Exposure Prioritization (Ex Priori): A Screening-Level High-Throughput Chemical Prioritization Tool
Abstract
:1. Introduction
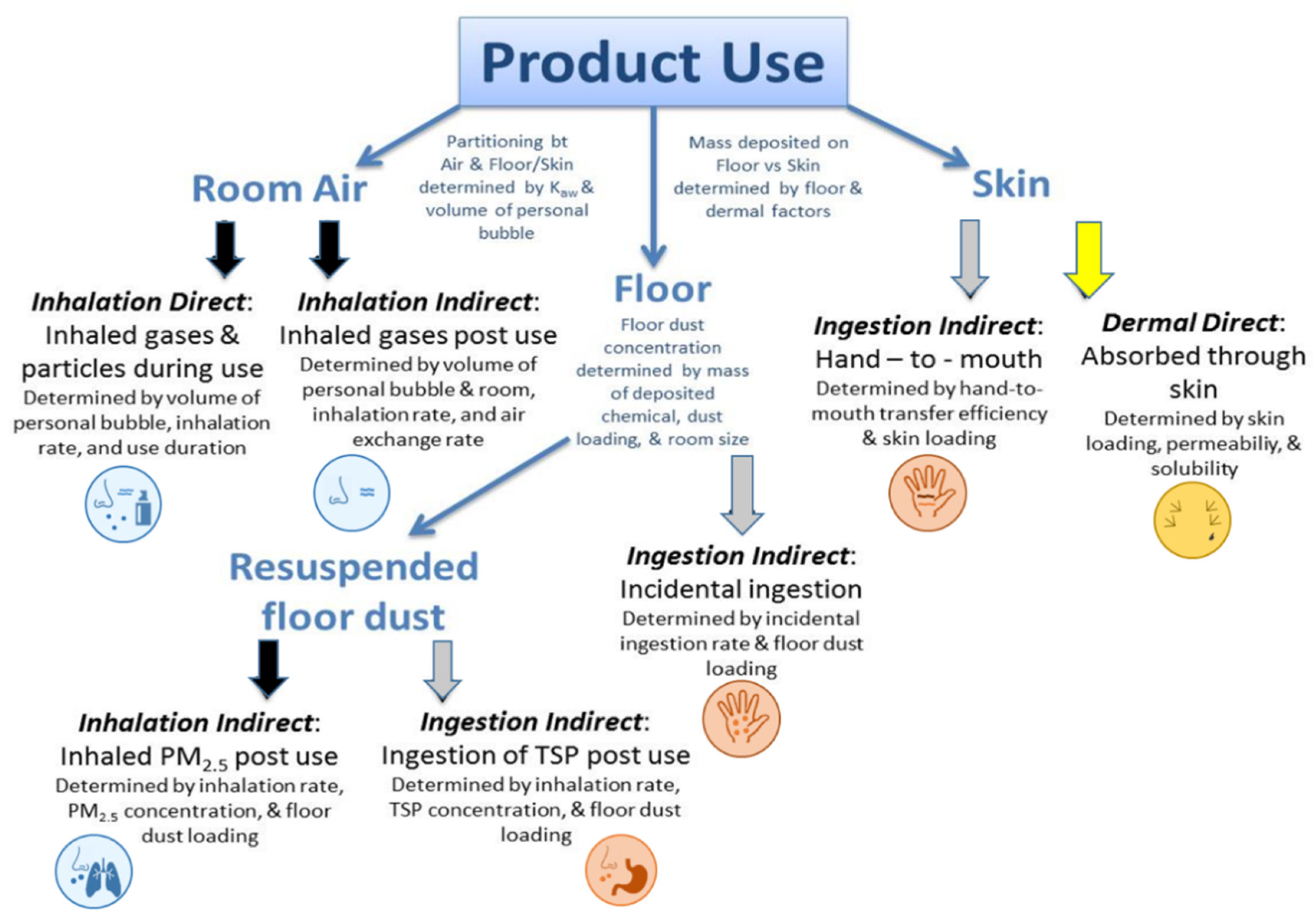
2. Materials and Methods
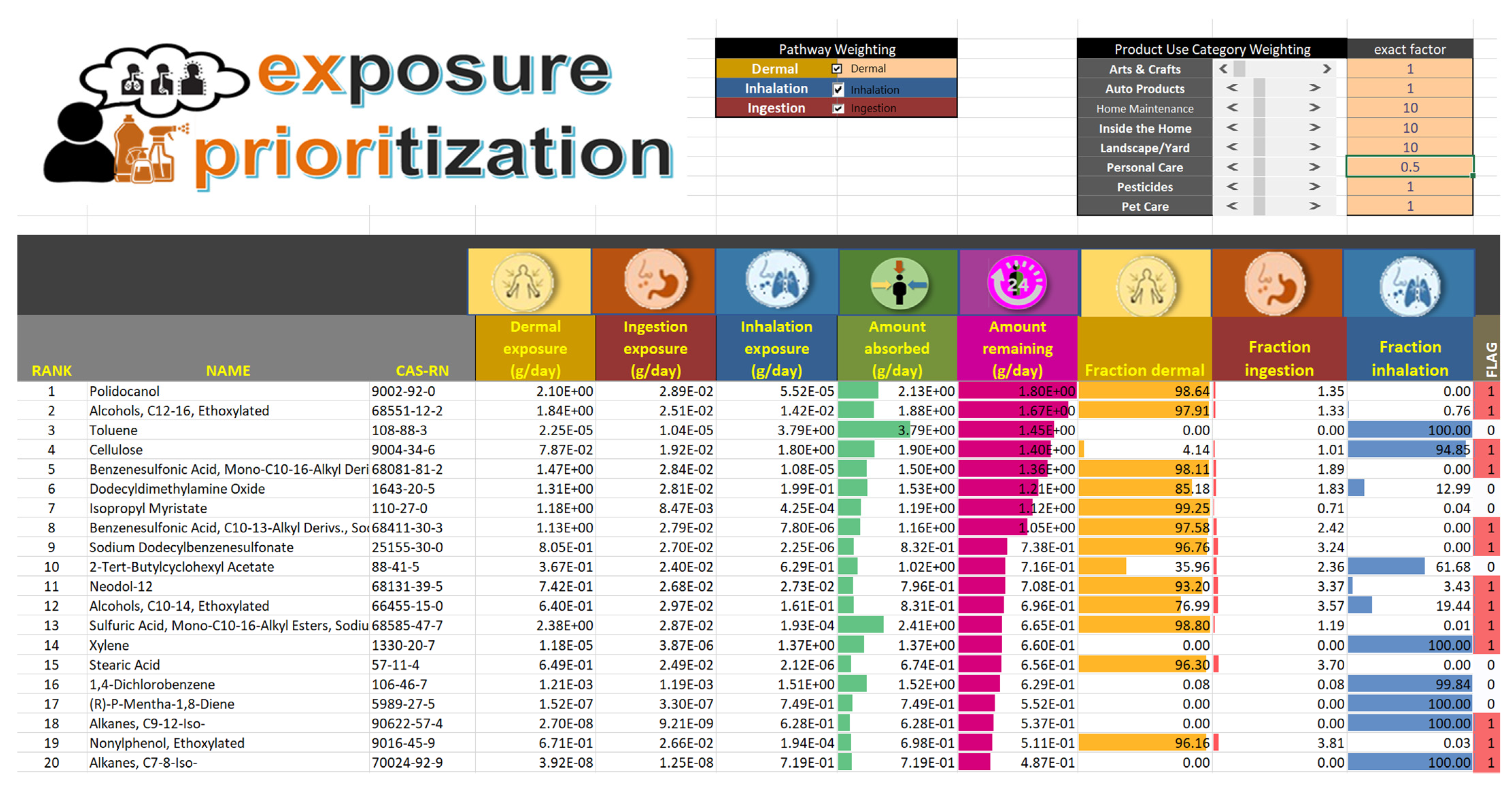
2.1. Product-Category Weights
2.2. Pathway Weighting
2.3. Sensitivity Analysis
2.4. Evaluation Using Exposures Inferred from NHANES Biomonitoring Data
3. Results
3.1. Chemical Rankings
3.2. Sensitivity Analysis
3.3. Evaluation by Comparing to NHANES-Inferred Exposures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chuprina, A.; Lukin, O.; Demoiseaux, R.; Buzko, A.; Shivanyuk, A. Drug- and lead-likeness, target class, and molecular diversity analysis of 7.9 million commercially available organic compounds provided by 29 suppliers. J. Chem. Inf. Model. 2010, 50, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Judson, R.; Richard, A.; Dix, D.J.; Houck, K.; Martin, M.; Kavlock, R.; Dellarco, V.; Henry, T.; Holderman, T.; Sayre, P.; et al. The toxicity data landscape for environmental chemicals. Environ. Health Perspect. 2009, 117, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Wambaugh, J.F.; Setzer, R.W.; Reif, D.M.; Gangwal, S.; Mitchell-Blackwood, J.; Arnot, J.A.; Joliet, O.; Frame, A.; Rabinowitz, J.; Knudsen, T.B.; et al. High-throughput models for exposure-based chemical prioritization in the ExpoCast project. Environ. Sci. Technol. 2013, 47, 8479–8488. [Google Scholar] [CrossRef] [PubMed]
- Egeghy, P.P.; Judson, R.; Gangwal, S.; Mosher, S.; Smith, D.; Vail, J.; Cohen Hubal, E.A. The exposure data landscape for manufactured chemicals. Sci. Total Environ. 2012, 414, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Goodson, W.H., 3rd; Lowe, L.; Carpenter, D.O.; Gilbertson, M.; Manaf Ali, A.; Lopez de Cerain Salsamendi, A.; Lasfar, A.; Carnero, A.; Azqueta, A.; Amedei, A.; et al. Assessing the carcinogenic potential of low-dose exposures to chemical mixtures in the environment: The challenge ahead. Carcinogenesis 2015, 36 (Suppl. 1), S254–S296. [Google Scholar] [CrossRef]
- Isaacs, K.K.; Glen, W.G.; Egeghy, P.; Goldsmith, M.R.; Smith, L.; Vallero, D.; Brooks, R.; Grulke, C.M.; Özkaynak, H. SHEDS-HT: An integrated probabilistic exposure model for prioritizing exposures to chemicals with near-field and dietary sources. Environ. Sci. Technol. 2014, 48, 12750–12759. [Google Scholar] [CrossRef]
- Csiszar, S.A.; Meyer, D.E.; Dionisio, K.L.; Egeghy, P.; Isaacs, K.K.; Price, P.S.; Scanlon, K.A.; Tan, Y.M.; Thomas, K.; Vallero, D.; et al. Conceptual Framework To Extend Life Cycle Assessment Using Near-Field Human Exposure Modeling and High-Throughput Tools for Chemicals. Environ. Sci. Technol. 2016, 50, 11922–11934. [Google Scholar] [CrossRef]
- Csiszar, S.A.; Ernstoff, A.S.; Fantke, P.; Jolliet, O. Stochastic modeling of near-field exposure to parabens in personal care products. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 152–159. [Google Scholar] [CrossRef]
- Egeghy, P.P.; Sheldon, L.S.; Isaacs, K.K.; Ozkaynak, H.; Goldsmith, M.R.; Wambaugh, J.F.; Judson, R.S.; Buckley, T.J. Computational Exposure Science: An Emerging Discipline to Support 21st-Century Risk Assessment. Environ. Health Perspect. 2016, 124, 697–702. [Google Scholar] [CrossRef]
- Vallero, D.; Isukapalli, S.; Zartarian, V.; McCurdy, T.; McKone, T.; Georgopoulos, P.; Dary, C. Modeling and Predicting Pesticide Exposures. In Hayes’ Handbook of Pesticide Toxicology; Krieger, R., Ed.; Elsevier Science: New York, NY, USA, 2010; pp. 995–1020. [Google Scholar] [CrossRef]
- Goldsmith, M.; Vallero, D.; Egeghy, P.; Chang, D.; Grulke, C.; Tan, C.; Wambaugh, J. Ex Priori: Exposure-based Prioritization across Chemical Space. In Proceedings of the International Society of Exposure Science Annual Conference, Cincinnati, OH, USA, 12–16 October 2014. [Google Scholar]
- Tan, Y.-M.; Chang, D.T.; Phillips, M.; Edwards, S.; Grulke, C.M.; Goldsmith, M.-R.; Sobus, J.; Conolly, R.; Tornero-Velez, R.; Dary, C.C. Biomarkers in computational toxicology. In Biomarkers in Toxicology; Gupta, R., Ed.; Elsevier: Waltham, MA, USA, 2014; pp. 1039–1055. [Google Scholar] [CrossRef]
- Goldsmith, M.-R.; Tan, C.; Chang, D.; Grulke, C.; Tornero-Velez, R.; Vallero, D.; Day, C.; Johnson, J.; Egeghy, P.; Mitchell-Blackwood, J.; et al. Summary Report for Personal Chemical Exposure Informatics: Visualization and Exploratory Research in Simulations and Systems (PerCEIVERS); Environmental Protection Agency: Washington, DC, USA, 2013; Report number EPA/600/R13/041 (NTIS PB2013-108926).
- Dionisio, K.L.; Frame, A.M.; Goldsmith, M.R.; Wambaugh, J.F.; Liddell, A.; Cathey, T.; Smith, D.; Vail, J.; Ernstoff, A.S.; Fantke, P.; et al. Exploring consumer exposure pathways and patterns of use for chemicals in the environment. Toxicol. Rep. 2015, 2, 228–237. [Google Scholar] [CrossRef]
- Fantke, P.; Chiu, W.A.; Aylward, L.; Judson, R.; Huang, L.; Jang, S.; Gouin, T.; Rhomberg, L.; Aurisano, N.; McKone, T.; et al. Exposure and toxicity characterization of chemical emissions and chemicals in products: Global recommendations and implementation in USEtox. Int. J. Life Cycle Assess. 2021, 26, 899–915. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, R.K.; Bachmann, T.M.; Gold, L.S.; Huijbregts, M.A.J.; Jolliet, O.; Juraske, R.; Koehler, A.; Larsen, H.F.; MacLeod, M.; Margni, M.; et al. USEtox—The UNEP-SETAC toxicity model: Recommended characterisation factors for human toxicity and freshwater ecotoxicity in life cycle impact assessment. Int. J. Life Cycle Assess. 2008, 13, 532–546. [Google Scholar] [CrossRef]
- Arnot, J.A.; Brown, T.N.; Wania, F.; Breivik, K.; McLachlan, M.S. Prioritizing chemicals and data requirements for screening-level exposure and risk assessment. Environ. Health Perspect. 2012, 120, 1565–1570. [Google Scholar] [CrossRef]
- Arnot, J.A.; Mackay, D. Policies for Chemical Hazard and Risk Priority Setting: Can Persistence, Bioaccumulation, Toxicity, and Quantity Information Be Combined? Environ. Sci. Technol. 2008, 42, 4648–4654. [Google Scholar] [CrossRef] [PubMed]
- Arnot, J.A.; Mackay, D.; Webster, E.; Southwood, J.M. Screening Level Risk Assessment Model for Chemical Fate and Effects in the Environment. Environ. Sci. Technol. 2006, 40, 2316–2323. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Westgate, J.N.; Hughes, L.; Zhang, X.; Givehchi, B.; Toose, L.; Armitage, J.M.; Wania, F.; Egeghy, P.; Arnot, J.A. A Model for Risk-Based Screening and Prioritization of Human Exposure to Chemicals from Near-Field Sources. Environ. Sci. Technol. 2018, 52, 14235–14244. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Exploring ToxCast™ Data Sets: Downloadable Data. Available online: https://www.epa.gov/chemical-research/exploring-toxcast-data-downloadable-data. (accessed on 18 January 2022).
- Goldsmith, M.R.; Grulke, C.M.; Brooks, R.D.; Transue, T.R.; Tan, Y.M.; Frame, A.; Egeghy, P.; Edwards, R.; Chang, D.; Tornero-Velez, R.; et al. Development of a consumer product ingredient database for chemical exposure screening and prioritization. Food Chem. Toxicol. 2014, 65, 269–279. [Google Scholar] [CrossRef]
- Dionisio, K.L.; Phillips, K.; Price, P.S.; Grulke, C.M.; Williams, A.; Biryol, D.; Hong, T.; Isaacs, K.K. The Chemical and Products Database, a resource for exposure-relevant data on chemicals in consumer products. Sci. Data 2018, 5, 180125. [Google Scholar] [CrossRef] [Green Version]
- Judson, R.; Richard, A.; Dix, D.; Houck, K.; Elloumi, F.; Martin, M.; Cathey, T.; Transue, T.R.; Spencer, R.; Wolf, M. ACToR--Aggregated Computational Toxicology Resource. Toxicol. Appl. Pharmacol. 2008, 233, 7–13. [Google Scholar] [CrossRef]
- Nicas, M. Estimating exposure intensity in an imperfectly mixed room. Am. Ind. Hyg. Assoc. J. 1996, 57, 542–550. [Google Scholar] [CrossRef]
- Nazaroff, W.W.; Weschler, C.J. Cleaning products and air fresheners: Exposure to primary and secondary air pollutants. Atmos. Environ. 2004, 38, 2841–2865. [Google Scholar] [CrossRef]
- Keil, C.B.; Nicas, M. Predicting Room Vapor Concentrations Due to Spills of Organic Solvents. AIHA J. 2003, 64, 445–454. [Google Scholar] [CrossRef]
- Woodrow, J.E.; Seiber, J.N.; Baker, L.W. Correlation Techniques for Estimating Pesticide Volatilization Flux and Downwind Concentrations. Environ. Sci. Technol. 1997, 31, 523–529. [Google Scholar] [CrossRef]
- Woodrow, J.E.; Seiber, J.N.; Dary, C. Predicting pesticide emissions and downwind concentrations using correlations with estimated vapor pressures. J. Agric. Food Chem. 2001, 49, 3841–3846. [Google Scholar] [CrossRef]
- Mackay, D.; van Wesenbeeck, I. Correlation of chemical evaporation rate with vapor pressure. Environ. Sci. Technol. 2014, 48, 10259–10263. [Google Scholar] [CrossRef]
- van Wesenbeeck, I.; Driver, J.; Ross, J. Relationship between the evaporation rate and vapor pressure of moderately and highly volatile chemicals. Bull. Environ. Contam. Toxicol. 2008, 80, 315–318. [Google Scholar] [CrossRef]
- Howard-Reed, C.; Corsi, R.L.; Moya, J. Mass Transfer of Volatile Organic Compounds from Drinking Water to Indoor Air: The Role of Residential Dishwashers. Environ. Sci. Technol. 1999, 33, 2266–2272. [Google Scholar] [CrossRef]
- McCready, D. A Comparison of Screening and Refined Exposure Models for Evaluating Toluene Air Emissions from a Washing Machine. Hum. Ecol. Risk Assess. Int. J. 2012, 19, 972–988. [Google Scholar] [CrossRef]
- McCready, D.; Arnold, S.M.; Fontaine, D.D. Evaluation of Potential Exposure to Formaldehyde Air Emissions from a Washing Machine Using the IAQX Model. Hum. Ecol. Risk Assess. Int. J. 2012, 18, 832–854. [Google Scholar] [CrossRef]
- Howard, C.; Corsi, R.L. Volatilization of chemicals from drinking water to indoor air: The role of residential washing machines. J. Air Waste Manag. Assoc. 1998, 48, 907–914. [Google Scholar] [CrossRef]
- Singer, B.C.; Destaillats, H.; Hodgson, A.T.; Nazaroff, W.W. Cleaning products and air fresheners: Emissions and resulting concentrations of glycol ethers and terpenoids. Indoor Air 2006, 16, 179–191. [Google Scholar] [CrossRef]
- Shin, H.M.; McKone, T.E.; Bennett, D.H. Volatilization of low vapor pressure--volatile organic compounds (LVP-VOCs) during three cleaning products-associated activities: Potential contributions to ozone formation. Chemosphere 2016, 153, 130–137. [Google Scholar] [CrossRef]
- Earnest, C.M. A Two-Zone Model to Predict Inhalation Exposure to Toxic Chemicals in Cleaning Products; University of Texas Austin: Austin, TX, USA, 2009. [Google Scholar]
- Buist, H.E.; Wit-Bos, L.; Bouwman, T.; Vaes, W.H. Predicting blood:air partition coefficients using basic physicochemical properties. Regul. Toxicol. Pharmacol. 2012, 62, 23–28. [Google Scholar] [CrossRef]
- Weschler, C.J.; Nazaroff, W.W. SVOC exposure indoors: Fresh look at dermal pathways. Indoor Air 2012, 22, 356–377. [Google Scholar] [CrossRef]
- Linnankoski, J.; Mäkelä, J.M.; Ranta, V.-P.; Urtti, A.; Yliperttula, M. Computational Prediction of Oral Drug Absorption Based on Absorption Rate Constants in Humans. J. Med. Chem. 2006, 49, 3674–3681. [Google Scholar] [CrossRef]
- Sarver, J.G.; White, D.; Erhardt, P.; Bachmann, K. Estimating Xenobiotic Half-Lives in Humans from Rat Data: Influence of log P. Environ. Health Perspect. 1997, 105, 1204–1209. [Google Scholar] [CrossRef]
- Mansouri, K.; Grulke, C.M.; Judson, R.S.; Williams, A.J. OPERA models for predicting physicochemical properties and environmental fate endpoints. J. Cheminform. 2018, 10, 10. [Google Scholar] [CrossRef]
- Williams, A.J.; Lambert, J.C.; Thayer, K.; Dorne, J.C.M. Sourcing data on chemical properties and hazard data from the US-EPA CompTox Chemicals Dashboard: A practical guide for human risk assessment. Environ. Int. 2021, 154, 106566. [Google Scholar] [CrossRef]
- U.S. Bureau of Labor Statistics. American Time Use Survey—May to December 2019 and 2020 Results. U.S. Department of Labor. 2021; Report Number USDL-21-1359. Available online: https://www.bls.gov/news.release/pdf/atus.pdf (accessed on 18 January 2022).
- U.S. Bureau of Labor Statistics. Consumer Expenditures in 2020. U.S. Department of Labor. 2021; Report Number 1096. Available online: https://www.bls.gov/opub/reports/consumer-expenditures/2020/home.htm (accessed on 18 January 2022).
- Stanfield, Z.; Setzer, R.W.; Hull, V.; Sayre, R.R.; Isaacs, K.K.; Wambaugh, J.F. Bayesian inference of chemical exposures from NHANES urine biomonitoring data. J. Expo. Sci. Environ. Epidemiol. 2022. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Consumer Exposure Model (CEM) Version 2.1 User Guide. 2019. Available online: https://www.epa.gov/tsca-screening-tools/consumer-exposure-model-cem-version-21-users-guide (accessed on 18 January 2022).
- Zhang, Y.; Banerjee, S.; Yang, R.; Lungu, C.; Ramachandran, G. Bayesian modeling of exposure and airflow using two-zone models. Ann. Occup. Hyg. 2009, 53, 409–424. [Google Scholar] [CrossRef]
- Nicas, M.; Plisko, M.J.; Spencer, J.W. Estimating benzene exposure at a solvent parts washer. J. Occup. Environ. Hyg. 2006, 3, 284–291. [Google Scholar] [CrossRef]
- Deshpande, B.K.; Frey, H.C.; Cao, Y.; Liu, X. Modeling of the Penetration of Ambient PM2.5 to Indoor Residential Microenvironment. In Proceedings of the 102nd Annual Conference and Exhibition, Air & Waste Management Association, Detroit, MI, USA, 16–19 June 2009. Paper No. 2009-A-86-AWMA. [Google Scholar]
- National Ambient Air Quality Standards for Particulate Matter. Fed. Reg. 2013; 78, 3086–3287, (January 15, 2013) (to be codified at 40 CFR Parts 50, 51, 52, 53 and 58).
- Wilson, R.; Jones-Otazo, H.; Petrovic, S.; Mitchell, I.; Bonvalot, Y.; Williams, D.; Richardson, G.M. Revisiting Dust and Soil Ingestion Rates Based on Hand-to-Mouth Transfer. Hum. Ecol. Risk Assess. 2013, 19, 158–188. [Google Scholar] [CrossRef]
- Ozkaynak, H.; Xue, J.; Zartarian, V.G.; Glen, G.; Smith, L. Modeled estimates of soil and dust ingestion rates for children. Risk Anal. 2011, 31, 592–608. [Google Scholar] [CrossRef]
- Klepeis, N.E.; Gabel, E.B.; Ott, W.R.; Switzer, P. Outdoor air pollution in close proximity to a continuous point source. Atmos. Environ. 2009, 43, 3155–3167. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Exposure Factors Handbook: 2011 Edition; National Center for Environmental Assessment: Washington, DC, USA, 2011; Report number EPA/600/R-090/052F. Available online: https://www.epa.gov/expobox/about-exposure-factors-handbook (accessed on 18 January 2022).
- Ring, C.L.; Arnot, J.A.; Bennett, D.H.; Egeghy, P.P.; Fantke, P.; Huang, L.; Isaacs, K.K.; Jolliet, O.; Phillips, K.A.; Price, P.S.; et al. Consensus Modeling of Median Chemical Intake for the U.S. Population Based on Predictions of Exposure Pathways. Environ. Sci. Technol. 2019, 53, 719–732. [Google Scholar] [CrossRef]
- Sarigiannis, D.A.; Hansen, U. Considering the cumulative risk of mixtures of chemicals-a challenge for policy makers. Environ. Health 2012, 11 (Suppl. 1), S18. [Google Scholar] [CrossRef] [Green Version]
- Zartarian, V.G.; Glen, G.; Smith, L.; Xue, J. SHEDS-Multimedia Model Version 3 (a) Technical Manual; (b) User Guide; and (c) Executable File to Launch SAS Program and Install Model; Environmental Protection Agency (Agency USEP): Washington, DC, USA, 2008; Report number EPA/600/R-08/118.


| Parameter | Definition | Range | Source of Default Values | Source of Low/High Values | ||
|---|---|---|---|---|---|---|
| Low | Default | High | ||||
| β | Air flow rate between user bubble and larger room (residential) | 60 m3/h | 82.008 m3/h | 300 m3/h | United States Environmental Protection Agency [48] | Derived from Zhang, Banerjee [49] (see Supplemental Material S3.4; see also [27,49,50] |
| CPM2.5 | Background indoor PM2.5 concentration | 5 µg/m3 | 7.16 µg/m3 | 9 µg/m3 | Deshpande, Frey [51] | Deshpande, Frey [51] |
| CTSP | Background indoor PM10 concentration | 40 µg/m3 | 75 µg/m3 | 150 µg/m3 | Assumed (half of NAAQS standard for PM10 [52], as a rough estimate) | Assumed (vary default by a factor of 2 in either direction) |
| Dust_floor_load | Mass of dust on the floor/unit area | 0.1 g/m2 | 0.52 g/m2 | 2.5 g/m2 | Wilson, Jones-Otazo [53] | Wilson, Jones-Otazo [53] |
| Frachand_mouth | Fraction of chemical that is transferred from hand to mouth | 0.05 | 0.2 | 0.8 | Ozkaynak, Xue [54] | Ozkaynak, Xue [54] |
| Inhdil | Dilution factor to account for increased ventilation and decreased exposure when using a product outdoors | 1 | 20 | 100 | Estimated based on Klepeis, Gabel [55] | Estimated based on Klepeis, Gabel [55] |
| Inhrate | Volumetric breathing rate | 6.8 m3/day | 16.2 m3/day | 71.2 m3/day | EPA [56] | EPA [56] (low value is average of age groups ≥ 21 for sedentary/resting; high value is average of age groups ≥ 21 for high intensity) |
| AER | Building air exchange rate (residential) | 0.1 air changes/h | 0.45 air changes/h | 3 air changes/h | EPA [56] | EPA [56] |
| Room dimension | Dimension of one side of square room | 2.8 m | 5.8 m | 14.2 m | EPA [56] | EPA [56] |
| Skin SA | Skin surface area of adult human | 1.61 m2 | 1.95 m2 | 2.425 m2 | EPA [56] | EPA [56] (low value is average of 5th percentile for adults; high value is average of 95th percentile for adults) |
| Vbubble | Near field volume during product use (user “bubble” as compared to room volume) | 0.125 m3 | 0.2 m3 | 27 m3 | Nicas [25] | Assumed |
| Ex Priori Rank (out of 1108 Chemicals) Based on… | Percent of Absorbed Dose via Route | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chemical Name | CASRN | Body Burden after 24 h | Absorbed Daily Intake | Dermal | Ingestion | Inhalation | Log10 Kow | Log10 Henry’s Law | Half-Life (Hours) |
| Alcohols, C12-16, Ethoxylated * | 68551-12-2 | 1 | 1 | 99.14 | 0.35 | 0.51 | 5.90 | −4.45 | 141.94 |
| Isopropyl Myristate | 110-27-0 | 2 | 4 | 99.93 | 0.06 | 0.02 | 6.90 | −6.12 | 274.90 |
| 2-Octyldodecan-1-Ol | 5333-42-6 | 3 | 20 | 99.85 | 0.15 | <0.01 | 8.83 | −6.33 | 987.02 |
| Decanoic Acid, Ester With 1, 2, 3-Propanetriol Octanoate * | 65381-09-1 | 4 | 15 | 99.80 | 0.20 | <0.01 | 4.97 | −7.39 | 76.27 |
| 2-Ethylhexyl Salicylate | 118-60-5 | 5 | 13 | >99.99 | <0.01 | <0.01 | 4.05 | −6.92 | 41.60 |
| 2-Cyano-3,3-Diphenyl-2-Propenoic Acid, 2-Ethylhexyl Ester | 6197-30-4 | 6 | 22 | 99.97 | 0.03 | <0.01 | 5.25 | −6.62 | 91.77 |
| Isopropyl Palmitate | 142-91-6 | 7 | 26 | 99.98 | <0.01 | 0.02 | 8.07 | −6.60 | 598.89 |
| Polyethylene Glycol Monostearate * | 9004-99-3 | 8 | 27 | >99.99 | <0.01 | <0.01 | 7.60 | −7.23 | 438.16 |
| Cetyl Alcohol | 36653-82-4 | 9 | 25 | 99.91 | 0.08 | 0.01 | 6.59 | −5.18 | 223.78 |
| Tetradecan-1-Ol, Propoxylated, Esters With Propionic Acid * | 74775-06-7 | 10 | 30 | >99.99 | <0.01 | <0.01 | 7.61 | −6.63 | 439.35 |
| Stearic Acid | 57-11-4 | 11 | 32 | 98.09 | 1.91 | <0.01 | 8.08 | −7.66 | 600.94 |
| 2-Ethylhexyl Palmitate | 29806-73-3 | 12 | 34 | 97.25 | 2.75 | <0.01 | 9.47 | −6.64 | 1514.11 |
| Stearic Acid, Monoester With Glycerol * | 31566-31-1 | 13 | 31 | 99.88 | 0.12 | <0.01 | 6.11 | −7.53 | 163.14 |
| Masoprocol | 500-38-9 | 14 | 19 | >99.99 | <0.01 | <0.01 | 3.55 | −5.78 | 29.85 |
| Cetostearyl Alcohol * | 67762-27-0 | 15 | 41 | 99.89 | 0.10 | 0.01 | 7.88 | −5.21 | 525.20 |
| Celgard * | 9003-07-0 | 16 | 45 | 98.13 | 1.87 | <0.01 | 8.75 | −7.13 | 934.75 |
| 4-Tert-Butyl-4′-Methoxydibenzoylmethane | 70356-09-1 | 17 | 35 | >99.99 | <0.01 | <0.01 | 4.64 | −3.87 | 61.43 |
| Alcohols, C16-18, Ethoxylated * | 68439-49-6 | 18 | 46 | 97.60 | 2.35 | 0.05 | 9.09 | −6.27 | 1171.97 |
| Homosalate | 118-56-9 | 19 | 28 | >99.99 | <0.01 | <0.01 | 3.92 | −6.92 | 38.08 |
| Pramocaine Hydrochloride | 637-58-1 | 20 | 36 | 99.99 | <0.01 | 0.01 | 4.04 | −7.58 | 41.38 |
| Ex Priori Rank (out of 1108 Chemicals) Based on… | Rank in Baseline Scenario Based on… | Percent of Absorbed Dose via Route… | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Name | CASRN | Body Burden after 24 h | Absorbed Daily Intake | Body Burden after 24 h | Dermal | Ingestion | Inhalation | Log10 Kow | Log10 Henry’s Law | Half-Life (Hours) |
| Polidocanol * | 9002-92-0 | 1 | 12 | 30 | 98.64 | 1.35 | <0.01 | 5.36 | −4.85 | 98.75 |
| Alcohols, C12-16, Ethoxylated * | 68551-12-2 | 2 | 16 | 1 | 97.91 | 1.33 | 0.76 | 5.90 | −4.45 | 141.94 |
| Toluene | 108-88-3 | 3 | 4 | 44 | <0.01 | <0.01 | >99.99 | 2.73 | −2.23 | 17.33 |
| Cellulose * | 9004-34-6 | 4 | 15 | 38 | 4.14 | 1.01 | 94.85 | 4.46 | −2.10 | 54.64 |
| Benzenesulfonic Acid, Mono-C10-16-Alkyl Derivs., Sodium Salts * | 68081-81-2 | 5 | 23 | 48 | 98.11 | 1.89 | <0.01 | 6.15 | −6.61 | 167.32 |
| Dodecyldimethylamine Oxide | 1643-20-5 | 6 | 20 | 32 | 85.18 | 1.83 | 12.99 | 4.86 | −4.33 | 71.28 |
| Isopropyl Myristate | 110-27-0 | 7 | 32 | 2 | 99.25 | 0.71 | 0.04 | 6.90 | −6.12 | 274.90 |
| Benzenesulfonic Acid, C10-13-Alkyl Derivs., Sodium Salts * | 68411-30-3 | 8 | 33 | 57 | 97.58 | 2.42 | <0.01 | 6.15 | −6.61 | 167.32 |
| Sodium Dodecylbenzenesulfonate * | 25155-30-0 | 9 | 42 | 35 | 96.76 | 3.24 | <0.01 | 5.88 | −6.57 | 140.00 |
| 2-Tert-Butylcyclohexyl Acetate | 88-41-5 | 10 | 36 | 41 | 35.96 | 2.36 | 61.68 | 4.24 | −3.64 | 47.04 |
| Neodol-12 * | 68131-39-5 | 11 | 46 | 65 | 93.20 | 3.37 | 3.43 | 5.90 | −4.45 | 141.94 |
| Alcohols, C10-14, Ethoxylated * | 66455-15-0 | 12 | 43 | 36 | 76.99 | 3.57 | 19.44 | 5.29 | −3.39 | 94.26 |
| Sulfuric Acid, Mono-C10-16-Alkyl Esters, Sodium Salts * | 68585-47-7 | 13 | 9 | 54 | 98.80 | 1.19 | 0.01 | 2.29 | −6.51 | 12.93 |
| Xylene * | 1330-20-7 | 14 | 27 | 72 | <0.01 | <0.01 | >99.99 | 3.14 | −2.17 | 22.77 |
| Stearic Acid | 57-11-4 | 15 | 53 | 11 | 96.30 | 3.70 | <0.01 | 8.08 | −7.66 | 600.94 |
| 1,4-Dichlorobenzene | 106-46-7 | 16 | 22 | 83 | 0.08 | 0.08 | 99.84 | 2.86 | −4.93 | 18.91 |
| (R)-P-Mentha-1,8-Diene | 5989-27-5 | 17 | 49 | 66 | <0.01 | <0.01 | >99.99 | 4.46 | −1.50 | 54.55 |
| Alkanes, C9-12-Iso-* | 90622-57-4 | 18 | 58 | 91 | <0.01 | <0.01 | >99.99 | 5.47 | −0.83 | 106.30 |
| Nonylphenol, Ethoxylated * | 9016-45-9 | 19 | 51 | 55 | 96.16 | 3.81 | 0.03 | 4.43 | −5.24 | 53.29 |
| Alkanes, C7-8-Iso- * | 70024-92-9 | 20 | 50 | 95 | <0.01 | <0.01 | >99.99 | 4.09 | −0.39 | 42.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hubbard, H.F.; Ring, C.L.; Hong, T.; Henning, C.C.; Vallero, D.A.; Egeghy, P.P.; Goldsmith, M.-R. Exposure Prioritization (Ex Priori): A Screening-Level High-Throughput Chemical Prioritization Tool. Toxics 2022, 10, 569. https://doi.org/10.3390/toxics10100569
Hubbard HF, Ring CL, Hong T, Henning CC, Vallero DA, Egeghy PP, Goldsmith M-R. Exposure Prioritization (Ex Priori): A Screening-Level High-Throughput Chemical Prioritization Tool. Toxics. 2022; 10(10):569. https://doi.org/10.3390/toxics10100569
Chicago/Turabian StyleHubbard, Heidi F., Caroline L. Ring, Tao Hong, Cara C. Henning, Daniel A. Vallero, Peter P. Egeghy, and Michael-Rock Goldsmith. 2022. "Exposure Prioritization (Ex Priori): A Screening-Level High-Throughput Chemical Prioritization Tool" Toxics 10, no. 10: 569. https://doi.org/10.3390/toxics10100569
APA StyleHubbard, H. F., Ring, C. L., Hong, T., Henning, C. C., Vallero, D. A., Egeghy, P. P., & Goldsmith, M.-R. (2022). Exposure Prioritization (Ex Priori): A Screening-Level High-Throughput Chemical Prioritization Tool. Toxics, 10(10), 569. https://doi.org/10.3390/toxics10100569







