Cumulative Risk Meets Inter-Individual Variability: Probabilistic Concentration Addition of Complex Mixture Exposures in a Population-Based Human In Vitro Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Designs
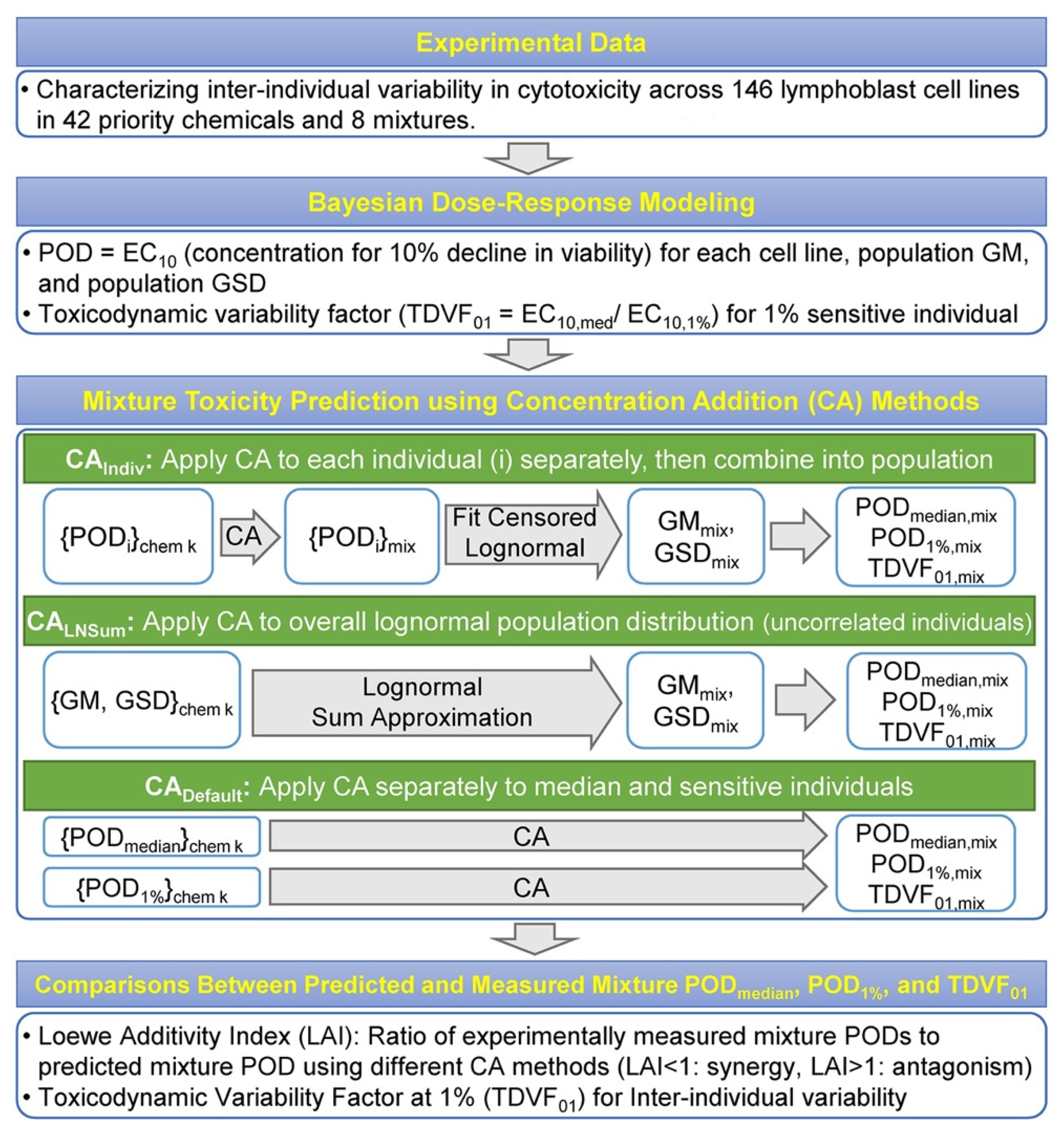
2.2. Bayesian Modeling of Concentration–Response for Cytotoxicity
2.3. Derivation of Points of Departure (PODs) and Toxicodynamic Variability Factor (TDVF01)
2.4. Mixture Concentration–Response Prediction Using Concentration Addition Approaches
2.5. Data Processing and Reproducibility
3. Results
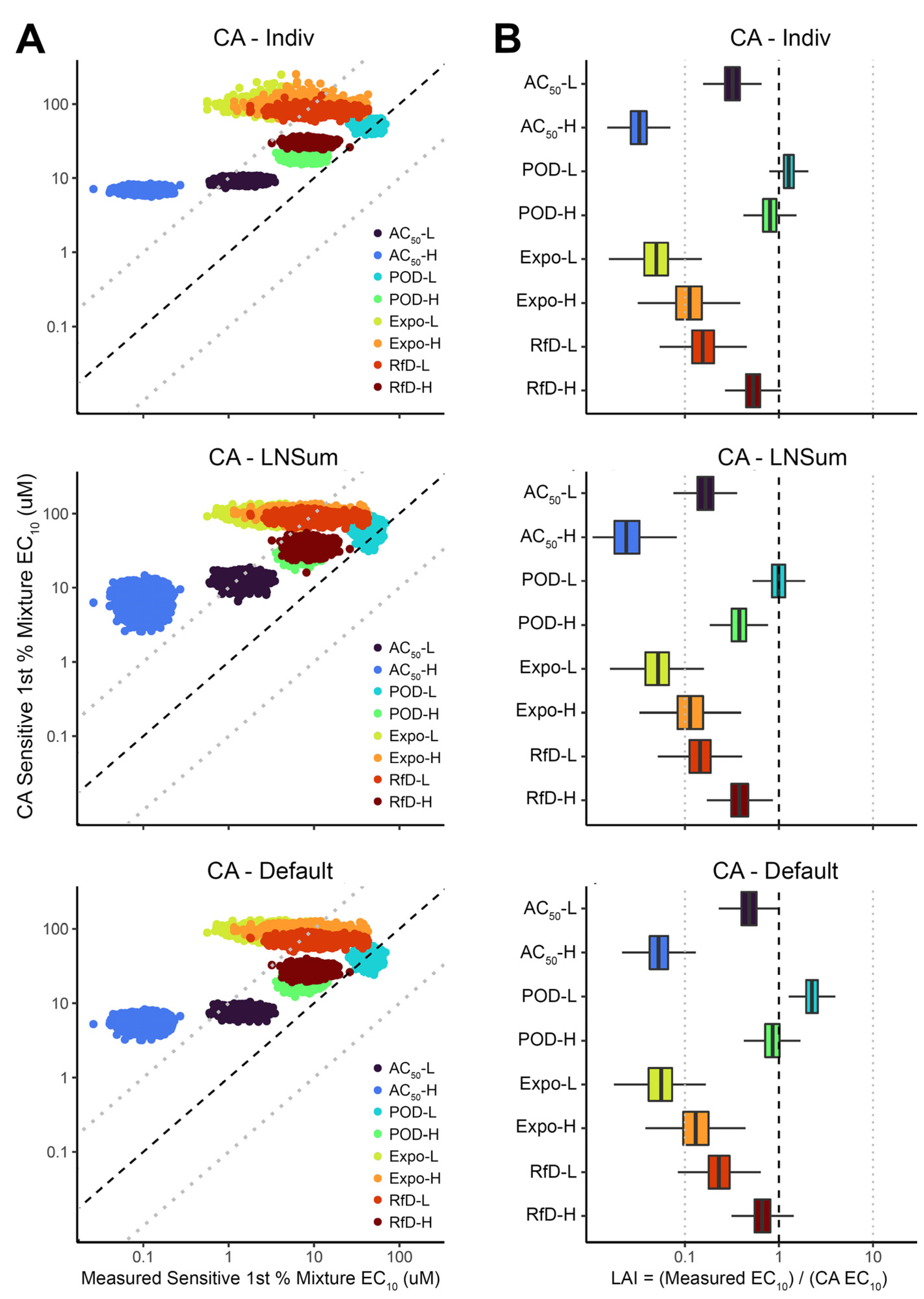
3.1. Population Variability in Accuracy of Concentration Addition across Individuals
3.2. Comparison of Concentration Addition Approaches for the Median and the Sensitive (First Percentile) Individuals
3.3. Concentration Addition Predictions for the Toxicodynamic Variability Factor (TDVF01)
3.4. Comparison of Loewe Additivity Index (LAI) across Cell Types
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
= PODm × CEFm.
= [PODm CEFm] × [Σ fk/(CEFm × PODk)]
= [PODm] × [Σ fk/PODk]
= PODm/PODCA = LAI
References
- EPA, U.S. Health Assessment Document For Diesel Engine Exhaust; EPA, U.S.: Washington, DC, USA, 2002. [Google Scholar]
- EPA, U.S. Integrated Science Assessment (ISA) for Particulate Matter; EPA, U.S.: Washington, DC, USA, 2019. [Google Scholar]
- FDA, U.S. Drug Development and Drug Interactions|Table of Substrates, Inhibitors and Inducers; EPA, U.S.: Washington, DC, USA, 2020. [Google Scholar]
- ATSDR. Interaction Profiles for Toxic Substances; ATSDR: Atlanta, GA, USA, 2006. [Google Scholar]
- EPA, U.S. Guidelines for the Health Risk Assessment of Chemical Mixtures; EPA, U.S.: Washington, DC, USA, 1986. [Google Scholar]
- EPA, U.S. Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures; EPA, U.S.: Washington, DC, USA, 2000. [Google Scholar]
- EPA, U.S. Framework for Cumulative Risk Assessment; EPA, U.S.: Washington, DC, USA, 2003. [Google Scholar]
- National Research Council. Phthalates and Cumulative Risk Assessment: The Tasks Ahead; The National Academies Press: Washington, DC, USA, 2008; p. 208. [Google Scholar]
- Loewe, S.; Muischnek, H. Über Kombinationswirkungen. Naunyn-Schmiedebergs Arch. Exp. Pathol. Und Pharmakol. 1926, 114, 313–326. [Google Scholar] [CrossRef]
- Bliss, C.I. The Toxicity of Poisons Applied Jointly1. Ann. Appl. Biol. 1939, 26, 585–615. [Google Scholar] [CrossRef]
- Kienzler, A.; Berggren, E.; Bessems, J.; Bopp, S.; van der Linden, S.; Worth, A.; Health, I.f.; Protection, C. Assessment of Mixtures: Review of Regulatory Requirements and Guidance; Publications Office: Luxembourg, 2014. [Google Scholar]
- Nisbet, I.C.; LaGoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharm. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- EPA, U.S. Recommended Toxicity Equivalence Factors (TEFs) for Human Health Risk Assessments of 2,3,7,8-Tetrachlorodibenzo-p-dioxin and Dioxin-Like Compounds; EPA, U.S.: Washington, DC, USA, 2010. [Google Scholar]
- EPA, U.S. Revised N-Methyl Carbamate Cumulative Risk Assessment; EPA, U.S.: Washington, DC, USA, 2007. [Google Scholar]
- Glauch, L.; Escher, B.I. The Combined Algae Test for the Evaluation of Mixture Toxicity in Environmental Samples. Environ. Toxicol. Chem. 2020, 39, 2496–2508. [Google Scholar] [CrossRef]
- Neale, P.A.; Braun, G.; Brack, W.; Carmona, E.; Gunold, R.; König, M.; Krauss, M.; Liebmann, L.; Liess, M.; Link, M.; et al. Assessing the Mixture Effects in In Vitro Bioassays of Chemicals Occurring in Small Agricultural Streams during Rain Events. Environ. Sci. Technol. 2020, 54, 8280–8290. [Google Scholar] [CrossRef] [PubMed]
- Muz, M.; Escher, B.I.; Jahnke, A. Bioavailable Environmental Pollutant Patterns in Sediments from Passive Equilibrium Sampling. Environ. Sci. Technol. 2020, 54, 15861–15871. [Google Scholar] [CrossRef] [PubMed]
- Beckers, L.-M.; Brack, W.; Dann, J.P.; Krauss, M.; Müller, E.; Schulze, T. Unraveling longitudinal pollution patterns of organic micropollutants in a river by non-target screening and cluster analysis. Sci. Total Environ. 2020, 727, 138388. [Google Scholar] [CrossRef]
- Muschket, M.; Di Paolo, C.; Tindall, A.J.; Touak, G.; Phan, A.; Krauss, M.; Kirchner, K.; Seiler, T.-B.; Hollert, H.; Brack, W. Identification of Unknown Antiandrogenic Compounds in Surface Waters by Effect-Directed Analysis (EDA) Using a Parallel Fractionation Approach. Environ. Sci. Technol. 2018, 52, 288–297. [Google Scholar] [CrossRef]
- Bart, S.; Short, S.; Jager, T.; Eagles, E.J.; Robinson, A.; Badder, C.; Lahive, E.; Spurgeon, D.J.; Ashauer, R. How to analyse and account for interactions in mixture toxicity with toxicokinetic-toxicodynamic models. Sci. Total Environ. 2022, 843, 157048. [Google Scholar] [CrossRef] [PubMed]
- Bart, S.; Jager, T.; Robinson, A.; Lahive, E.; Spurgeon, D.J.; Ashauer, R. Predicting Mixture Effects over Time with Toxicokinetic–Toxicodynamic Models (GUTS): Assumptions, Experimental Testing, and Predictive Power. Environ. Sci. Technol. 2021, 55, 2430–2439. [Google Scholar] [CrossRef]
- Ashauer, R.; Escher, B.I. Advantages of toxicokinetic and toxicodynamic modelling in aquatic ecotoxicology and risk assessment. J. Environ. Monit. 2010, 12, 2056–2061. [Google Scholar] [CrossRef] [PubMed]
- Jager, T.; Albert, C.; Preuss, T.G.; Ashauer, R. General Unified Threshold Model of Survival-A Toxicokinetic-Toxicodynamic Framework for Ecotoxicology. Environ. Sci. Technol. 2011, 45, 2529–2540. [Google Scholar] [CrossRef]
- Tod, M.; Bourguignon, L.; Bleyzac, N.; Goutelle, S. A Model for Predicting the Interindividual Variability of Drug-Drug Interactions. AAPS J. 2017, 19, 497–509. [Google Scholar] [CrossRef]
- Peters, S.A.; Schroeder, P.E.; Giri, N.; Dolgos, H. Evaluation of the use of static and dynamic models to predict drug-drug interaction and its associated variability: Impact on drug discovery and early development. Drug Metab. Dispos. 2012, 40, 1495–1507. [Google Scholar] [CrossRef]
- Bois, F.Y.; Jamei, M.; Clewell, H.J. PBPK modelling of inter-individual variability in the pharmacokinetics of environmental chemicals. Toxicology 2010, 278, 256–267. [Google Scholar] [CrossRef]
- Drakvik, E.; Altenburger, R.; Aoki, Y.; Backhaus, T.; Bahadori, T.; Barouki, R.; Brack, W.; Cronin, M.T.D.; Demeneix, B.; Hougaard Bennekou, S.; et al. Statement on advancing the assessment of chemical mixtures and their risks for human health and the environment. Environ. Int. 2020, 134, 105267. [Google Scholar] [CrossRef]
- Chen, Z.; Lloyd, D.; Zhou, Y.H.; Chiu, W.A.; Wright, F.A.; Rusyn, I. Risk Characterization of Environmental Samples Using In Vitro Bioactivity and Polycyclic Aromatic Hydrocarbon Concentrations Data. Toxicol. Sci. 2021, 179, 108–120. [Google Scholar] [CrossRef]
- Chen, Z.; Jang, S.; Kaihatu, J.M.; Zhou, Y.H.; Wright, F.A.; Chiu, W.A.; Rusyn, I. Potential Human Health Hazard of Post-Hurricane Harvey Sediments in Galveston Bay and Houston Ship Channel: A Case Study of Using In Vitro Bioactivity Data to Inform Risk Management Decisions. Int. J. Environ. Res. Public Health 2021, 18, 13378. [Google Scholar] [CrossRef]
- Hsieh, N.H.; Chen, Z.; Rusyn, I.; Chiu, W.A. Risk Characterization and Probabilistic Concentration-Response Modeling of Complex Environmental Mixtures Using New Approach Methodologies (NAMs) Data from Organotypic in Vitro Human Stem Cell Assays. Environ. Health Perspect. 2021, 129, 17004. [Google Scholar] [CrossRef]
- House, J.S.; Grimm, F.A.; Klaren, W.D.; Dalzell, A.; Kuchi, S.; Zhang, S.D.; Lenz, K.; Boogaard, P.J.; Ketelslegers, H.B.; Gant, T.W.; et al. Grouping of UVCB substances with dose-response transcriptomics data from human cell-based assays. ALTEX 2022, 39, 388–404. [Google Scholar] [CrossRef]
- House, J.S.; Grimm, F.A.; Klaren, W.D.; Dalzell, A.; Kuchi, S.; Zhang, S.D.; Lenz, K.; Boogaard, P.J.; Ketelslegers, H.B.; Gant, T.W.; et al. Grouping of UVCB substances with new approach methodologies (NAMs) data. ALTEX 2021, 38, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Grimm, F.A.; Iwata, Y.; Sirenko, O.; Chappell, G.A.; Wright, F.A.; Reif, D.M.; Braisted, J.; Gerhold, D.L.; Yeakley, J.M.; Shepard, P.; et al. A chemical-biological similarity-based grouping of complex substances as a prototype approach for evaluating chemical alternatives. Green Chem. 2016, 18, 4407–4419. [Google Scholar] [CrossRef] [PubMed]
- Rusyn, I.; Chiu, W.A.; Wright, F.A. Model systems and organisms for addressing inter- and intra-species variability in risk assessment. Regul. Toxicol. Pharmacol. 2022, 132, 105197. [Google Scholar] [CrossRef] [PubMed]
- Rusyn, I.; Chiu, W.A. Decision-Making with New Approach Methodologies: Time to Replace Default Uncertainty Factors with Data. Toxicol. Sci. 2022, 189, 148–149. [Google Scholar] [CrossRef]
- Chiu, W.A.; Rusyn, I. Advancing chemical risk assessment decision-making with population variability data: Challenges and opportunities. Mamm. Genome 2018, 29, 182–189. [Google Scholar] [CrossRef]
- Blanchette, A.D.; Burnett, S.D.; Rusyn, I.; Chiu, W.A. A tiered approach to population-based in vitro testing for cardiotoxicity: Balancing estimates of potency and variability. J. Pharmacol. Toxicol. Methods 2022, 114, 107154. [Google Scholar] [CrossRef]
- Burnett, S.D.; Blanchette, A.D.; Chiu, W.A.; Rusyn, I. Human induced pluripotent stem cell (iPSC)-derived cardiomyocytes as an in vitro model in toxicology: Strengths and weaknesses for hazard identification and risk characterization. Expert Opin. Drug Metab. Toxicol. 2021, 17, 887–902. [Google Scholar] [CrossRef]
- Blanchette, A.D.; Burnett, S.D.; Grimm, F.A.; Rusyn, I.; Chiu, W.A. A Bayesian Method for Population-wide Cardiotoxicity Hazard and Risk Characterization Using an In Vitro Human Model. Toxicol. Sci. 2020, 178, 391–403. [Google Scholar] [CrossRef]
- Burnett, S.D.; Blanchette, A.D.; Grimm, F.A.; House, J.S.; Reif, D.M.; Wright, F.A.; Chiu, W.A.; Rusyn, I. Population-based toxicity screening in human induced pluripotent stem cell-derived cardiomyocytes. Toxicol. Appl. Pharmacol. 2019, 381, 114711. [Google Scholar] [CrossRef]
- Abdo, N.; Xia, M.; Brown, C.C.; Kosyk, O.; Huang, R.; Sakamuru, S.; Zhou, Y.H.; Jack, J.R.; Gallins, P.; Xia, K.; et al. Population-based in vitro hazard and concentration-response assessment of chemicals: The 1000 genomes high-throughput screening study. Environ. Health Perspect. 2015, 123, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Abdo, N.; Wetmore, B.A.; Chappell, G.A.; Shea, D.; Wright, F.A.; Rusyn, I. In vitro screening for population variability in toxicity of pesticide-containing mixtures. Environ. Int. 2015, 85, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Eduati, F.; Mangravite, L.M.; Wang, T.; Tang, H.; Bare, J.C.; Huang, R.; Norman, T.; Kellen, M.; Menden, M.P.; Yang, J.; et al. Prediction of human population responses to toxic compounds by a collaborative competition. Nat. Biotechnol. 2015, 33, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.A.; Wright, F.A.; Rusyn, I. A tiered, Bayesian approach to estimating of population variability for regulatory decision-making. ALTEX 2017, 34, 377–388. [Google Scholar] [CrossRef]
- World Health Organization. Chemical-Specific Adjustment Factors for Interspecies Differences and Human Variability: Guidance Document for Use of Data in Dose/Concentration-Response Assessment; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- EPA, U.S. Guidance for Applying Quantitative Data to Develop Data-Derived Extrapolation Factors for Interspecies and Intraspecies Extrapolation; EPA, U.S.: Washington, DC, USA, 2014. [Google Scholar]
- Ford, L.C.; Jang, S.; Chen, Z.; Zhou, Y.H.; Gallins, P.J.; Wright, F.A.; Chiu, W.A.; Rusyn, I. A Population-Based Human In Vitro Approach to Quantify In-ter-Individual Variability in Responses to Chemical Mixtures. Toxics 2022, 10, 441. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, Y.; Wright, F.A.; Chiu, W.A.; Rusyn, I. Rapid hazard characterization of environmental chemicals using a compendium of human cell lines from different organs. ALTEX 2020, 37, 623–638. [Google Scholar] [CrossRef]
- Williams, A.J.; Grulke, C.M.; Edwards, J.; McEachran, A.D.; Mansouri, K.; Baker, N.C.; Patlewicz, G.; Shah, I.; Wambaugh, J.F.; Judson, R.S.; et al. The CompTox Chemistry Dashboard: A community data resource for environmental chemistry. J. Cheminform. 2017, 9, 61. [Google Scholar] [CrossRef]
- Wambaugh, J.F.; Setzer, R.W.; Reif, D.M.; Gangwal, S.; Mitchell-Blackwood, J.; Arnot, J.A.; Joliet, O.; Frame, A.; Rabinowitz, J.; Knudsen, T.B.; et al. High-throughput models for exposure-based chemical prioritization in the ExpoCast project. Environ. Sci. Technol. 2013, 47, 8479–8488. [Google Scholar] [CrossRef]
- Wignall, J.A.; Shapiro, A.J.; Wright, F.A.; Woodruff, T.J.; Chiu, W.A.; Guyton, K.Z.; Rusyn, I. Standardizing benchmark dose calculations to improve science-based decisions in human health assessments. Environ. Health Perspect. 2014, 122, 499–505. [Google Scholar] [CrossRef]
- U.S. EPA. Benchmark Dose Technical Guidance; Risk Assessment Forum; U.S. EPA: Washington, DC, USA, 2012. [Google Scholar]
- Gelman, A.; Goodrich, B.; Gabry, J.; Vehtari, A. R-squared for Bayesian regression models. Am. Stat. 2019, 73, 307–309. [Google Scholar] [CrossRef]
- Gelman, A.; Rubin, D.B. Inference from iterative simulation using multiple sequences. Stat. Sci. 1992, 7, 457–472. [Google Scholar] [CrossRef]
- Roell, K.R.; Reif, D.M.; Motsinger-Reif, A.A. An Introduction to Terminology and Methodology of Chemical Synergy-Perspectives from Across Disciplines. Front. Pharmacol. 2017, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Escher, B.; Braun, G.; Zarfl, C. Exploring the Concepts of Concentration Addition and Independent Action Using a Linear Low-Effect Mixture Model. Environ. Toxicol. Chem. 2020, 39, 2552–2559. [Google Scholar] [CrossRef] [PubMed]
- Boik, J.C.; Newman, R.A.; Boik, R.J. Quantifying synergism/antagonism using nonlinear mixed-effects modeling: A simulation study. Stat. Med. 2008, 27, 1040–1061. [Google Scholar] [CrossRef] [PubMed]
- Greco, W.; Unkelbach, H.-D.; Pöch, G.; Sühnel, J.; Kundi, M.; Bödeker, W. Consensus on concepts and terminology for combined-action assessment: The Saariselkä agreement. Arch. Complex Environ. Stud. 1992, 4, 65–69. [Google Scholar]
- Poch, G.; Reiffenstein, R.J.; Unkelbach, H.D. Application of the isobologram technique for the analysis of combined effects with respect to additivity as well as independence. Can. J. Physiol. Pharmacol. 1990, 68, 682–688. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Lo, C.F. WKB Approximation for the Sum of Two Correlated Lognormal Random Variables. Appl. Math. Sci. 2013, 7, 6355–6367. [Google Scholar] [CrossRef]
- Limpert, E.; Stahel, W.A.; Abbt, M. Log-normal Distributions across the Sciences: Keys and Clues: On the charms of statistics, and how mechanical models resembling gambling machines offer a link to a handy way to characterize log-normal distributions, which can provide deeper insight into variability and probability—Normal or log-normal: That is the question. BioScience 2001, 51, 341–352. [Google Scholar] [CrossRef]
- EFSA Scientific Committee; More, S.J.; Bampidis, V.; Benford, D.; Bragard, C.; Hernandez-Jerez, A.; Bennekou, S.H.; Halldorsson, T.I.; Koutsoumanis, K.P.; Lambre, C.; et al. Guidance Document on Scientific criteria for grouping chemicals into assessment groups for human risk assessment of combined exposure to multiple chemicals. EFSA J. 2021, 19, e07033. [Google Scholar] [CrossRef]
- EFSA Scientific Committee; More, S.J.; Bampidis, V.; Benford, D.; Bennekou, S.H.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Koutsoumanis, K.; Naegeli, H. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA J. 2019, 17, e05634. [Google Scholar]
- OECD. Considerations for Assessing the Risks of Combined Exposure to Multiple Chemicals; Series on Testing and Assessment No. 296; OECD Publishing: Berlin, Germany, 2018. [Google Scholar]
- Kumar, V.; Boobis, A.R.; Moretto, A. Test and Risk Assessment Strategies for combined exposure to multiple chemicals. Food Chem. Toxicol. 2020, 144, 111607. [Google Scholar] [CrossRef] [PubMed]
- Kienzler, A.; Bopp, S.K.; van der Linden, S.; Berggren, E.; Worth, A. Regulatory assessment of chemical mixtures: Requirements, current approaches and future perspectives. Regul. Toxicol. Pharmacol. 2016, 80, 321–334. [Google Scholar] [CrossRef]
- Sexton, K.; Hattis, D. Assessing cumulative health risks from exposure to environmental mixtures- hree fundamental questions. Environ. Health Perspect. 2007, 115, 825–832. [Google Scholar] [CrossRef]
- Luo, Y.S.; Chen, Z.; Hsieh, N.H.; Lin, T.E. Chemical and biological assessments of environmental mixtures: A review of current trends, advances, and future perspectives. J. Hazard Mater. 2022, 432, 128658. [Google Scholar] [CrossRef] [PubMed]
- Valdiviezo, A.; Luo, Y.S.; Chen, Z.; Chiu, W.A.; Rusyn, I. Quantitative In Vitro-to-In Vivo Extrapolation for Mixtures: A Case Study of Superfund Priority List Pesticides. Toxicol. Sci. 2021, 183, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, B.A. Quantitative in vitro-to-in vivo extrapolation in a high-throughput environment. Toxicology 2015, 332, 94–101. [Google Scholar] [CrossRef]
- DeBord, D.G.; Carreon, T.; Lentz, T.J.; Middendorf, P.J.; Hoover, M.D.; Schulte, P.A. Use of the “Exposome” in the Practice of Epidemiology: A Primer on -Omic Technologies. Am. J. Epidemiol. 2016, 184, 302–314. [Google Scholar] [CrossRef]
- Grimm, F.A.; Blanchette, A.; House, J.S.; Ferguson, K.; Hsieh, N.H.; Dalaijamts, C.; Wright, A.A.; Anson, B.; Wright, F.A.; Chiu, W.A.; et al. A human population-based organotypic in vitro model for cardiotoxicity screening. ALTEX 2018, 35, 441–452. [Google Scholar] [CrossRef]
- Armitage, J.M.; Wania, F.; Arnot, J.A. Application of mass balance models and the chemical activity concept to facilitate the use of in vitro toxicity data for risk assessment. Environ. Sci. Technol. 2014, 48, 9770–9779. [Google Scholar] [CrossRef]
- Kramer, N.I. Measuring, Modeling, and Increasing the Free Concentration of Test Chemicals in Cell Assays; Utrecht University: Utrecht, The Netherlands, 2010. [Google Scholar]
- Fischer, F.C.; Cirpka, O.A.; Goss, K.U.; Henneberger, L.; Escher, B.I. Application of Experimental Polystyrene Partition Constants and Diffusion Coefficients to Predict the Sorption of Neutral Organic Chemicals to Multiwell Plates in in Vivo and in Vitro Bioassays. Environ. Sci. Technol. 2018, 52, 13511–13522. [Google Scholar] [CrossRef]
- Armitage, J.M.; Sangion, A.; Parmar, R.; Looky, A.B.; Arnot, J.A. Update and Evaluation of a High-Throughput In Vitro Mass Balance Distribution Model: IV-MBM EQP v2.0. Toxics 2021, 9, 315. [Google Scholar] [CrossRef]
- Arnot, J.A. The Exposure And Safety Estimation (EAS-E) Suite. Available online: Eas-e-suite.com (accessed on 29 August 2022).
- Van Broekhuizen, F.A.; Posthuma, L.; Traas, T.P. Addressing combined effects of chemicals in environmental safety assessment under REACH-A thought starter. RIVM Lett. Rep. 2016-0162 2017. Available online: https://www.rivm.nl/publicaties/addressing-combined-effects-of-chemicals-in-environmental-safety-assessment-under-reach (accessed on 29 August 2022).
- Kortenkamp, A.; Faust, M. Regulate to reduce chemical mixture risk. Science 2018, 361, 224–226. [Google Scholar] [CrossRef] [Green Version]
- Rudén, C. Future chemical risk management, Accounting for combination effects and assessing chemicals in groups. Swed. Gov. Inq.-Swed. Gov. Off. Rep. SOU 2019, 45. Available online: https://www.government.se/4adb1a/contentassets/ee36b3e49c354bb8967f3a33a2d5ca50/future-chemical-risk-management---accounting-for-combination-effects-and-assessing-chemicals-in-groups-sou-201945 (accessed on 29 August 2022).




| Cell Type 1 | Phenotype 2 | LAI [95% CI] 3 |
|---|---|---|
| LCL (CA-Indiv) | Viability (Median) | 10−0.26 [−0.96, 0.45] |
| LCL (CA-LNSum) | Viability (Median) | 10−0.23 [−0.76, 0.43] |
| LCL (CA-Default) | Viability (Median) | 10−0.37 [−1.06, 0.25] |
| LCL (CA-Indiv) | Viability (Sens01) | 10−0.59 [−1.62, 0.17] |
| LCL (CA-LNSum) | Viability (Sens01) | 10−0.77 [−1.79, 0.09] |
| LCL (CA-Default) | Viability (Sens01) | 10−0.44 [−1.48, 0.43] |
| LCL (CA-Indiv) | Viability (TDVF01) | 10−0.61 [−1.32, −0.19] |
| LCL (CA-LNSum) | Viability (TDVF01) | 10−0.8 [−1.34, −0.31] |
| LCL (CA-Default) | Viability (TDVF01) | 10−0.52 [−1.18, −0.05] |
| iCell Cardiomyocytes | Cell Number | 100.51 [−0.64, 2.88] |
| iCell Endothelial cells | Cell Number | 100.3 [−1.82, 1.4] |
| iCell Hepatocytes | Cell Number | 100.52 [−0.11, 5.01] |
| HUVECs | Cell Number | 101.15 [0.13, 3.45] |
| iCell Neurons | Cell Number | 100.48 [−0.98, 1.43] |
| iCell Cardiomyocytes | Other phenotypes | 100.25 [−1.14, 1.62] |
| iCell Endothelial cells | Other phenotypes | 100.53 [−2.59, 1.85] |
| iCell Hepatocytes | Other phenotypes | 100.67 [−1.35, 3.34] |
| HUVECs | Other phenotypes | 100.58 [−1.95, 2.1] |
| iCell Neurons | Other phenotypes | 100.4 [−1.02, 1.43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.; Ford, L.C.; Rusyn, I.; Chiu, W.A. Cumulative Risk Meets Inter-Individual Variability: Probabilistic Concentration Addition of Complex Mixture Exposures in a Population-Based Human In Vitro Model. Toxics 2022, 10, 549. https://doi.org/10.3390/toxics10100549
Jang S, Ford LC, Rusyn I, Chiu WA. Cumulative Risk Meets Inter-Individual Variability: Probabilistic Concentration Addition of Complex Mixture Exposures in a Population-Based Human In Vitro Model. Toxics. 2022; 10(10):549. https://doi.org/10.3390/toxics10100549
Chicago/Turabian StyleJang, Suji, Lucie C. Ford, Ivan Rusyn, and Weihsueh A. Chiu. 2022. "Cumulative Risk Meets Inter-Individual Variability: Probabilistic Concentration Addition of Complex Mixture Exposures in a Population-Based Human In Vitro Model" Toxics 10, no. 10: 549. https://doi.org/10.3390/toxics10100549
APA StyleJang, S., Ford, L. C., Rusyn, I., & Chiu, W. A. (2022). Cumulative Risk Meets Inter-Individual Variability: Probabilistic Concentration Addition of Complex Mixture Exposures in a Population-Based Human In Vitro Model. Toxics, 10(10), 549. https://doi.org/10.3390/toxics10100549








