Clinical Assessment of Acute Organophosphorus Pesticide Poisoning in Pediatric Patients Admitted to the Toxicology Emergency Department
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Determination of Laboratory Parameters
2.2.1. Hematological Assay
2.2.2. Biochemical Assay
2.3. Statistical Analysis
2.4. Ethics Considerations
3. Results
3.1. Clinical and Demographic Characteristics of the Patient’s Groups
3.2. Profile of the Hematological Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sabarwal, A.; Kumar, K.; Singh, R.P. Hazardous effects of chemical pesticides on human health–Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 2018, 63, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Koutroubas, S.D. Farmers’ exposure to pesticides: Toxicity types and ways of prevention. Toxics 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rial-Berriel, C.; Acosta-Dacal, A.; Zumbado, M.; Henríquez-Hernández, L.A.; Rodríguez-Hernández, A.; Macías-Montes, A.; Boada, L.D.; Travieso-Aja, M.D.M.; Martin-Cruz, B.; Suárez-Pérez, A.; et al. Epidemiology of Animal Poisonings in the Canary Islands (Spain) during the Period 2014–2021. Toxics 2021, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.; Duca, R.C.; Lovas, S.; Creta, M.; Scheepers, P.T.; Godderis, L.; Ádám, B. Systematic review of comparative studies assessing the toxicity of pesticide active ingredients and their product formulations. Environ. Res. 2020, 181, 108926. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef] [PubMed]
- Requena, M.; Parrón, T.; Navarro, A.; García, J.; Ventura, M.I.; Hernández, A.F.; Alarcón, R. Association between environmental exposure to pesticides and epilepsy. Neurotoxicology 2018, 68, 13–18. [Google Scholar] [CrossRef]
- Karalexi, M.A.; Tagkas, C.F.; Markozannes, G.; Tseretopoulou, X.; Hernández, A.F.; Schüz, J.; Halldorsson, T.I.; Psaltopoulou, T.; Petridou, E.T.; Tzoulaki, I.; et al. Exposure to pesticides and childhood leukemia risk: A systematic review and meta-analysis. Environ. Pollut. 2021, 285, 117376. [Google Scholar] [CrossRef]
- Muñoz-Quezada, M.T.; Lucero, B.A.; Barr, D.B.; Steenland, K.; Levy, K.; Ryan, P.B.; Iglesias, V.; Alvarado, S.; Concha, C.; Rojas, E.; et al. Neurodevelopmental effects in children associated with exposure to organophosphate pesticides: A systematic review. Neurotoxicology 2013, 39, 158–168. [Google Scholar] [CrossRef] [Green Version]
- Georgiadis, N.; Tsarouhas, K.; Tsitsimpikou, C.; Vardavas, A.; Rezaee, R.; Germanakis, I.; Tsatsakis, A.; Stagos, D.; Kouretas, D. Pesticides and cardiotoxicity. Where do we stand? Toxicol. Appl. Pharmacol. 2018, 353, 1–14. [Google Scholar] [CrossRef]
- Chittrakul, J.; Sapbamrer, R.; Sirikul, W. Insecticide exposure and risk of asthmatic symptoms: A systematic review and meta-Analysis. Toxics 2021, 9, 228. [Google Scholar] [CrossRef]
- Mohamed, Y.A.; Meabed, M.H.; Abougaba, K.M.; Sayed, F.A.; Welson, N.N.; Ibrahim, R.E. A comparative study: Rural versus urban children as regard exposure to organophosphorus pesticides using cholinesterase enzyme activity. J. Basic Appl. Sci. 2022, 11, 1–10. [Google Scholar] [CrossRef]
- Srivastava, A.; Gupta, B.; Bihari, V.; Mathur, N.; Srivastava, L.; Pangtey, B.; Bharti, R.; Kumar, P. Clinical, biochemical and neurobehavioural studies of workers engaged in the manufacture of quinalphos. Food Chem. Toxicol. 2000, 38, 65–69. [Google Scholar] [CrossRef]
- Ejigu, D.; Mekonnen, Y. Pesticide use on agricultural fields and health problems in various activities. East Afr. Med. J. 2005, 82, 8. [Google Scholar]
- Meller, D.; Fraser, I.; Kryger, M. Hyperglycemia in anticholinesterase poison-ing. Can. Med. Assoc. J. 1998, 124, 745. [Google Scholar]
- Eltayyeb, A.A.; Gad, E.F.; Abd Elaal, A.N. Management of organophosphorus pesticide poisoning in Assiut University Children’s Hospital: A clinical audit. J. Curr. Med. Res. Pract. 2021, 6, 174. [Google Scholar] [CrossRef]
- Suwannakul, B.; Sapbamrer, R.; Wiwattanadittakul, N.; Hongsibsong, S. Different Timing of Prenatal Organophosphate Pesticides Exposure Is Influenced Different Aspects of Infant Developmental Performance. Toxics 2021, 9, 99. [Google Scholar] [CrossRef]
- Sgargi, D.; Adam, B.; Budnik, L.T.; Dinelli, G.; Moldovan, H.R.; Perry, M.J.; Scheepers, P.T.; Schlunssen, V.; Teixeira, J.P.; Mandrioli, D.; et al. Protocol for a systematic review and meta-analysis of human exposure to pesticide residues in honey and other bees’ products. Environ. Res. 2020, 186, 109470. [Google Scholar] [CrossRef]
- Bai, S.H.; Ogbourne, S.M. Glyphosate: Environmental contamination, toxicity and potential risks to human health via food contamination. Environ. Sci. Pollut. Res. 2016, 23, 18988–19001. [Google Scholar] [CrossRef]
- Elgueta, S.; Valenzuela, M.; Fuentes, M.; Ulloa, P.E.; Ramos, C.; Correa, A.; Molinett, S. Analysis of multi-pesticide residues and dietary risk assessment in fresh tomatoes (Lycopersicum esculentum) from local supermarkets of the metropolitan region, Chile. Toxics 2021, 9, 249. [Google Scholar] [CrossRef]
- Pal, E.; Almasri, H.; Paris, L.; Diogon, M.; Pioz, M.; Cousin, M.; Sené, D.; Tchamitchian, S.; Tavares, D.A.; Delbac, F.; et al. Toxicity of the Pesticides Imidacloprid, Difenoconazole and Glyphosate Alone and in Binary and Ternary Mixtures to Winter Honey Bees: Effects on Survival and Antioxidative Defenses. Toxics 2022, 10, 104. [Google Scholar] [CrossRef]
- Kittelmann, A.; Müller, C.; Rohn, S.; Michalski, B. Transfer of Pesticide Residues from Grapes (Vitis vinifera) into Wine—Correlation with Selected Physicochemical Properties of the Active Substances. Toxics 2022, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Alkon, A.; Gunier, R.B.; Hazard, K.; Castorina, R.; Hoffman, P.D.; Scott, R.P.; Anderson, K.A.; Bradman, A. Preschool-Age Children’s Pesticide Exposures in Child Care Centers and at Home in Northern California. J. Pediatr. Health Care 2022, 36, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Fan, N.C.; Yao, T.C.; Hsia, S.H.; Lee, E.P.; Huang, J.L.; Wu, H.P. Clinical spectrum of acute poisoning in children admitted to the pediatric emergency department. Pediatr. Neonatol. 2019, 60, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gokalp, G. Evaluation of poisoning cases admitted to pediatric emergency department. Int. J. Pediatr. Adolesc. Med. 2019, 6, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, H.; Birincioglu, I.; Turna, O.; Ketenci, H.C.; Beyhun, N.E. Fatal poisoning of chilhood in the Eastern Black Sea region of Turkey (2009–2013). J. Forensic Leg. Med. 2015, 34, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T. Pediatric Suicide. J. PeriAnesthesia Nurs. 2021, 36, 438–440. [Google Scholar] [CrossRef]
- Wu, J.-H.; Wu, H.-P.; Liu, H.-L.; Yang, M.-C.; Chou, C.-C.; Chang, C.-F.; Lin, H.-M.; Tsai, T.-H.; Lin, Y.-R. Factors associated with outcomes of children treated at an emergency department for nonpharmaceutical poison exposure. J. Acute Med. 2011, 1, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Snodgrass, W.R. Diagnosis and treatment of poisoning due to pesticides. In Hayes’ Handbook of Pesticide Toxicology; Academic Press: Cambridge, MA, USA, 2010; pp. 1295–1311. [Google Scholar]
- Nistor, N.; Frasinariu, O.E.; Rugină, A.; Ciomaga, I.M.; Jităreanu, C.; Ştreangă, V. Epidemiological study on accidental poisonings in children from northeast Romania. Medicine 2018, 97, e11469. [Google Scholar] [CrossRef]
- Kazanasmaz, H.; Kazanasmaz, Ö.; Çalık, M. Epidemiological and socisociocultural asessment of childhood poisonings. Turk. J. Emerg. Med. 2019, 19, 127–131. [Google Scholar] [CrossRef]
- Simonelli, A.; Carfora, A.; Basilicata, P.; Liguori, B.; Mascolo, P.; Policino, F.; Niola, M.; Campobasso, C.P. Suicide by pesticide (phorate) ingestion: Case report and review of literature. Toxics 2022, 10, 205. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Fareed, M.; Pathak, M.K.; Bihari, V.; Kamal, R.; Srivastava, A.K.; Kesavachandran, C.N. Adverse respiratory health and hematological alterations among agricultural workers occupationally exposed to organophosphate pesticides: A cross-sectional study in North India. PLoS ONE 2013, 8, e69755. [Google Scholar] [CrossRef]
- Hernández, A.F.; López, O.; Rodrigo, L.; Gil, F.; Pena, G.; Serrano, J.L.; Pla, A. Changes in erythrocyte enzymes in humans long-term exposed to pesticides: Influence of several markers of individual susceptibility. Toxicol. Lett. 2005, 159, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Hundekari, I.A.; Suryakar, A.N.; Rathi, D.B. Acute organo-phosphorus pesticide poisoning in North Karnataka, India: Oxidative damage, haemoglobin level and total leukocyte. Afr. Health Sci. 2013, 13, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Thompson, C.M.; Gerdes, J.M.; VanBrocklin, H.F. Positron emission tomography studies of organophosphate chemical threats and oxime countermeasures. Neurobiol. Dis. 2020, 133, 104455. [Google Scholar] [CrossRef]
- Giyanwani, P.R.; Zubair, U.; Salam, O.; Zubair, Z. Respiratory failure following organophosphate poisoning: A literature review. Cureus 2017, 9, e1651. [Google Scholar] [CrossRef] [Green Version]
- Leibson, T.; Lifshitz, M. Organophosphate and carbamate poisoning: Review of the current literature and summary of clinical and laboratory experience in southern Israel. Isr. Med. Assoc. J. 2008, 10, 767. [Google Scholar]
- Hassanin, N.M.; Awad, O.M.; El-Fiki, S.; Abou-Shanab, R.A.; Abou-Shanab, A.R.; Amer, R.A. Association between exposure to pesticides and disorder on hematological parameters and kidney function in male agricultural workers. Environ. Sci. Pollut. Res. 2018, 25, 30802–30807. [Google Scholar] [CrossRef]
- Minton, N.A.; Murray, V.S. A review of organophosphate poisoning. Med. Toxicol. Advers. Drug Exp. 1988, 3, 350–375. [Google Scholar] [CrossRef]
- Bardin, P.G.; Van Eeden, S.F.; Moolman, J.A.; Foden, A.P.; Joubert, J.R. Organophosphate and carbamate poisoning. Arch. Intern. Med. 1994, 154, 1433–1441. [Google Scholar] [CrossRef]
- Kwong, T.C. Organophosphate pesticides: Biochemistry and clinical toxicology. Ther. Drug Monit. 2002, 24, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Iza, S.C.; Rodríguez, A.I.; Prieto-Suarez, E. Assessment of hematological parameters in workers exposed to organophosphorus pesticides, carbamates and pyrethroids in Cundinamarca 2016–2017. Rev. De Salud Pública 2017, 19, 468–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karami-Mohajeri, S.; Ahmadipour, A.; Rahimi, H.R.; Abdollahi, M. Adverse effects of organophosphorus pesticides on the liver: A brief summary of four decades of research. Arh. Za Hig. Rada I Toksikol. 2017, 68, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Nanda, R.; Mangaraj, M.; Rathod, K.P.; Mishra, P.K. Glycemic status in organophosphorus poisoning. J Nepal Health Res Counc 2015, 13, 214–219. [Google Scholar] [PubMed]
- Nistor, N.; Jitareanu, C.; Frasinariu, O.E.; Ciomaga, I.M.; Rugina, A.L.; Streanga, V. Epidemiologic profile and triggering factors of voluntary poisoning in teenagers. Medicine 2017, 96, e5831. [Google Scholar] [CrossRef]
- London, L.; Flisher, A.J.; Wesseling, C.; Mergler, D.; Kromhout, H. Suicide and exposure to organophosphate insecticides: Cause or effect? Am. J. Ind. Med. 2005, 47, 308–321. [Google Scholar] [CrossRef]
- Pope, C.N.; Chakraborti, T.K.; Chapman, M.L.; Farrar, J.D. Long-term neurochemical and behavioral effects induced by acute chlorpyrifos treatment. Pharmacol. Biochem. Behav. 1992, 42, 251–256. [Google Scholar] [CrossRef]
- Insecticides, Organophosphorus. A General introduction. World Health organization, Geneva, 1986, 13–181. Available online: https://apps.who.int/iris/bitstream/handle/10665/40198/9241542632-eng.pdf?sequence=1&isAllowed=y (accessed on 20 August 2022).
- Jokanović, M.; Kosanović, M. Neurotoxic effects in patients poisoned with organophosphorus pesticides. Environ. Toxicol. Pharmacol. 2010, 29, 195–201. [Google Scholar] [CrossRef]
- Wagner, S.l.; Orwick, D.L. Chronic Organophosphate Exposure Associated With Transient Hypertonia in an Infant. Pediatrics 1994, 94, 94–97. [Google Scholar]
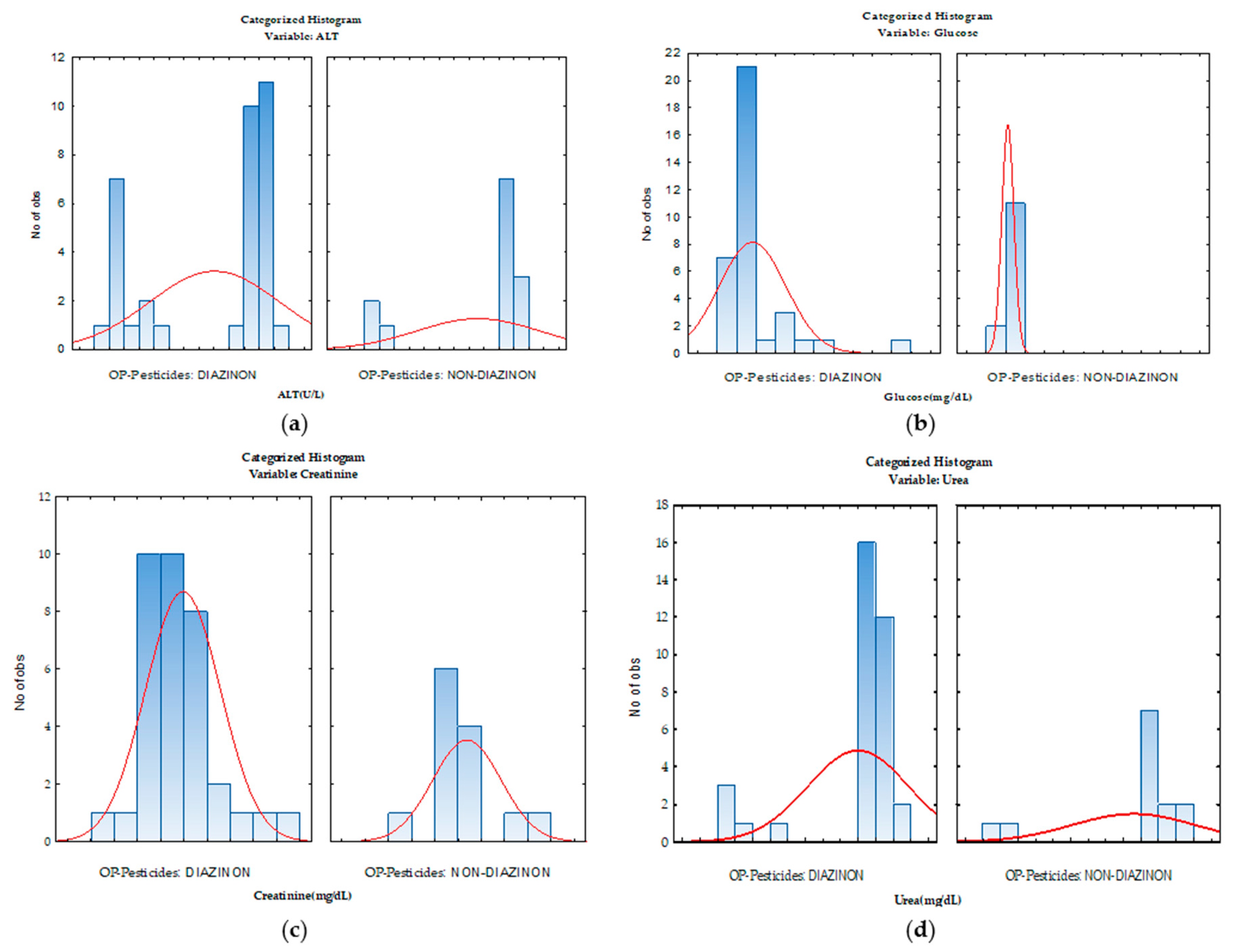
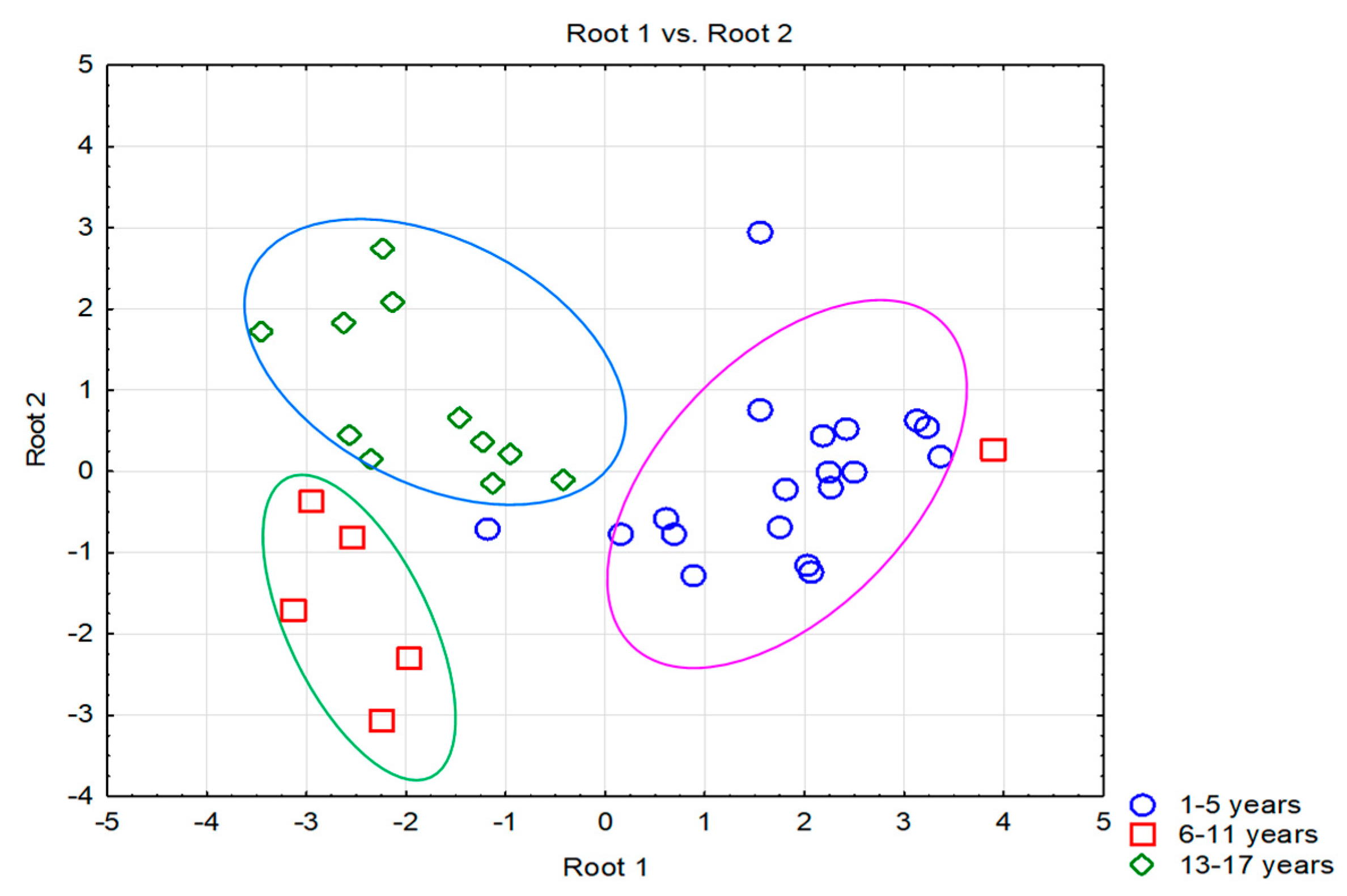
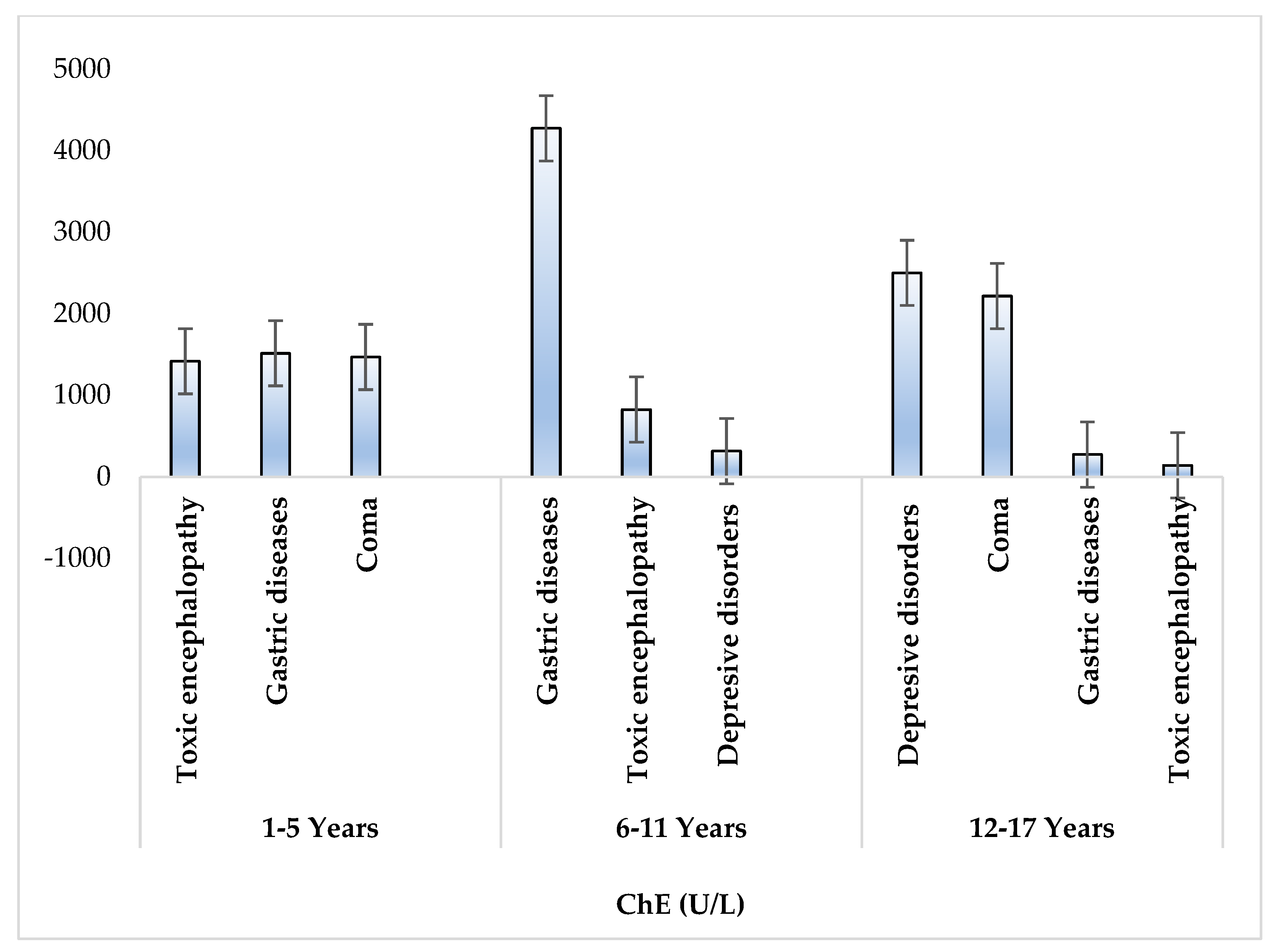
| Characteristics | Details |
|---|---|
| number of cases | 48 |
| environment (urban/rural) | 4/44 |
| age groups | 1–5 years (n = 31) 6–11 years (n = 6) 12–17 years (n = 11) |
| diseases due to acute intoxication with organophosphorus pesticides | toxic encephalopathy (n = 21), coma (n = 8), depressive disorder (n = 5), gastrointestinal disorders (n = 13), respiratory failure (n = 6) |
| type of intoxication (voluntary/accidental) | 9/39 |
| entry route (ingestion/dermal absorption) | 46/2 |
| place of exposure | at home |
| name of the involved pesticide (trade name) | Diazinon (80% of cases), others like Lindavet, Calypso, and Byemite (20% of cases) |
| Parameter | Children 1–5 Years (n = 31) | Children 6–11 Years (n = 6) | Adolescents 12–17 Years (n = 11) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Stdev | Median | Range | Mean | Stdev | Median | Range | Mean | Stdev | Median | Range | |
| WBC (×103/µL) | 16.83 | 9.47 | 13.1 | 4.68–42.69 | 11.89 | 4.75 | 11.76 | 5.9–18.59 | 14.18 | 5.22 | 13.27 | 7.65–25.44 |
| NEU (%) | 66.56 | 20.66 | 71.7 | 8.76–90.7 | 70.93 | 11.99 | 72.8 | 55.7–88.5 | 75.48 | 17.08 | 80.9 | 29.7–93.9 |
| LY (%) | 23.06 | 16.96 | 19.85 | 4.28–66.6 | 20.6 | 8.8 | 21.35 | 7.5–31.2 | 18.16 | 16.26 | 13.5 | 3–64.7 |
| MO (%) | 7.23 | 8.14 | 5 | 1.2–48.8 | 7.23 | 3.12 | 6.8 | 3.3–11.5 | 5.35 | 1.77 | 5 | 2.9–8.7 |
| EOS (%) | 2.48 | 6.71 | 0.9 | 0–38.2 | 1.02 | 0.96 | 0.85 | 0–2.9 | 0.9 | 0.82 | 0.6 | 0–2.5 |
| BAS (%) | 0.51 | 1.66 | 0.2 | 0–9.4 | 0.22 | 0.15 | 0.15 | 0.1–0.5 | 0.21 | 0.09 | 0.2 | 0–0.3 |
| RBC (×106/µL) | 4.47 | 0.89 | 4.52 | 0.26–5.74 | 4.41 | 0.4 | 4.32 | 4.01–4.94 | 4.45 | 0.5 | 4.65 | 3.26–4.97 |
| HGB (g/dL) | 10.92 | 1.89 | 11.3 | 3–13 | 12.18 | 0.74 | 12.15 | 11.2–13.3 | 12.66 | 1.21 | 12.8 | 10.5–14.8 |
| HCT (%) | 33.11 | 6.59 | 34 | 0.60–40.4 | 36.63 | 2.53 | 35.9 | 33.7–41.3 | 38.6 | 4.77 | 38.1 | 30.5–47.8 |
| MCV/fL | 72.37 | 14.37 | 74.3 | 4.59–88.2 | 83.33 | 3.08 | 83.85 | 78.9–86.9 | 86.8 | 5.21 | 86.1 | 79.1–96.1 |
| MCH/pg | 24.06 | 3.76 | 24.8 | 12.9–28.8 | 27.72 | 1.14 | 27.85 | 25.8–29.4 | 28.58 | 1.82 | 28.1 | 26.6–33.4 |
| MCHC (g/dL) | 32.74 | 1.95 | 32.9 | 27–36.4 | 33.3 | 1.19 | 32.75 | 32.2–35 | 32.95 | 1.73 | 32.4 | 31–36.4 |
| RDW-Cv/fL | 16.43 | 11.07 | 14.2 | 10.12–75.8 | 12.62 | 0.65 | 12.35 | 11.8–13.6 | 12.77 | 0.66 | 12.9 | 11.8–13.9 |
| RDW-SD/fL | 39.87 | 4.47 | 40.8 | 28.1–49.2 | 39.5 | 3.48 | 39.25 | 34.5–45.1 | 42.65 | 4.1 | 44 | 35.7–51.1 |
| PLT (×103/µL) | 364.45 | 142.64 | 354 | 37.1–698 | 276.67 | 104.39 | 246 | 185–485 | 290 | 106.59 | 288 | 119–522 |
| MPV/fL | 8.4 | 1.34 | 8 | 6.4–12.3 | 9.5 | 1.57 | 9.8 | 7–11.8 | 9.25 | 1.31 | 9.4 | 6.8–11.1 |
| PCT/fL | 1.38 | 5.88 | 0.29 | 0.164–33.6 | 0.25 | 0.06 | 0.25 | 0.15–0.34 | 0.26 | 0.08 | 0.25 | 0.11–0.39 |
| p-LCR (%) | 30.36 | 61.66 | 17.75 | 8.4–361 | 26.82 | 10.86 | 22.95 | 12.1–43.7 | 29.45 | 8.79 | 31.3 | 12.8–42.6 |
| PDW/fL | 13.51 | 2.6 | 15 | 8.8–17.5 | 13.75 | 2.48 | 14.95 | 10.3–16.3 | 15.34 | 1.59 | 15.9 | 12.2–17.6 |
| Parameter | Children 1–5 Years (n = 31) | Children 6–11 Years (n = 6) | Adolescents 12–17 Years (n = 11) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Stdev | Median | Range | Mean | Stdev | Median | Range | Mean | Stdev | Median | Range | |
| AST | 66.23 | 161.69 | 29 | 18–942 | 30 | 8.7 | 28.5 | 19–44 | 26.55 | 12.27 | 26 | 12–51 |
| ALT | 46.94 | 123.58 | 19 | 12–717 | 21.67 | 11.97 | 21 | 8.00–45 | 13.27 | 6.85 | 11 | 5–27 |
| ChE | 1449.42 | 2779.47 | 180 | 41–12,017 | 2380.33 | 3058.14 | 786 | 290–8843 | 1211 | 2197.16 | 182 | 71–7036 |
| Urea | 30.47 | 9.04 | 28 | 17–52 | 24.17 | 6.28 | 24 | 14–32 | 25.18 | 9.19 | 23 | 8–45 |
| Creatinine | 0.58 | 0.16 | 0.58 | 0.26–1.02 | 0.68 | 0.17 | 0.64 | 0.45–0.997 | 0.65 | 0.1 | 0.6 | 0.56–0.92 |
| Glucose | 157.77 | 95.37 | 112 | 64–513 | 156.33 | 41.61 | 150.5 | 110–241 | 127.45 | 51.81 | 104 | 77–238 |
| Clinical Parameters | r | p |
|---|---|---|
| ChE × ALT | 0.47 | 0.001 |
| ChE × AST | 0.43 | 0.001 |
| ChE × PLT | 0.48 | 0.001 |
| ChE × Creatinine | 0.38 | 0.008 |
| ChE × Glucose | 0.39 | 0.009 |
| ChE × RDW-cv | 0.47 | 0.001 |
| Variable | Wilks’-λ | λ-Partial | F | p | R2 | 1-R2 |
|---|---|---|---|---|---|---|
| Leucocyte | 0.233 | 0.498 | 9.55 | 0.001 | 0.216 | 0.783 |
| Urea | 0.207 | 0.560 | 7.44 | 0.004 | 0.378 | 0.621 |
| ALT | 0.160 | 0.724 | 3.605 | 0.047 | 0.085 | 0.914 |
| Creatinine | 0.173 | 0.671 | 4.647 | 0.022 | 0.391 | 0.608 |
| Lymphocyte | 0.168 | 0.690 | 4.252 | 0.029 | 0.171 | 0.828 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caba, I.-C.; Ștreangă, V.; Dobrin, M.-E.; Jităreanu, C.; Jităreanu, A.; Profire, B.-Ș.; Apotrosoaei, M.; Focșa, A.-V.; Caba, B.; Agoroaei, L. Clinical Assessment of Acute Organophosphorus Pesticide Poisoning in Pediatric Patients Admitted to the Toxicology Emergency Department. Toxics 2022, 10, 582. https://doi.org/10.3390/toxics10100582
Caba I-C, Ștreangă V, Dobrin M-E, Jităreanu C, Jităreanu A, Profire B-Ș, Apotrosoaei M, Focșa A-V, Caba B, Agoroaei L. Clinical Assessment of Acute Organophosphorus Pesticide Poisoning in Pediatric Patients Admitted to the Toxicology Emergency Department. Toxics. 2022; 10(10):582. https://doi.org/10.3390/toxics10100582
Chicago/Turabian StyleCaba, Ioana-Cezara, Violeta Ștreangă, Mona-Elisabeta Dobrin, Cristina Jităreanu, Alexandra Jităreanu, Bianca-Ștefania Profire, Maria Apotrosoaei, Alin-Viorel Focșa, Bogdan Caba, and Luminița Agoroaei. 2022. "Clinical Assessment of Acute Organophosphorus Pesticide Poisoning in Pediatric Patients Admitted to the Toxicology Emergency Department" Toxics 10, no. 10: 582. https://doi.org/10.3390/toxics10100582
APA StyleCaba, I.-C., Ștreangă, V., Dobrin, M.-E., Jităreanu, C., Jităreanu, A., Profire, B.-Ș., Apotrosoaei, M., Focșa, A.-V., Caba, B., & Agoroaei, L. (2022). Clinical Assessment of Acute Organophosphorus Pesticide Poisoning in Pediatric Patients Admitted to the Toxicology Emergency Department. Toxics, 10(10), 582. https://doi.org/10.3390/toxics10100582







