Xia, B.; Shi, X.; Qu, K. Polystyrene microplastics alter the behavior, energy reserve and nutritional composition of marine jacopever (Sebastes schlegelii). J. Hazard. Mater. 2018, 360, 97–105. [Google Scholar] [CrossRef]
- Espinosa, C.; Esteban, M.; Cuesta, A. Dietary administration of PVC and PE microplastics produces histological damage, oxidative stress and immunoregulation in European sea bass (Dicentrarchus labrax L.). Fish Shellfish Immunol. 2019, 95, 574–583. [Google Scholar] [CrossRef]
- Araújo, A.P.D.C.; Gomes, A.R.; Malafaia, G. Hepatotoxicity of pristine polyethylene microplastics in neotropical physalaemus cuvieri tadpoles (Fitzinger, 1826). J. Hazard. Mater. 2019, 386, 121992. [Google Scholar] [CrossRef] [PubMed]
- Iheanacho, S.C.; Odo, G.E. Dietary exposure to polyvinyl chloride microparticles induced oxidative stress and hepatic damage in Clarias gariepinus (Burchell, 1822). Environ. Sci. Pollut. Res. 2020, 27, 21159–21173. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiong, H.; Mi, K.; Xue, W.; Wei, W.; Zhang, Y. Toxicity comparison of nano-sized and micron-sized microplastics to Goldfish Carassius auratus Larvae. J. Hazard. Mater. 2020, 388, 122058. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shi, M.; Wang, Y.; Xiao, Y.; Cai, D.; Xiao, F. Keap1-Nrf2 pathway up-regulation via hydrogen sulfide mitigates polystyrene microplastics induced-hepatotoxic effects. J. Hazard. Mater. 2020, 402, 123933. [Google Scholar] [CrossRef]
- Luo, T.; Wang, C.; Pan, Z.; Jin, C.; Fu, Z.; Jin, Y. Maternal Polystyrene Microplastic Exposure during Gestation and Lactation Altered Metabolic Homeostasis in the Dams and Their F1 and F2 Offspring. Environ. Sci. Technol. 2019, 53, 10978–10992. [Google Scholar] [CrossRef]
- Umamaheswari, S.; Priyadarshinee, S.; Bhattacharjee, M.; Kadirvelu, K.; Ramesh, M. Exposure to polystyrene microplastics induced gene modulated biological responses in zebrafish (Danio rerio). Chemosphere 2020, 281, 128592. [Google Scholar] [CrossRef]
- Jovanović, B.; Gökdağ, K.; Guven, O.; Emre, Y.; Whitley, E.M.; Kideys, A.E. Virgin microplastics are not causing imminent harm to fish after dietary exposure. Mar. Pollut. Bull. 2018, 130, 123–131. [Google Scholar] [CrossRef]
- Li, L.; Xu, M.; He, C.; Wang, H.; Hu, Q. Polystyrene nanoplastics potentiate the development of hepatic fibrosis in high fat diet fed mice. Environ. Toxicol. 2021, 37, 362–372. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, F.; Liang, K.; Niu, W.; Duan, X.; Jia, X.; Wu, X.; Xu, P.; Zhou, L. Polystyrene nanoplastics affect digestive function and growth in juvenile groupers. Sci. Total Environ. 2021, 808, 152098. [Google Scholar] [CrossRef]
- Kim, L.; Cui, R.; Kwak, J.I.; An, Y.-J. Sub-acute exposure to nanoplastics via two-chain trophic transfer: From brine shrimp Artemia franciscana to small yellow croaker Larimichthys polyactis. Mar. Pollut. Bull. 2022, 175, 113314. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Amereh, F.; Eslami, A.; Fazelipour, S.; Rafiee, M.; Zibaii, M.I.; Babaei, M. Thyroid endocrine status and biochemical stress responses in adult male Wistar rats chronically exposed to pristine polystyrene nanoplastics. Toxicol. Res. 2019, 8, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Li, X.; Zhou, Y.; Yu, H.; Xie, Y.; Guo, H.; Wang, H.; Li, Y.; Feng, Y.; Wang, Y. Polystyrene microplastics induce hepatotoxicity and disrupt lipid metabolism in the liver organoids. Sci. Total Environ. 2021, 806, 150328. [Google Scholar] [CrossRef] [PubMed]
- LaPlaca, S.B.; Hurk, P.V.D. Toxicological effects of micronized tire crumb rubber on mummichog (Fundulus heteroclitus) and fathead minnow (Pimephales promelas). Ecotoxicology 2020, 29, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Solomando, A.; Capó, X.; Alomar, C.; Compa, M.; Valencia, J.M.; Sureda, A.; Deudero, S. Assessment of the effect of long-term exposure to microplastics and depuration period in Sparus aurata Linnaeus, 1758: Liver and blood biomarkers. Sci. Total Environ. 2021, 786, 147479. [Google Scholar] [CrossRef] [PubMed]
- Magni, S.; Della Torre, C.; Garrone, G.; D’Amato, A.; Parenti, C.; Binelli, A. First evidence of protein modulation by polystyrene microplastics in a freshwater biological model. Environ. Pollut. 2019, 250, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Lu, K.; Deng, Y.; Ren, H.; Zhang, Y. Combined effects of polystyrene microplastics and natural organic matter on the accumulation and toxicity of copper in zebrafish. Sci. Total Environ. 2019, 682, 128–137. [Google Scholar] [CrossRef]
- Lee, M.T.; Lin, W.C.; Yu, B.; Lee, T.-T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals—A review. Asian Australas. J. Anim. Sci. 2016, 30, 299–308. [Google Scholar] [CrossRef]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant Defenses in Fish: Biotic and Abiotic Factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Pamplona, R.; Costantini, D. Molecular and structural antioxidant defenses against oxidative stress in animals. Am. J. Physiol. Integr. Comp. Physiol. 2011, 301, R843–R863. [Google Scholar] [CrossRef]
- De Andrade, L.L.; Pereira, A.D.E.S.; Fraceto, L.F.; Martinez, C.B.D.R. Can atrazine loaded nanocapsules reduce the toxic effects of this herbicide on the fish Prochilodus lineatus? A multibiomarker approach. Sci. Total Environ. 2019, 663, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Liu, Z.; Wu, D.; Chen, M.; Lv, W.; Zhao, Y. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol. 2018, 200, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gu, E.; Li, H.; Tian, C.; Feng, C. Oxidative stress and histological changes in Corbicula fluminea exposed to nano-Al13 and monomeric Al coagulants. Environ. Sci. Nano 2019, 6, 2736–2748. [Google Scholar] [CrossRef]
- Wegner, A.; Besseling, E.; Foekema, E.; Kamermans, P.; Koelmans, A. Effects of nanopolystyrene on the feeding behavior of the blue mussel (Mytilus edulis L.). Environ. Toxicol. Chem. 2012, 31, 2490–2497. [Google Scholar] [CrossRef] [PubMed]
- Alimi, O.S.; Budarz, J.F.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Wu, Y.; Wei, M.; Feng, C. A novel understanding of residual nano-Al13 formation and degradation during coagulation and flocculation: A proof based on ESI-TOF-MS. Environ. Sci. Nano 2018, 5, 2712–2721. [Google Scholar] [CrossRef]
- Li, Z.; Feng, C.; Wu, Y.; Guo, X. Impacts of nanoplastics on bivalve: Fluorescence tracing of organ accumulation, oxidative stress and damage. J. Hazard. Mater. 2020, 392, 122418. [Google Scholar] [CrossRef]
- Kang, H.-M.; Byeon, E.; Jeong, H.; Kim, M.-S.; Chen, Q.; Lee, J.-S. Different effects of nano- and microplastics on oxidative status and gut microbiota in the marine medaka Oryzias melastigma. J. Hazard. Mater. 2020, 405, 124207. [Google Scholar] [CrossRef]
- Brown, D.M.; Wilson, M.R.; MacNee, W.; Stone, V.; Donaldson, K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: A role for surface area and oxidative stress in the enhanced activity of Ultrafines. Toxicol. Appl. Pharmacol. 2001, 175, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Kaplan, G. The Role of TNFα in Ulcerative Colitis. J. Clin. Pharmacol. 2007, 47, 930–941. [Google Scholar] [CrossRef]
- Zhao, Y.; Bao, Z.; Wan, Z.; Fu, Z.; Jin, Y. Polystyrene microplastic exposure disturbs hepatic glycolipid metabolism at the physiological, biochemical, and transcriptomic levels in adult zebrafish. Sci. Total Environ. 2019, 710, 136279. [Google Scholar] [CrossRef]
- Opal, S.M.; DePalo, V.A. Anti-Inflammatory Cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, F. Signaling pathways of oxidative stress in aquatic organisms exposed to xenobiotics. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2020, 333, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, B.; Raza, S.H.A.; Zhang, D.; Wu, T.; Zhang, Z.; Ullah, I.; Khan, R.; Yang, G.; Wang, C.; et al. Role of Myeloperoxidase of northern snakehead (Channa argus) in Aeromonas veronii infection. Microb. Pathog. 2019, 135, 103622. [Google Scholar] [CrossRef] [PubMed]
- Van der Veen, B.S.; de Winther, M.P.; Heeringa, P. Myeloperoxidase: Molecular Mechanisms of Action and Their Relevance to Human Health and Disease. Antioxid. Redox Signal. 2009, 11, 2899–2937. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Ahmadifar, E.; Kalhor, N.; Dawood, M.A.; Ahmadifar, M.; Moghadam, M.S.; Abarghouei, S.; Hedayati, A. Effects of polystyrene microparticles on inflammation, antioxidant enzyme activities, and related gene expression in Nile tilapia (Oreochromis niloticus). Environ. Sci. Pollut. Res. 2020, 28, 14909–14916. [Google Scholar] [CrossRef]
- Zhao, L.; Shi, W.; Hu, F.; Song, X.; Cheng, Z.; Zhou, J. Prolonged oral ingestion of microplastics induced inflammation in the liver tissues of C57BL/6J mice through polarization of macrophages and increased infiltration of natural killer cells. Ecotoxicol. Environ. Saf. 2021, 227, 112882. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Capó, X.; Company, J.; Alomar, C.; Compa, M.; Sureda, A.; Grau, A.; Hansjosten, B.; López-Vázquez, J.; Quintana, J.; Rodil, R.; et al. Long-term exposure to virgin and seawater exposed microplastic enriched-diet causes liver oxidative stress and inflammation in gilthead seabream Sparus aurata, Linnaeus. Sci. Total Environ. 2021, 767, 144976. [Google Scholar] [CrossRef]
- Usman, S.; Razis, A.F.A.; Shaari, K.; Amal, M.N.A.; Saad, M.Z.; Isa, N.M.; Nazarudin, M.F. Polystyrene Microplastics Exposure: An Insight into Multiple Organ Histological Alterations, Oxidative Stress and Neurotoxicity in Javanese Medaka Fish (Oryzias javanicus Bleeker, 1854). Int. J. Environ. Res. Public Health 2021, 18, 9449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wen, K.; Ding, D.; Liu, J.; Lei, Z.; Chen, X.; Ye, G.; Zhang, J.; Shen, H.; Yan, C.; et al. Size-dependent adverse effects of microplastics on intestinal microbiota and metabolic homeostasis in the marine medaka (Oryzias melastigma). Environ. Int. 2021, 151, 106452. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Park, J.W.; Lim, Y.; Seo, S.; Hwang, D.Y. In vivo impact assessment of orally administered polystyrene nanoplastics: Biodistribution, toxicity, and inflammatory response in mice. Nanotoxicology 2021, 15, 1180–1198. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Sun, J.; Li, Z.; Zhang, W.; Liu, Z.; Li, C.; Peng, C.; Cui, G.; Shao, H.; Du, Z. Activation of pyroptosis and ferroptosis is involved in the hepatotoxicity induced by polystyrene microplastics in mice. Chemosphere 2021, 291, 132944. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, J.; Wei, X.; Chang, L.; Liu, S. Proinflammatory properties and lipid disturbance of polystyrene microplastics in the livers of mice with acute colitis. Sci. Total Environ. 2020, 750, 143085. [Google Scholar] [CrossRef]
- Yin, L.; Liu, H.; Cui, H.; Chen, B.; Li, L.; Wu, F. Impacts of polystyrene microplastics on the behavior and metabolism in a marine demersal teleost, black rockfish (Sebastes schlegelii). J. Hazard. Mater. 2019, 380, 120861. [Google Scholar] [CrossRef]
- Brandts, I.; Teles, M.; Tvarijonaviciute, A.; Pereira, M.; Martins, M.; Tort, L.; Oliveira, M. Effects of polymethylmethacrylate nanoplastics on Dicentrarchus labrax. Genomics 2018, 110, 435–441. [Google Scholar] [CrossRef]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. BBA Mol. Basis Dis. 2011, 1812, 1007–1022. [Google Scholar] [CrossRef]
- Rakhshandehroo, M.; Knoch, B.; Müller, M.; Kersten, S. Peroxisome Proliferator-Activated Receptor Alpha Target Genes. PPAR Res. 2010, 2010, 612089. [Google Scholar] [CrossRef]
- Minamikawa, J.; Tanaka, S.; Yamauchi, M.; Inoue, D.; Koshiyama, H. Potent inhibitory effect of troglitazone on carotid arterial wall thickness in type 2 diabetes. J. Clin. Endocrinol. Metab. 1998, 83, 1818. [Google Scholar] [CrossRef]
- Hegarty, B.D.; Turner, N.; Cooney, G.J.; Kraegen, E.W. Insulin resistance and fuel homeostasis: The role of AMP-activated protein kinase. Acta Physiol. 2009, 196, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Bruhs, A.; Schwarz, T. The Short-Chain Fatty Acid Sodium Butyrate Functions as a Regulator of the Skin Immune System. J. Investig. Dermatol. 2016, 137, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Ortega, D.; del Pulgar, T.G.; Valdés-Mora, F.; Cebrián, A.; Lacal, J.C. Involvement of human choline kinase alpha and beta in carcinogenesis: A different role in lipid metabolism and biological functions. Adv. Enzym. Regul. 2011, 51, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between Lipids and Branched-Chain Amino Acids in Development of Insulin Resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-F.; Chen, C.-Y.; Lu, T.-H.; Liao, C.-M. Toxicity-based toxicokinetic/toxicodynamic assessment for bioaccumulation of polystyrene microplastics in mice. J. Hazard. Mater. 2018, 366, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Schrage, A.; Hempel, K.; Schulz, M.; Kolle, S.N.; van Ravenzwaay, B.; Landsiedel, R. Refinement and Reduction of Acute Oral Toxicity Testing: A Critical Review of the Use of Cytotoxicity Data. Altern. Lab. Anim. 2011, 39, 273–295. [Google Scholar] [CrossRef]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef]
- Wright, S.L.; Rowe, D.; Thompson, R.; Galloway, T.S. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. 2013, 23, R1031–R1033. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The Impact of Polystyrene Microplastics on Feeding, Function and Fecundity in the Marine Copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef]
- Cedervall, T.; Hansson, L.A.; Lard, M.; Frohm, B.; Linse, S. Food Chain Transport of Nanoparticles Affects Behaviour and Fat Metabolism in Fish. PLoS ONE 2012, 7, e32254. [Google Scholar] [CrossRef]
- Yu, W.; Naim, J.O.; McGowan, M.; Ippolito, K.; Lanzafame, R.J. Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem. Photobiol. 1997, 66, 866–871. [Google Scholar] [CrossRef]
- Wei, L.; Liao, P.; Wu, H.; Li, X.; Pei, F.; Li, W.; Wu, Y. Toxicological effects of cinnabar in rats by NMR-based metabolic profiling of urine and serum. Toxicol. Appl. Pharmacol. 2008, 227, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Tataranni, P.A.; Larson, D.E.; Snitker, S.; Young, J.B.; Flatt, J.P.; Ravussin, E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am. J. Physiol. Metab. 1996, 271, E317–E325. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hou, M.; Shang, K.; Wang, H.; Wang, J. Microplastics (Polystyrene) Exposure Induces Metabolic Changes in the Liver of Rare Minnow (Gobiocypris rarus). Molecules 2022, 27, 584. [Google Scholar] [CrossRef] [PubMed]
- Wood, T. Physiological functions of the pentose phosphate pathway. Cell Biochem. Funct. 1986, 4, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Riganti, C.; Gazzano, E.; Polimeni, M.; Aldieri, E.; Ghigo, D. The pentose phosphate pathway: An antioxidant defense and a crossroad in tumor cell fate. Free Radic. Biol. Med. 2012, 53, 421–436. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, X.; Zhu, H.; Zhang, Q.; He, Y.; Shen, Y.; Xu, X.; Li, J. The effects of exposure to microplastics on grass carp (Ctenopharyngodon idella) at the physiological, biochemical, and transcriptomic levels. Chemosphere 2021, 286, 131831. [Google Scholar] [CrossRef]
- Li, Z.; Ah, S.J.; Choi, C. Oxidative Stress and Apoptosis in Goldfish (Carassius auratus) Caused by Exposure to Different Concentrations of Micro-polystyrene. Ocean. Polar Res. 2021, 43, 141–148. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Xu, M.; Ma, J.; Zhang, S.; Liu, S.; Wang, K.; Tian, H.; Cui, J. Enhanced hepatic cytotoxicity of chemically transformed polystyrene microplastics by simulated gastric fluid. J. Hazard. Mater. 2020, 410, 124536. [Google Scholar] [CrossRef]
- Kaloyianni, M.; Dimitriadi, A.; Ovezik, M.; Stamkopoulou, D.; Feidantsis, K.; Kastrinaki, G.; Gallios, G.; Tsiaoussis, I.; Koumoundouros, G.; Bobori, D. Magnetite nanoparticles effects on adverse responses of aquatic and terrestrial animal models. J. Hazard. Mater. 2020, 383, 121204. [Google Scholar] [CrossRef]
- Kaloyianni, M.; Bobori, D.C.; Xanthopoulou, D.; Malioufa, G.; Sampsonidis, I.; Kalogiannis, S.; Feidantsis, K.; Kastrinaki, G.; Dimitriadi, A.; Koumoundouros, G.; et al. Toxicity and Functional Tissue Responses of Two Freshwater Fish after Exposure to Polystyrene Microplastics. Toxics 2021, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Takle, H.; Andersen, O. Caspases and apoptosis in fish. J. Fish Biol. 2007, 71, 326–349. [Google Scholar] [CrossRef]
- Pan, L.; Yu, D.; Zhang, Y.; Zhu, C.; Yin, Q.; Hu, Y.; Zhang, X.; Yue, R.; Xiong, X. Polystyrene microplastics-triggered mitophagy and oxidative burst via activation of PERK pathway. Sci. Total Environ. 2021, 781, 146753. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. BBA Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Hozayen, W.G.; Ramadan, S.M. Berberine ameliorates methotrexate-induced liver injury by activating Nrf2/HO-1 pathway and PPARγ, and suppressing oxidative stress and apoptosis in rats. Biomed. Pharmacother. 2017, 94, 280–291. [Google Scholar] [CrossRef]
- Qiao, Y.; Xiao, F.; Li, W.; Yu, M.; Du, P.; Fang, Z.; Sun, J. Hepatocellular HO-1 mediated iNOS-induced hepatoprotection against liver ischemia reperfusion injury. Biochem. Biophys. Res. Commun. 2020, 521, 1095–1100. [Google Scholar] [CrossRef]
- Cheng, H.; Duan, Z.; Wu, Y.; Wang, Y.; Zhang, H.; Shi, Y.; Zhang, H.; Wei, Y.; Sun, H. Immunotoxicity responses to polystyrene nanoplastics and their related mechanisms in the liver of zebrafish (Danio rerio) larvae. Environ. Int. 2022, 161, 107128. [Google Scholar] [CrossRef]
- Bobori, D.C.; Dimitriadi, A.; Feidantsis, K.; Samiotaki, A.; Fafouti, D.; Sampsonidis, I.; Kalogiannis, S.; Kastrinaki, G.; Lambropoulou, D.A.; Kyzas, G.Z.; et al. Differentiation in the expression of toxic effects of polyethylene-microplastics on two freshwater fish species: Size matters. Sci. Total Environ. 2022, 830, 154603. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, H.; He, C.; Jin, Y.; Fu, Z. Polystyrene nanoparticles trigger the activation of p38 MAPK and apoptosis via inducing oxidative stress in zebrafish and macrophage cells. Environ. Pollut. 2020, 269, 116075. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef]
- Schroder, K.; Zhou, R.; Tschopp, J. The NLRP3 inflammasome: A sensor for metabolic danger? Science 2010, 327, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Forcina, G.C.; Dixon, S.J. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics 2019, 19, e1800311. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, J.; Saunders, J.M.; Moraes, V.W.; Madhavan, A.; Madrazo, N.; Anthony, M.C.; Wiseman, R.L. The PERK Arm of the Unfolded Protein Response Regulates Mitochondrial Morphology during Acute Endoplasmic Reticulum Stress. Cell Rep. 2018, 22, 2827–2836. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, H.; Wang, C.; Su, X.-L.; Song, Y.; Wu, P.; Yang, Z.; Wong, M.-H.; Cai, Z.; Zheng, C. Metabolomics Reveal Nanoplastic-Induced Mitochondrial Damage in Human Liver and Lung Cells. Environ. Sci. Technol. 2022, 56, 12483–12493. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Lee, Y.-H.; Hsu, Y.-H.; Chiu, I.-J.; Huang, C.C.-Y.; Huang, C.-C.; Chia, Z.-C.; Lee, C.-P.; Lin, Y.-F.; Chiu, H.-W. The Kidney-Related Effects of Polystyrene Microplastics on Human Kidney Proximal Tubular Epithelial Cells HK-2 and Male C57BL/6 Mice. Environ. Health Perspect. 2021, 129, ehp7612. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, B.; Zhang, B.; Ye, Y.; Jiang, W. Polystyrene micro(nano)plastics damage the organelles of RBL-2H3 cells and promote MOAP-1 to induce apoptosis. J. Hazard. Mater. 2022, 438, 129550. [Google Scholar] [CrossRef]
- Klionsky, D. Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy (3rd edition) (vol 12, pg 1, 2015). Autophagy 2016, 12, 443. [Google Scholar] [CrossRef]
- Dimitriadi, A.; Papaefthimiou, C.; Genizegkini, E.; Sampsonidis, I.; Kalogiannis, S.; Feidantsis, K.; Bobori, D.C.; Kastrinaki, G.; Koumoundouros, G.; Lambropoulou, D.A.; et al. Adverse effects polystyrene microplastics exert on zebrafish heart—Molecular to individual level. J. Hazard. Mater. 2021, 416, 125969. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Koike, M.; Sou, Y.-S.; Ueno, T.; Hara, T.; Mizushima, N.; Iwata, J.-I.; Ezaki, J.; Murata, S.; et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 2007, 131, 1149–1163. [Google Scholar] [CrossRef]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.-A.; Outzen, H.; Øvervatn, A.; Bjørkøy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef]
- Yang, M.; Wang, W.-X. Differential cascading cellular and subcellular toxicity induced by two sizes of nanoplastics. Sci. Total Environ. 2022, 829, 154593. [Google Scholar] [CrossRef] [PubMed]
- Missawi, O.; Venditti, M.; Cappello, T.; Zitouni, N.; Marco, G.D.; Boughattas, I.; Bousserrhine, N.; Belbekhouche, S.; Minucci, S.; Maisano, M.; et al. Autophagic event and metabolomic disorders unveil cellular toxicity of environmental microplastics on marine polychaete Hediste diversicolor. Environ. Pollut. 2022, 302, 119106. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Wang, D.; Zhao, H.; Wang, Y.; Zhang, Y.; Liu, Y.; Li, B.; Xing, M. Polystyrene microplastics up-regulates liver glutamine and glutamate synthesis and promotes autophagy-dependent ferroptosis and apoptosis in the cerebellum through the liver-brain axis. Environ. Pollut. 2022, 307, 119449. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Yin, K.; Zhao, H.; Lu, H.; Meng, X.; Hou, L.; Li, J.; Xing, M. Endoplasmic reticulum stress-controlled autophagic pathway promotes polystyrene microplastics-induced myocardial dysplasia in birds. Environ. Pollut. 2022, 311, 119963. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ni, M.; Lee, B.; Barron, E.; Hinton, D.R.; Lee, A. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008, 15, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Zielke, S.; Kardo, S.; Zein, L.; Mari, M.; Covarrubias-Pinto, A.; Kinzler, M.N.; Meyer, N.; Stolz, A.; Fulda, S.; Reggiori, F.; et al. ATF4 links ER stress with reticulophagy in glioblastoma cells. Autophagy 2020, 17, 2432–2448. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Guo, M.; Mu, M.; Zong, H.; Xing, M. Co-administration of zinc for treating and preventing arsenism in common carp Cyprinus carpio: An alternative to avoid physiological and cellular damages. Aquaculture 2021, 531, 735965. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Xu, C.Y.; Zhang, B.B.; Gu, C.J.; Shen, C.S.; Yin, S.S.; Aamir, M.; Li, F. Are we underestimating the sources of microplastic pollution in terrestrial environment? J. Hazard. Mater. 2020, 400, 123228. [Google Scholar] [CrossRef]
- Stanton, T.; Johnson, M.; Nathanail, P.; MacNaughtan, W.; Gomes, R.L. Freshwater and airborne textile fibre populations are dominated by ‘natural’, not microplastic, fibres. Sci. Total Environ. 2019, 666, 377–389. [Google Scholar] [CrossRef]
- De Witte, B.; Devriese, L.; Bekaert, K.; Hoffman, S.; Vandermeersch, G.; Cooreman, K.; Robbens, J. Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Mar. Pollut. Bull. 2014, 85, 146–155. [Google Scholar] [CrossRef]
- Baalkhuyur, F.M.; Bin Dohaish, E.-J.A.; Elhalwagy, M.E.; Alikunhi, N.M.; AlSuwailem, A.M.; Røstad, A.; Coker, D.J.; Berumen, M.L.; Duarte, C.M. Microplastic in the gastrointestinal tract of fishes along the Saudi Arabian Red Sea coast. Mar. Pollut. Bull. 2018, 131, 407–415. [Google Scholar] [CrossRef]
- Pegado, T.D.S.E.S.; Schmid, K.; Winemiller, K.O.; Chelazzi, D.; Cincinelli, A.; Dei, L.; Giarrizzo, T. First evidence of microplastic ingestion by fishes from the Amazon River estuary. Mar. Pollut. Bull. 2018, 133, 814–821. [Google Scholar] [CrossRef]
- Teng, J.; Wang, Q.; Ran, W.; Wu, D.; Liu, Y.; Sun, S.; Liu, H.; Cao, R.; Zhao, J. Microplastic in cultured oysters from different coastal areas of China. Sci. Total Environ. 2018, 653, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic Pollution in Table Salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef] [PubMed]
- Peeken, I.; Primpke, S.; Beyer, B.; Gütermann, J.; Katlein, C.; Krumpen, T.; Bergmann, M.; Hehemann, L.; Gerdts, G. Arctic sea ice is an important temporal sink and means of transport for microplastic. Nat. Commun. 2018, 9, 1505. [Google Scholar] [CrossRef]
- Poulain, M.; Mercier, M.J.; Brach, L.; Martignac, M.; Routaboul, C.; Perez, E.; Desjean, M.C.; ter Halle, A. Small Microplastics As a Main Contributor to Plastic Mass Balance in the North Atlantic Subtropical Gyre. Environ. Sci. Technol. 2018, 53, 1157–1164. [Google Scholar] [CrossRef]
- Gu, H.; Wei, S.; Hu, M.; Wei, H.; Wang, X.; Shang, Y.; Li, L.; Shi, H.; Wang, Y. Microplastics aggravate the adverse effects of BDE-47 on physiological and defense performance in mussels. J. Hazard. Mater. 2020, 398, 122909. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Jin, S.-R.; Chen, Z.-Z.; Gao, J.-Z.; Liu, Y.-N.; Liu, J.-H.; Feng, X.-S. Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environ. Pollut. 2018, 243, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Jang, F.H.; Wong, C.; Choo, J.; Sia, E.S.A.; Mujahid, A.; Müller, M. Increased transfer of trace metals and Vibrio sp. from biodegradable microplastics to catfish Clarias gariepinus. Environ. Pollut. 2022, 298, 118850. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, L.; Liu, J.; Cheng, Y.; Waiho, K.; Chen, A.; Wang, Y. Polystyrene microplastics increase Pb bioaccumulation and health damage in the Chinese mitten crab Eriocheir sinensis. Sci. Total Environ. 2022, 829, 154586. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xie, S.; Zhang, C.; Pan, Z.; Sun, D.; Zhou, A.; Xu, G.; Zou, J. Interactions effects of nano-microplastics and heavy metals in hybrid snakehead (Channa maculata female x Channa argus male). Fish Shellfish Immunol. 2022, 124, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Rios-Fuster, B.; Arechavala-Lopez, P.; García-Marcos, K.; Alomar, C.; Compa, M.; Álvarez, E.; Julià, M.M.; Martí, A.S.; Sureda, A.; Deudero, S. Experimental evidence of physiological and behavioral effects of microplastic ingestion in Sparus aurata. Aquat. Toxicol. 2021, 231, 105737. [Google Scholar] [CrossRef]
- Barnes, D.K.A. Invasions by marine life on plastic debris. Nature 2002, 416, 808–809. [Google Scholar] [CrossRef]
- Gall, S.; Thompson, R. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Lin, S.; Cao, G.; Wu, J.; Jin, H.; Wang, C.; Wong, M.H.; Yang, Z.; Cai, Z. Absorption, distribution, metabolism, excretion and toxicity of microplastics in the human body and health implications. J. Hazard. Mater. 2022, 437, 129361. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Tirelli, V.; O’Connor, I.; Officer, R. Microplastics in Arctic polar waters: The first reported values of particles in surface and sub-surface samples. Sci. Rep. 2015, 5, 14947. [Google Scholar] [CrossRef]
- Abel, S.M.; Primpke, S.; Wu, F.; Brandt, A.; Gerdts, G. Human footprints at hadal depths: Interlayer and intralayer comparison of sediment cores from the Kuril Kamchatka trench. Sci. Total Environ. 2022, 838, 156035. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Kannan, K. Microplastics in house dust from 12 countries and associated human exposure. Environ. Int. 2019, 134, 105314. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Soliman, H.A.M.; Osman, A.G.M.; Sayed, A.E.-D.H. Antioxidants and molecular damage in Nile Tilapia (Oreochromis niloticus) after exposure to microplastics. Environ. Sci. Pollut. Res. 2020, 27, 14581–14588. [Google Scholar] [CrossRef]
- Abbasi, S.; Soltani, N.; Keshavarzi, B.; Moore, F.; Turner, A.; Hassanaghaei, M. Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere 2018, 205, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Collard, F.; Gilbert, B.; Compère, P.; Eppe, G.; Das, K.; Jauniaux, T.; Parmentier, E. Microplastics in livers of European anchovies (Engraulis encrasicolus L.). Environ. Pollut. 2017, 229, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.H.; Ronda, A.C.; Oliva, A.L.; Marcovecchio, J.E. Evidence of Microplastic Ingestion by Fish from the Bahía Blanca Estuary in Argentina, South America. Bull. Environ. Contam. Toxicol. 2019, 102, 750–756. [Google Scholar] [CrossRef] [PubMed]


| Research Object | Particle Size | Material | Number of Particles | Concentration | Mode of Exposure | Exposure Time | Summary | Reference |
|---|---|---|---|---|---|---|---|---|
| Wistar male rats | 25, 50 nm | PS | - | 1, 3, 6, 10 mg/kg bw/day | Oral gavage | 5 weeks | In the high-dose gavage group, the accumulation of PS NPs in the liver was confirmed by whole-body scanning. | [28] |
| Zebrafish | 42 nm | PS | - | 1 mg/g bw | In diet | 7 days | The co-parentally exposed F1 larvae at 120 hpf had a significant amount of fluorescence in the liver, indicating that PS-NPs accumulate in the liver of zebrafish larvae. | [45] |
| Zebrafish liver cells | 65 nm | PS | - | 5, 50 mg/L | In culture medium | 6–72 h | 100% of ZFL cells took up the fluorescent PS-NPs after 6 h of incubation. Additionally, the ZFL internalization dynamics followed a dose–response pattern, with cells incubated at higher doses and longer times presenting higher fluorescence. | [42] |
| Zebrafish | 70 nm | PS | - | 0.5, 1.5, 5 ppm | In water | 30 days | After 30 days of incubation with 1.5 ppm florescence PS-NPs, green fluorescence was seen in the liver. | [51] |
| Hepatocytes of the large yellow croaker | 80 nm | PS | - | 1, 10, 100 mg/L | In diet | 3, 12 h | Results showed that PS-NPs could be accumulated in hepatocytes. Moreover, the uptake of PS-NPs by hepatocytes increased with the increase in the time and concentrations of PS-NPs treatment. | [52] |
| HL7702 cells | 100 nm | PS | - | 1 mg/L | In culture medium | 24 h | There was a large number of PS-NPs in the cytoplasm. | [46] |
| C57 male mice | 100 nm | PS | - | 0.1, 1 mg/L | In water | 60 days | High-dose group of PS-NPs accumulated in mouse liver. | [46] |
| Goldfish | 250 nm | PS | - | 300 mg/L | In water | 168 h | PS-NPs could accumulate in the liver, increasing over time within 7 days. | [47] |
| Mice | 200~300 nm | PS | - | 578 μg/mL | Oral gavage | 48 h | Uptake of [64Cu] Cu-DOTA-polystyrene was observed in the liver at 48 h after administration. | [53] |
| CD-1 female mice | 790 nm | PS | - | 30 mg/kg | Oral gavage | 35 days | The concentration of PS-NPs in the liver was (69.86 ± 25.31) μg/g. | [54] |
| Research Object | Particle Size | Material | Number of Particles | Concentration | Mode of Exposure | Exposure Time | Summary | Reference |
|---|---|---|---|---|---|---|---|---|
| Zebrafish | 2.34 ± 0.07 μm | PLABio MPs | - | 2.5, 5 mg/L | In water | 30 days | PLABio MPs at concentrations of 2.5 and 5 mg/L could accumulate in the liver. | [45,55] |
| Zebrafish | 5 μm | PS | 2.9 × 102, 2.9 × 103, 2.9 × 104 particles/mL | 20, 200, 2000 μg/L | In water | 7 days | PS-MPs accumulated in the fish liver after 7 days of exposure. | [27] |
| Male crab C. japonica | 5 μm | PS | 1.0 × 103 particles/mL | 0.68 × 10−4 mg/mL | In water | 7 days | PS-MPs accumulated the most in crab hepatopancreas. | [56] |
| Male mice | 5, 20 μm | PS | 1.46 × 106 items (5 μm), 2.27× 104 items (20 μm) | 0.1 mg/day | Oral gavage | 28 days | After 4 weeks of exposure, the maximum tissue concentration of PS-MPs in the liver was 0.303 ± 0.029 mg/g. | [29] |
| Marine medaka | 10 μm | PS | - | 2, 20, 200 μg/L | In water | 60 days | PS-MPs were accumulated in the liver of marine medaka at all exposure doses. | [53,57] |
| Marine medaka | 10 μm | PS | 1.82 × 1010 particles/m3 | 10 mg/L | In water | 30–60 days | It was observed that over 30 spherical PS with a diameter ≤3 μm accumulated in the exposed group. | [48] |
| Male Swiss mice | 35.46 ± 18.17 μm | PE | - | 60 mg/L | Oral gavage | 7 days | PE-MPs could accumulate in the mouse liver. | [54,58] |
| Red tilapia | 70~90 μm | PS | 3.51 × 104 particles/mL | 100 μg/L | In water | 14 days | PS-MPs could accumulate in the tilapia liver, and it showed a generally increasing tendency with time. | [44] |
| Research Object | Particle Size | Material | Number of Particles | Concentration | Mode of Exposure | Exposure Time | Summary | Reference |
|---|---|---|---|---|---|---|---|---|
| C57BL/6 female mice | 42 nm | PS | - | 10, 50 μg/mL | Inject via tail vein | 5 injections in 15 days | Hepatocyte binucleation increased after exposure. Fatty degeneration and ballooning were significantly increased in the liver tissue of the high-fat-fed mice, and the perilobular steatosis was severe. | [68] |
| Fish | 60 nm | PS | - | 5 mg/L | In water | 7 days | Hepatocytes in the exposed group were destroyed with aggregated and condensed nuclei. | [5] |
| Zebrafish | 70 nm | PS | 1.1 × 108, 1.1 × 109, 1.1 × 1010 particles/mL | 20, 200, 2000 μg/L | In water | 3 weeks | Necrosis, infiltration, and fat droplets were observed in hepatocytes. | [27] |
| Male C57 mice | 100 nm | PS | - | 0.1, 1 mg/L | In water | 60 days | Hepatocellular edema and vacuolar degeneration, enlarged nucleus, cell dikaryon, irregularly arranged hepatic cords, the proliferation of bile ducts, as well as the inflammation of portal areas were found in the PS-NPs exposed groups. | [46] |
| Juvenile groupers | 100.86 ± 7.15 nm | PS | - | 300, 3000 μg/L | In water | 14 days | Hepatocyte vacuolization was observed in the 300 and 3000 μg/L exposure groups. | [69] |
| Zebrafish | 100~120 nm | PS | - | 10, 100 μg/L | In water | 35 days | PS-MPs caused histopathological damage such as inflammation, degeneration, and hemorrhage in zebrafish liver tissue. | [66] |
| Larimichthys polyactis | 190 nm | PS | - | 1 mg/L | In diet | 8 days | There was some zonal necrosis, a decrease in tissue density, and normal-stained nuclei in the liver of the NPs-exposed fish. | [70] |
| Goldfish | 250 nm | PS | - | 0, 0.05, 0.5, 5 mg/L | In water | 28 days | Necrosis, cellular swelling, and hemorrhage were observed in the PS-NPs-exposed liver. | [47] |
| Research Object | Particle Size | Material | Number of Particles | Concentration | Mode of Exposure | Exposure Time | Summary | Reference |
|---|---|---|---|---|---|---|---|---|
| Zebrafish | 5 μm | PS | 2.9 × 102, 2.9 × 103, 2.9 × 104 particles/mL | 20, 200, 2000 μg/L | In water | 3 weeks | Necrosis, infiltration, and fat droplets were observed in PS-MPs exposed hepatocytes. | [27] |
| Goldfish | 5 μm | PS | - | 10, 100, 1000 μg/L | In water | 7 days | After exposure in the high-dose group, there was blood cell infiltration in the liver of the goldfish. | [63] |
| ICR mice | 5 μm | PS | - | 100, 1000 μg/L | In water | 6 weeks | Liver pathological sections showed increased liver ballooning degeneration in mice. | [65] |
| C57 male mice | 5 μm | PS | 1.46 × 106 particles | 20 mg/kg bw/day | In water | 30 days | The PS-MPs exposed group showed severe vacuolar degeneration, chronic inflammatory infiltration, and hepatocyte edema. | [64] |
| Goldfish | 8 μm | PS | - | 0, 0.05, 0.5, 5 mg/L | In water | 28 days | Necrosis, cellular swelling, and hemorrhage were observed in PS-MPs-exposed liver. | [47] |
| Marine medaka | 10 μm | PS | - | 2, 20, 200 μg/L | In water | 60 days | Compared with the control group, a significant decrease in the hepatosomatic index was found in adult male marine medaka exposed to PS-MPs. | [57] |
| Marine jacopever | 15 μm | PS | 1 × 106 microspheres/L | - | In water | 7 days of decontamination after 14 days of exposure | The Liver pathological section showed hyperemia in the PS-MPs exposed group. | [59] |
| Grouper | 22.3 μm | PS | - | 2, 20 mg/g df | In water | 25 days | Eosinophilic infiltration was observed in the exposed liver. | [50] |
| Tadpoles | 35.46 ± 18.17 μm | PE | 4.24 × 10−6 particles/m3 | 60 mg/L | In water | 7 days | Vasodilation, infiltration, hyperemia, edema degeneration, hypertrophy, and hyperplasia occurred in the exposed liver. | [61] |
| Clarias gariepinus | 95.41 ± 4.23 μm | PVC | - | 0.5, 1.5, 3.0% | In diet | 30 days of decontamination after 45 days of exposure | Hepatocyte necrosis, fat vacuolization and degeneration, and glycogen depletion were observed in PVC group. | [62] |
| European sea bass | 40~150 μm | PVC/PE | - | 0, 100, 500 mg/kg·di | In diet | 3 weeks | Hepatocytes showed vacuolation, infiltration, and focal necrosis after exposure to MPs. | [60] |
| Research Object | Particle Size | Material | Number of Particles | Concentration | Mode of Exposure | Exposure Time | Summary | Reference |
|---|---|---|---|---|---|---|---|---|
| Zebrafish | 70 nm, 5 μm | PS | 1.1 × 108–10 particles/mL (70 nm), 2.9 × 102–4 particles/mL (5 μm) | 0, 20, 200, 2000 μg/L | In water | 3 weeks | Histopathological analysis showed that inflammation occurred in the liver of zebrafish in the 70 nm and 5 μm 2000 μg/L PS-MP groups. | [27] |
| Nile tilapia | 350 μm, 9 μm | PS | - | 5 mg/L | In water | 28 days | Expression of interferon-γ (IFN-γ) genes was upregulated in the livers of fish exposed to 0.35 μm and 9 μm compared with controls; interleukin 8 (IL8), interleukin (IL-1β), and tumor necrosis factor (TNF-α) gene expression was upregulated in the 9 μm group. | [97] |
| Gilthead seabream | 100~500 μm | PE | - | 10% | In diet | 90 days (30 days of purification) | The level of inflammatory factor MPO in the liver of the exposed group increased. | [100] |
| Sparus aurata Linnaeus | 200~500 μm | PE | - | 10% | In diet | 90 days (30 days of purification) | Exposure induced an inflammatory response in the liver, manifested by elevated MPO levels. | [75] |
| Zebrafish | 100~120 nm | PS | - | 0, 10, 100 μg/L | In water | 35 days | PS-MPs caused inflammatory damage in zebrafish liver tissue. | [66] |
| Javanese medaka fish | 5 µm | PS | 1.46 × 103~5 particles | 0, 100, 500, 1000 µg/L | In water | 21 days | Significant inflammatory changes were observed in the liver. | [101] |
| Oryzias melastigma | 2, 10, 200 μm | PS | - | 10 mg/L | In water | 60 days | Fish in the 2 and 10 μm MPs-exposed groups exhibited liver damage, mainly manifested by the presence of inflammation. | [102] |
| Male mice (Mus musculus, ICR) | 5, 20 μm | PS | 1.46 × 106 items (5 µm) 2.27 × 104 items (20 µm) | 0.01, 0.1, 0.5 mg/day | Oral gavage | 28 days | Two particle sizes of liver HE staining showed inflammation. | [29] |
| ICR mice | - | PS | - | 0, 5, 25, 50 mg/kg bw | Oral gavage | 2 weeks | In the liver of NP-treated mice, the expression of inflammatory response proteins (iNOS, COX-2) and the mRNA levels of inflammatory cytokines were significantly increased. | [103] |
| High-fat diet C57BL/6 female mice | 42 nm | PS | - | 0, 10, 50 μg/mL | Inject via tail vein | 5 times in 15 days | Liver Kupffer cell (KC) infiltration was enhanced, and proinflammatory factor expression was elevated. | [68] |
| Mice | 5 μm | PS | 1.46 × 106 particles | 20 mg/kg bw/day | In water | 30 days | The liver in the mic-P group showed severe vacuolar degeneration, chronic inflammatory infiltration, and hepatocyte edema. | [64] |
| ICR male mice | 5 μm | PS | - | 0, 0.1, 0.5, 1 mg/mL | In water | 4 weeks | The expression of interleukins IL-1β and IL-18 increased in the microplastic exposure group. | [104] |
| C57BL/6J mice | 500 nm | PS | - | 0.5 mg/100 µL | In water | 4 weeks | MPs upregulated the mRNA expressions of inflammatory factors IFN-γ, TNF-α, IL-1β, IL-6, and IL-33, and downregulated IL-4, IL-5, IL-10, IL-18, and TGF-β1 in the liver. | [98] |
| C57 mice | 5 μm | PS | - | 500 μg/L | In water | 28 days | Exposure to MP results in the expression of inflammatory factors in the liver. | [105] |
| Research Object | Particle Size | Material | Number of Particles | Concentration | Mode of Exposure | Exposure Time | Summary | Reference |
|---|---|---|---|---|---|---|---|---|
| Zebrafish | 70 nm, 5 μm | PS | 1.1 × 108–10 particles/mL, 2.9 × 102–4 particles/mL | 0, 20, 200, 2000 μg/L | In water | 3 weeks | Lipid droplets were found in the liver pathological pictures of the 2000 μg/L 70 nm, 5 μm exposure group. | [27] |
| Juvenile D. labrax | 45 nm | PMMA | - | 0, 0.02, 0.2, 2, 20 mg/L | In water | 96 h | The transcription levels of the genes related to lipid metabolism, pparα, pparγ (peroxisome proliferator-activated receptor), and nd5, were upregulated in the liver at 96 h exposure. | [107] |
| Black rockfish | 500 nm, 15 μm | PS | - | 190 μg/L | In water | 21 days | The HIS (liver index) value was generally positively correlated with the liver metabolism (e.g., lipid, protein) of the fish, and the 15μm group had a larger liver index than the other groups. | [106] |
| Larimichthys crocea | 80 nm | PS | - | 0, 1, 10, 100 mg/kg·di | In diet | 21 days | The liver of the high-dose group was enlarged and appeared white, and the contents of TG and lipids in the liver after PSNPs exposure were significantly higher than those in the control group. Oil red O staining showed that the higher the exposure dose, the higher the accumulation of lipid droplets in the liver; the expression of the genes involved in the process of lipid synthesis, decomposition, and transport changed. | [52] |
| Yellow croaker hepatocytes | 80 nm | PS | - | 0, 5, 20, 80 mg/L | In culture medium | 24 h | The expression of lipid synthesis genes fas, srebp1, and pparγ in hepatocytes increased, and the lipid catabolism atg1, pparα, and aco genes increased and then decreased. | [52] |
| Oryzias melastigmas | 10, 200 μm | PS | 1.82 × 1010 particles/m3, 2.27 × 106 particles/m3 | 10 mg/L | In water | 30, 45, 60 days | PS exposure inhibited the accumulation of fatty acid, fatty acid methyl ester, and fatty acid ethyl ester in the liver of marine medaka. | [48] |
| Oryzias melastigma | 2, 10, 200 μm | PS | - | 10 mg/L | In water | 60 days | The hepatic lipid content was significantly increased in the 200 μm PS-MPs exposure group. | [102] |
| ICR male mice | 5, 20 μm | PS | 1.46 × 106 items, 2.27 × 104 items | 0.01, 0.1, 0.5 mg/day | Oral gavage | 28 days | Hepatic HE staining showed lipid droplets; the levels of TC and TG in the liver decreased. | [29] |
| ICR mice | 5 μm | PS | - | 0, 100, 1000 μg/L | In water | 6 weeks | The levels of TCH and TG in the liver of the parental female mice were significantly higher in a concentration-dependent manner than those of the control group, and the level of TCH in the F1 liver was significantly decreased; the level of TG in the liver of the F1 male mice changed, and the level of TG in the F1 female mice decreased; | [65] |
| Mice Mus musculus | 5, 20 μm | PS | - | 0, 0.01, 0.1, 0.5 mg/mL | Oral gavage | 4 weeks | Exposure to 5 and 20 μm PS-MPs inhibited liver TG levels in mice. | [115] |
| High-fat diet C57BL/6 female mice | 42 nm | PS | - | 0, 10, 50 μg/ml | Inject via tail vein | 5 times in 15 days | Liver TC, TG, pparα, and pparγ gene mRNA expression were not affected, unlike the fat and cpt1α gene levels, which were. | [68] |
| C57 mice | 5 μm | PS | - | 500 μg/L | In water | 28 days | Exposure to PSMP caused TG accumulation. | [105] |
| Research Object | Particle Size | Material | Number of Particles | Concentration | Mode of Exposure | Exposure Time | Summary | Reference |
|---|---|---|---|---|---|---|---|---|
| Zebrafish | 70 nm, 5, | PS | 1.1 × 108–10 particles/mL, 2.9 × 102–4 particles/mL | 0, 20, 200, 2000 μg/L | In water | 3 weeks | Energy metabolism is altered in zebrafish liver after exposure to MPs. | [27] |
| ICR male mice | 5, 20 μm | PS | 1.46 × 106 items, 2.27 × 104 items | 0.01, 0.1, 0.5 mg/day | Oral gavage | 28 days | After exposure to MPs, ATP decreased and LDH levels increased in the livers of mice. | [29] |
| ICR mice | 5 μm | PS | - | 0, 100, 1000 μg/L | In water | 6 weeks | MPs affect many activities in mouse livers related to energy metabolism. | [65] |
| Black rockfish | 500 nm, 15 μm | PS | - | 190 μg/L | In water | 21 days | MPs affect crude protein and crude fat content in fish livers. | [106] |
| Zebrafish | 70 nm | PS | - | 0.5, 1.5, 5 ppm | In water | 30 days | NPs affect energy metabolism in zebrafish liver. | [51] |
| Grass carp | 32~40 μm | PS | - | 100, 1000 μg/L | In water | 21 days | The results of pathway analysis showed that MPs affected the signaling pathways related to energy metabolism in grass carp liver. | [127] |
| Minnows | 1 μm | PS | (3.71 ± 0.1) × 108 items/L | 200 μg/L | In water | 28 days | MP exposure interferes with hepatic energy metabolism in the minnow. | [124] |
| Research Object | Particle Size | Material | Number of Particles | Concentration | Mode of Exposure | Exposure Time | Reference |
|---|---|---|---|---|---|---|---|
| Grouper | 22.3 μm | PS | - | 2, 20 mg/g·dt | In water | 25 days | [50] |
| Zebrafish, perch | 5~12 μm | PS | - | 0.0075/0.85 g/day | In water | 21 days | [131] |
| Mice | 5 μm | PS | 1.46 × 106 particles | 20 mg/kg·bw/day | In water | 30 days | [64] |
| Goldfish | 1 μm | PS | 0, 10, 100, 1000 particles/mL | - | In water | 7 days | [128] |
| Male mice | 5 μm | PS | 1.46 × 106 particles | 0.1 mg/day | In water | 90 days | [133] |
| SMMC-7721 | 500 nm | PS | - | 20 μg/mL | In culture medium | 24 h | [129] |
| Zebrafish | 50,100 nm | PS | - | 0.1, 0.5, 2, 10 mg/L | In water | 120 hpf | [137] |
| Zebrafish, perch | 10~45 μm 106~125 μm | PE | - | 10 mg/g·df | In water | 21 days | [138] |
| RAW264.7 | 42 nm | PS | - | 0, 1, 5, 10 mg/mL | In water | 24 h | [139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Ju, Y.; Qian, H.; Wang, J.; Miao, X.; Zhu, Y.; Zhou, L.; Ye, L. Nanoplastics and Microplastics May Be Damaging Our Livers. Toxics 2022, 10, 586. https://doi.org/10.3390/toxics10100586
Yin J, Ju Y, Qian H, Wang J, Miao X, Zhu Y, Zhou L, Ye L. Nanoplastics and Microplastics May Be Damaging Our Livers. Toxics. 2022; 10(10):586. https://doi.org/10.3390/toxics10100586
Chicago/Turabian StyleYin, Jianli, Ye Ju, Honghao Qian, Jia Wang, Xiaohan Miao, Ying Zhu, Liting Zhou, and Lin Ye. 2022. "Nanoplastics and Microplastics May Be Damaging Our Livers" Toxics 10, no. 10: 586. https://doi.org/10.3390/toxics10100586
APA StyleYin, J., Ju, Y., Qian, H., Wang, J., Miao, X., Zhu, Y., Zhou, L., & Ye, L. (2022). Nanoplastics and Microplastics May Be Damaging Our Livers. Toxics, 10(10), 586. https://doi.org/10.3390/toxics10100586