Metals and Metalloids Increase in Cycas micronesica Seed Gametophyte Tissue in Shaded Growth Conditions
Abstract
:1. Introduction
2. Materials and Methods
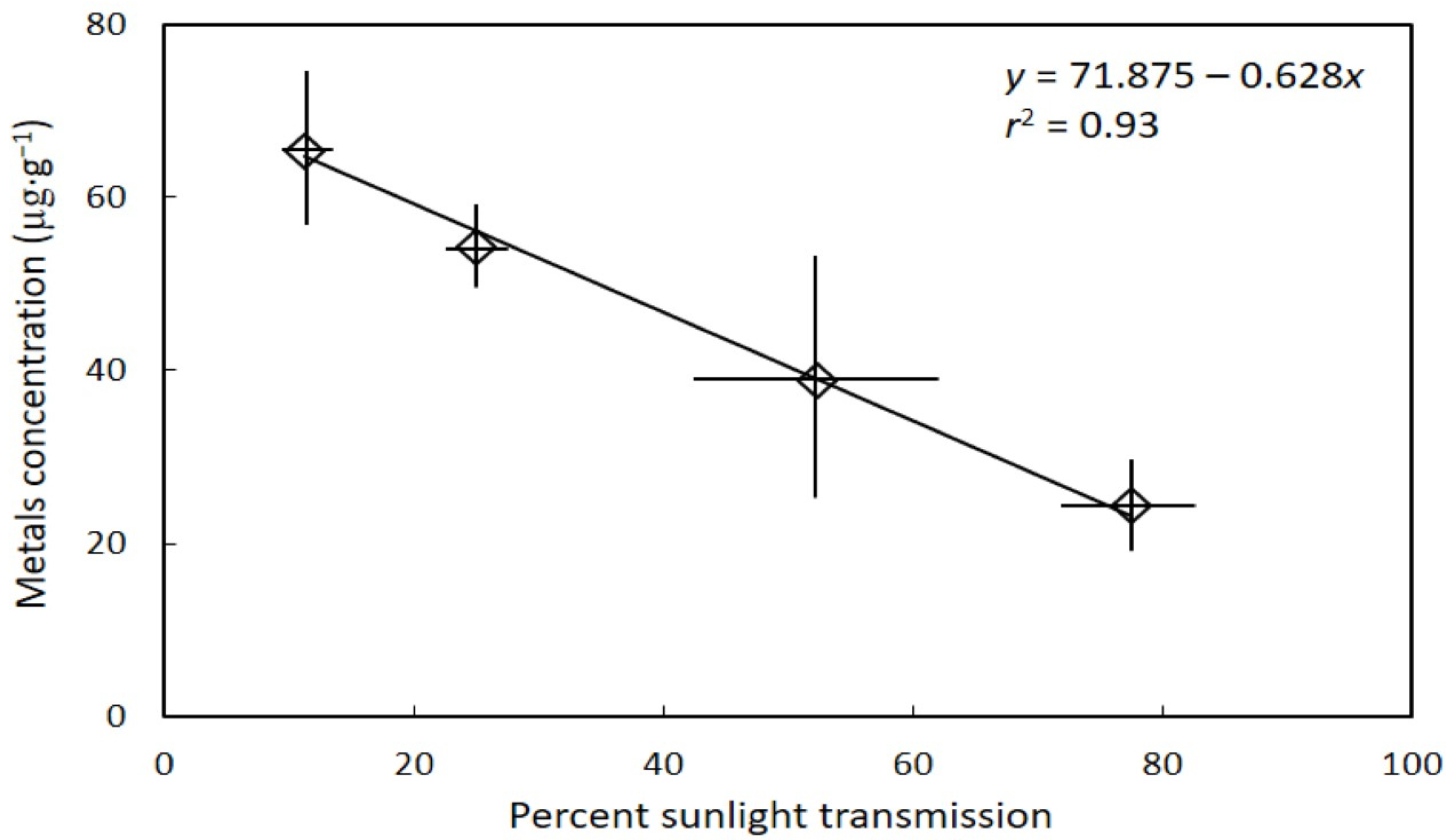
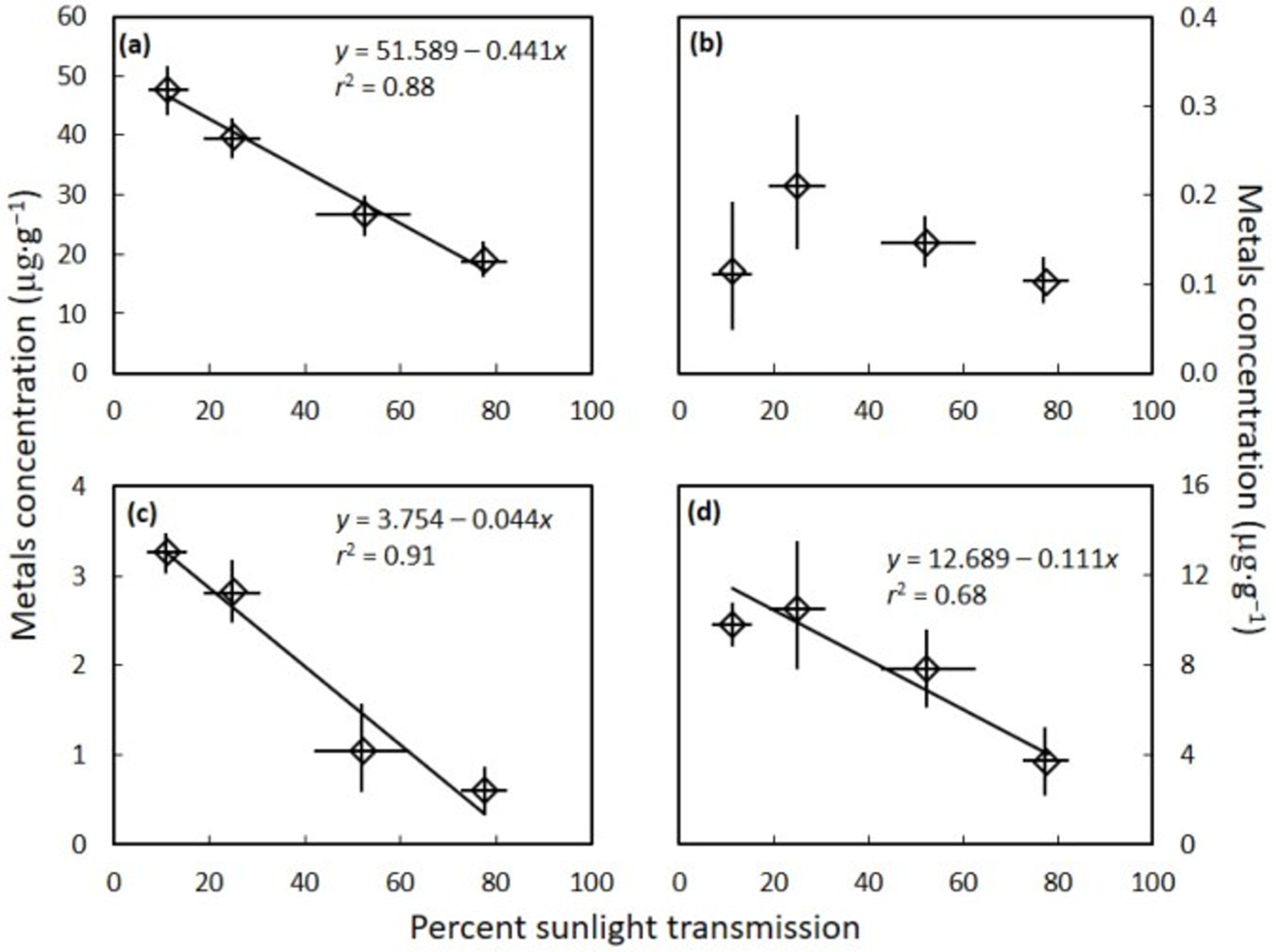
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmerman, H. Progress Report of Work in the Laboratory of Pathology during May 1945, Guam; U.S. Naval Medical Research Unit: Washington, DC, USA, 1945. [Google Scholar]
- Kurland, L.T.; Mulder, D.W. Epidemiologic investigations of amyotrophic lateral sclerosis. I. Preliminary report on geographic distribution, with special reference to the Mariana Islands, including clinical and pathologic observations. Neurology 1954, 4, 355–378. [Google Scholar] [CrossRef]
- Steele, J.C. Parkinsonism-dementia complex of Guam. Mov. Disord. 2005, 20, S99–S107. [Google Scholar] [CrossRef] [PubMed]
- Plato, C.C.; Garruto, R.M.; Galasko, D.; Craig, U.K.; Plato, M.; Gamst, A.; Torres, J.M.; Wiederholt, W. Amyotrophic lateral sclerosis and parkinsonism-dementia complex of Guam: Changing incidence rates during the past 60 years. Am. J. Epidemiol. 2003, 157, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, R.T.; Garruro, R.M.; Gajdusek, D.C. Epidemiological surveillance of amyotrophic lateral sclerosis and parkinsonism-dementia in the Commonwealth of the Northern Mariana Islands. Ann. Neurol. 1983, 13, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.A.; Marler, T.E. The Lessons of ALS-PDC—Environmental Factors in ALS Etiology. In Spectrums of Amyotrophic Lateral Sclerosis; Shaw, C.A., Morrice, J.R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 57–79. [Google Scholar] [CrossRef]
- Whiting, M.G. Toxicity of cycads. Econ. Bot. 1963, 17, 270–302. [Google Scholar] [CrossRef]
- Spencer, P.S.; Palmer, V.S.; Kisby, G.E. Seeking environmental causes of neurodegenerative disease and envisioning primary prevention. NeuroToxicology 2016, 56, 269–283. [Google Scholar] [CrossRef]
- Spencer, P.S.; Palmer, V.S.; Kisby, G.E. Western Pacific ALS-PDC: Evidence implicating cycad genotoxins. J. Neurol. Sci. 2020, 419, 117185. [Google Scholar] [CrossRef]
- Giménez-Roldán, S.; Steele, J.C.; Palmer, V.S.; Spencer, P.S. Lytico-bodig in Guam: Historical links between diet and illness during and after Spanish colonization. J. Hist. Neurosci. 2021, 30, 335–374. [Google Scholar] [CrossRef]
- Kurland, L.T. Amyotrophic lateral sclerosis and Parkinson’s disease complex on Guam linked to an environmental neurotoxin. Trends Neurosci. 1988, 11, 51–54. [Google Scholar] [CrossRef]
- Tabata, R.C. Neuropathology Induced by Sterol Glucosides in Mice. Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 2008. [Google Scholar]
- Cox, P.A.; Sacks, O.W. Cycad neurotoxins, consumption of flying foxes, and ALS-PDC disease in Guam. Neurology 2002, 58, 956–959. [Google Scholar] [CrossRef]
- Van Kampen, J.M.; Baranowski, D.C.; Robertson, H.A.; Shaw, C.A.; Kay, D.G. The progressive BSSG rat model of Parkinson’s: Recapitulating multiple key features of the human disease. PLoS ONE 2015, 10, e0139694. [Google Scholar] [CrossRef]
- Shaw, C.A. Neural Dynamics of Neurological Disease; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Garruto, R.M.; Fukatsu, R.; Yanagihara, R.; Gajdusek, D.C.; Hook, G.; Fiori, C.E. Imaging of calcium and aluminum in neurofibrillary tangle-bearing neurons in parkinsonism-dementia of Guam. Proc. Natl. Acad. Sci. USA 1984, 81, 1875–1879. [Google Scholar] [CrossRef]
- Yanagihara, R.; Garruto, R.M.; Gajdusek, D.C.; Tomita, A.; Uchikawa, T.; Konagaya, Y.; Chen, K.M.; Sobue, I.; Plato, C.C.; Gibbs, C.J., Jr. Calcium and vitamin D metabolism in Guamanian Chamorros with amyotrophic lateral sclerosis and parkinsonism-dementia. Ann. Neurol. 1984, 15, 42–48. [Google Scholar] [CrossRef]
- McLachlan, D.R.; McLachlan, C.D.; Krishnan, B.; Krishnan, S.S.; Dalton, A.J.; Steele, J.C. Aluminum and calcium in soil and food from Guam, Palau and Jamaica: Implications for amyotrophic lateral sclerosis and parkinsonism-dementia syndromes of Guam. Environ. Geochem. Health 1989, 11, 45–53. [Google Scholar]
- Vinceti, M.; Bottecchi, I.; Fan, A.; Finkelstein, Y.; Mandrioli, J. Are environmental exposures to selenium, heavy metals, and pesticides risk factors for amyotrophic lateral sclerosis? Rev. Environ. Health 2012, 27, 19–41. [Google Scholar] [CrossRef]
- Marler, T.E.; Lee, V.; Shaw, C.A. Habitat heterogeneity of Cycas micronesica seed chemistry in Guam’s forest. Micronesica 2007, 39, 297–314. [Google Scholar]
- Marler, T.E.; Krishnapillai, M.V. Incident light and leaf age influence leaflet element concentrations of Cycas micronesica trees. Horticulturae 2019, 5, 58. [Google Scholar] [CrossRef]
- Marler, T.E.; Lee, V.; Shaw, C.A. Cycad toxins and neurological diseases in Guam: Defining theoretical and experimental standards for correlating human disease with environmental toxins. HortScience 2005, 40, 1598–1606. [Google Scholar] [CrossRef]
- Young, F.J. Soil Survey of Territory of Guam; U.S. Department of Agriculture Natural Resources Conservation Service: Washington, DC, USA, 1988.
- Hou, X.; Jones, B.T. Inductively Coupled Plasma/Optical Emission Spectrometry. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons: Chichester, UK, 2000; pp. 9468–9485. [Google Scholar]
- Walton, J.R.; Wang, M.X. APP expression, distribution and accumulation are altered by aluminum in a rodent model for Alzheimer’s disease. J. Inorg. Biochem. 2009, 103, 1548–1554. [Google Scholar] [CrossRef]
- Exley, C.; House, E.R. Aluminium in the human brain. Mon. Für. Chem.-Chem. Mon. 2011, 142, 357–363. [Google Scholar] [CrossRef]
- Petrik, M.S.; Wong, M.C.; Tabata, R.C.; Garry, R.F.; Shaw, C.A. Aluminum adjuvant linked to Gulf War illness induces motor neuron death in mice. NeuroMol. Med. 2007, 9, 83–100. [Google Scholar] [CrossRef]
- Shaw, C.A.; Petrik, M.S. Aluminum hydroxide injections lead to motor deficits and motor neuron degeneration. J. Inorg. Biochem. 2009, 103, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.A.; Tomljenovic, L. Aluminum in the central nervous system (CNS): Toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol. Res. 2013, 56, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Rivadeneyra-Domínguez, E.; Rodríguez-Landa, J.F. Las cícadas y su relación con algunas enfermedades neurodegenerativas. Neurología 2014, 29, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.C.; Williams, D.B. Calcium and Aluminium in the Chamorro Diet: Unlikely Causes of Alzheimer-Type Neurofibrillary Degeneration on Guam. In Motor Neuron Disease; Leigh, P.N., Swash, M., Eds.; Springer: London, UK, 1995; pp. 189–200. [Google Scholar] [CrossRef]
- Stommel, E.W.; Field, N.C.; Caller, T.A. Aerosolization of cyanobacteria as a risk factor for amyotrophic lateral sclerosis. Med. Hypotheses 2013, 80, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Terry, L.I.; Marler, T.E. Habitats, trade winds, and pollination of the endangered Cycas micronesica: Is there a role for wind as pollen vector on the island of Guam? Int. J. Plant Sci. 2015, 176, 525–543. [Google Scholar] [CrossRef]
- Yu, M.H. Environmental Toxicology-Biological and Health Effects of Pollutants, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Rai, P.K.; Leeb, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Afonne, O.J.; Ifediba, E.C. Heavy metals risks in plant foods—Need to step up precautionary measures. Curr. Opin. Toxicol. 2020, 22, 1–6. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, K.K.; Kumar, V.; Prasad, S.; Cabral-Pinto, M.M.S.; Jeon, B.-H.; Kumar, S.; Abdellattif, M.H.; Alsukaibia, A.K.D. Investigation of heavy metal accumulation in vegetables and health risk to humans from their consumption. Front. Environ. Sci. 2022, 10, 791052. [Google Scholar] [CrossRef]
- Parveen, N.; Berni, R.; Sudhakaran, S.; Bhat, J.A.; Shinde, S.; Ramawat, N.; Singh, V.P.; Sahi, S.; Deshmukh, R.; Chauhan, D.K.; et al. Metalloids in plants: A systematic discussion beyond description. Ann. Appl. Biol. 2022, 180, 7–25. [Google Scholar] [CrossRef]
- Kumar, P.; Gacem, A.; Ahmad, M.T.; Yadav, V.K.; Singh, S.; Yadav, K.K.; Alam, M.M.; Dawane, V.; Piplode, S.; Maurya, P.; et al. Environmental and human health implications of metal(loid)s: Source identification, contamination, toxicity, and sustainable clean-up technologies. Front. Environ. Sci. 2022, 10, 949581. [Google Scholar] [CrossRef]
- Song, W.; Zhang, H.; Li, X.; Song, H.; Niu, B.; Shi, X.; Li, J. Safe utilization of cultivated land in high-risk areas of soil heavy metal pollution based on soil resilience. Front. Environ. Sci. 2022, 10, 889069. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H. Shade and Cd conditions strongly impact the physiological responses of purple Perilla. Front. Environ. Sci. 2022, 10, 896963. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, H.; Zhang, Z.; Chen, C.; Yu, F.; Guy, R.D. Effects of fruit shading on gene and protein expression during starch and oil accumulation in developing Styrax tonkinensis kernels. Front. Plant Sci. 2022, 13, 905633. [Google Scholar] [CrossRef]
- Sušin, J.; Kmecl, V.; Gregorčič, A. A survey of nitrate and nitrite content of fruit and vegetable grown in Slovenia during 1996–2002. Food Add. Cont. 2006, 23, 385–390. [Google Scholar] [CrossRef]
- De Cires, A.; de la Torre, A.; Delgado, B.; Lara, C. Role of light and CO2 fixation in the control of nitrate-reductase activity in barley leaves. Planta 1993, 190, 277–283. [Google Scholar] [CrossRef]
- Ferretti, M.; Merlo, L.; Passera, C.; Ghisi, R. Influence of irradiance on enzymes of sulphate and nitrate assimilation pathways in maize leaves. Plant Physiol. Biochem. 1995, 33, 111–114. [Google Scholar]
- Premuzic, Z.; Gárate, A.; Bonilla, I. Production of lettuce under different fertilisation treatments, yield and quality. Acta Hortic. 2002, 571, 65–72. [Google Scholar] [CrossRef]
- He, J.; Cheok, L.; Qin, L. Nitrate accumulation, productivity and photosynthesis of temperate butter head lettuce under different nitrate availabilities and growth irradiances. Open Hortic. J. 2011, 4, 17–24. [Google Scholar] [CrossRef]
- Signore, A.; Bell, L.; Santamaria, P.; Wagstaff, C.; Van Labeke, M.-C. Red light is effective in reducing nitrate concentration in rocket by increasing nitrate reductase activity, and contributes to increased total glucosinolates content. Front. Plant Sci. 2020, 11, 604. [Google Scholar] [CrossRef]
- Cibrián-Jaramillo, A.; Daly, A.C.; Brenner, E.; Desalle, R.; Marler, T.E. When north and south don’t mix: Genetic connectivity of a recently endangered oceanic cycad, Cycas micronesica, in Guam using EST-microsatellites. Molecular Ecol. 2010, 19, 2364–2379. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E. Reciprocal garden study reveals acute spatial-edaphic adaptation for Cycas micronesica. Diversity 2021, 13, 237. [Google Scholar] [CrossRef]
- James, K. Nutrients in Native Plant Seeds. In The Food Potential of Seeds from Australian Native Plants; Jones, G.P., Ed.; Deakin University Press: Geelong, Australia, 1985; pp. 46–58. [Google Scholar]
- Miller, J.B.; James, K.W.; Maggiore, P.M. Tables of Composition of Australian Aboriginal Foods; Aboriginal Studies Press: Canberra, Australia, 1993. [Google Scholar]
- Kumar, N.R.; Reddy, J.S.; Gopikrishna, G.; Solomon, K.A. GC-MS determination of bioactive constituents of Cycas beddomei cones. Int. J. Pharm. Bio. Sci. 2012, 3, 344–350. [Google Scholar]
- Noora, B.K.P.; Sangeetha, N.; Gousaliyaa. Effect of fermentation on the properties of porridge from Cycas flour (Cycas circinalis L.). Int. J. Adv. Sci. Eng. Inf. Technol. 2018, 6, 19–22. [Google Scholar]
- Kamila, P.K.; Bal, P.; Ray, A.; Kar, S.K.; Panda, P.C. Nutritional value, phytochemical composition and antioxidant potential of the seed flour of Cycas sphaerica, endemic to India. S. Afr. J. Bot. 2022, 150, 965–973. [Google Scholar] [CrossRef]

| Metal | Variability Index |
|---|---|
| Aluminum | 71% |
| Arsenic | 63% |
| Cadmium | 83% |
| Chromium | 60% |
| Cobalt | 93% |
| Lead | 99% |
| Nickel | 96% |
| Selenium | 92% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marler, T.E.; Shaw, C.A. Metals and Metalloids Increase in Cycas micronesica Seed Gametophyte Tissue in Shaded Growth Conditions. Toxics 2022, 10, 550. https://doi.org/10.3390/toxics10100550
Marler TE, Shaw CA. Metals and Metalloids Increase in Cycas micronesica Seed Gametophyte Tissue in Shaded Growth Conditions. Toxics. 2022; 10(10):550. https://doi.org/10.3390/toxics10100550
Chicago/Turabian StyleMarler, Thomas E., and Christopher A. Shaw. 2022. "Metals and Metalloids Increase in Cycas micronesica Seed Gametophyte Tissue in Shaded Growth Conditions" Toxics 10, no. 10: 550. https://doi.org/10.3390/toxics10100550
APA StyleMarler, T. E., & Shaw, C. A. (2022). Metals and Metalloids Increase in Cycas micronesica Seed Gametophyte Tissue in Shaded Growth Conditions. Toxics, 10(10), 550. https://doi.org/10.3390/toxics10100550







