Microencapsulation of Horseradish (Armoracia rusticana L.) Juice Using Spray-Drying
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Encapsulating Agents
2.2. Chemicals and Reagents
2.3. Infeed Solution Preparation
2.4. Encapsulation of Horseradish Juice by Spray-Drying
2.5. Measurement of Physical Parameters
2.6. Calculation of Retention Efficiency (RE) and Stability
2.7. Chemical Properties of Encapsulated Horseradish-Juice Powder
2.7.1. Spectrophotometric Determination of Bioactive Compounds and Antioxidant Activities
2.7.2. HPLC Analysis of Individual Phenolic Compounds
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics of Microencapsulated Horseradish Leaf and Root Juices
3.2. Characteristics of Bioactive Compounds in Microencapsulated Horseradish Leaf and Root Juices
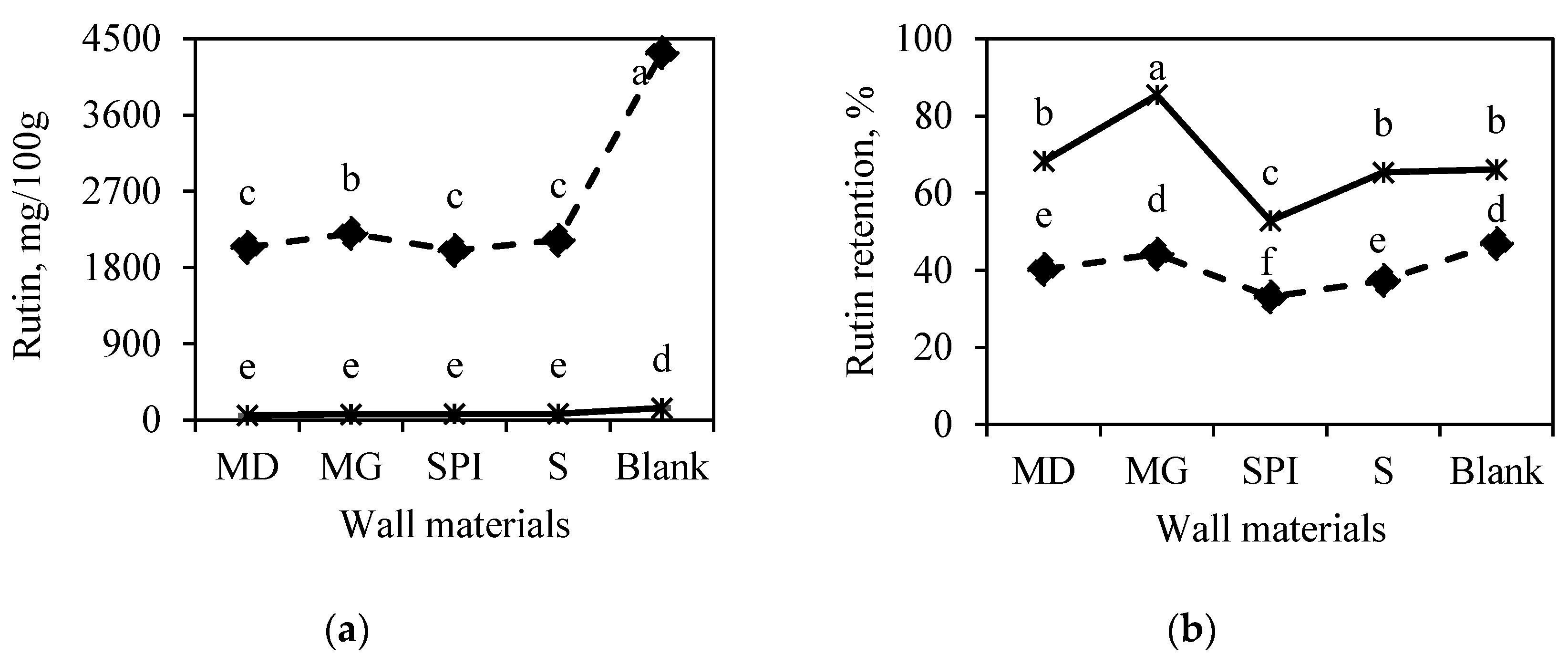
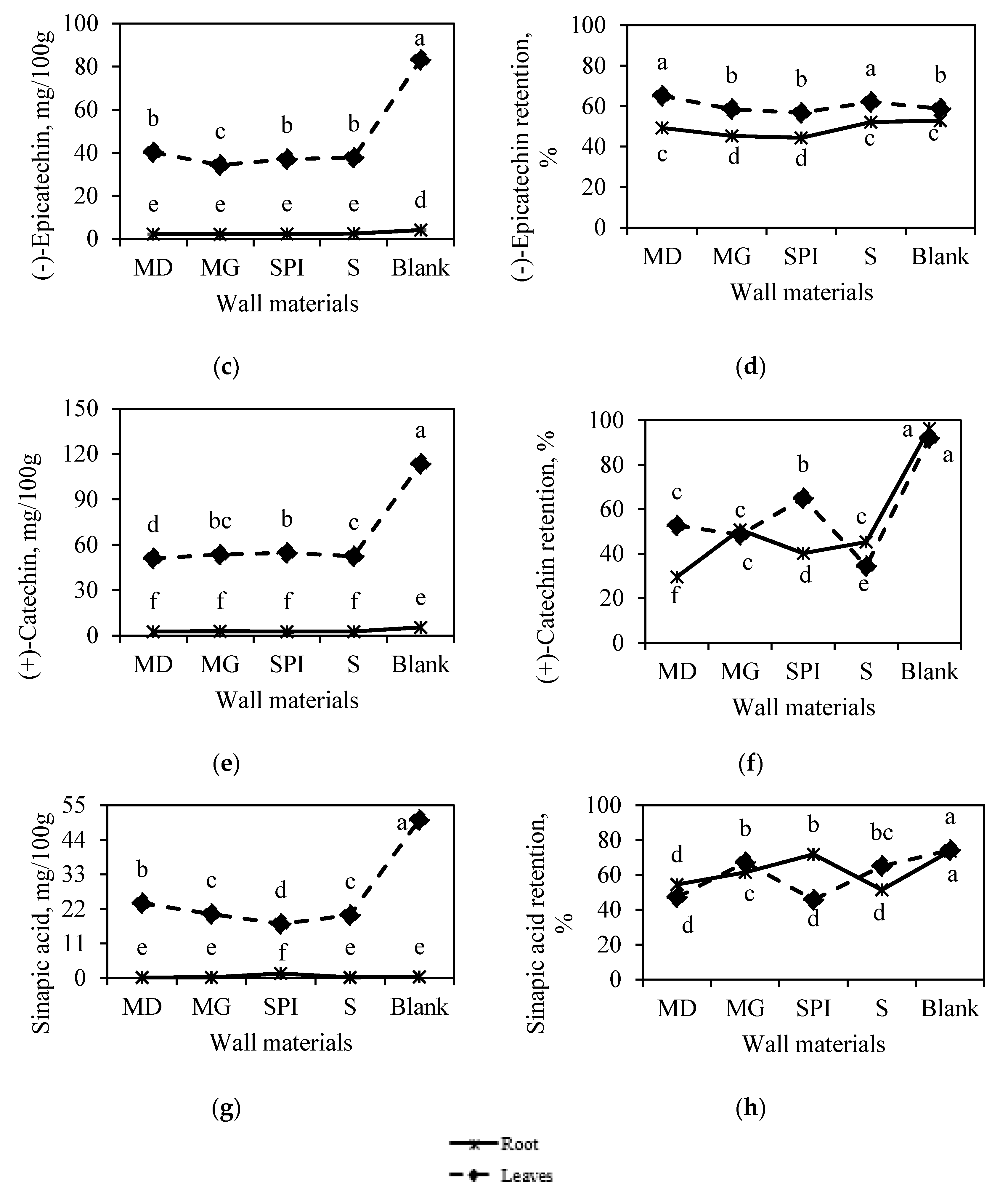
3.2.1. Effect of Horseradish Plant Part on Bioactive Compounds in Microcapsules
3.2.2. Effect of Wall Material Type on Bioactive-Compound RE
- for horseradish leaf juice, MG > M > S > SP;
- for horseradish root juice, M > MG > SP > S.
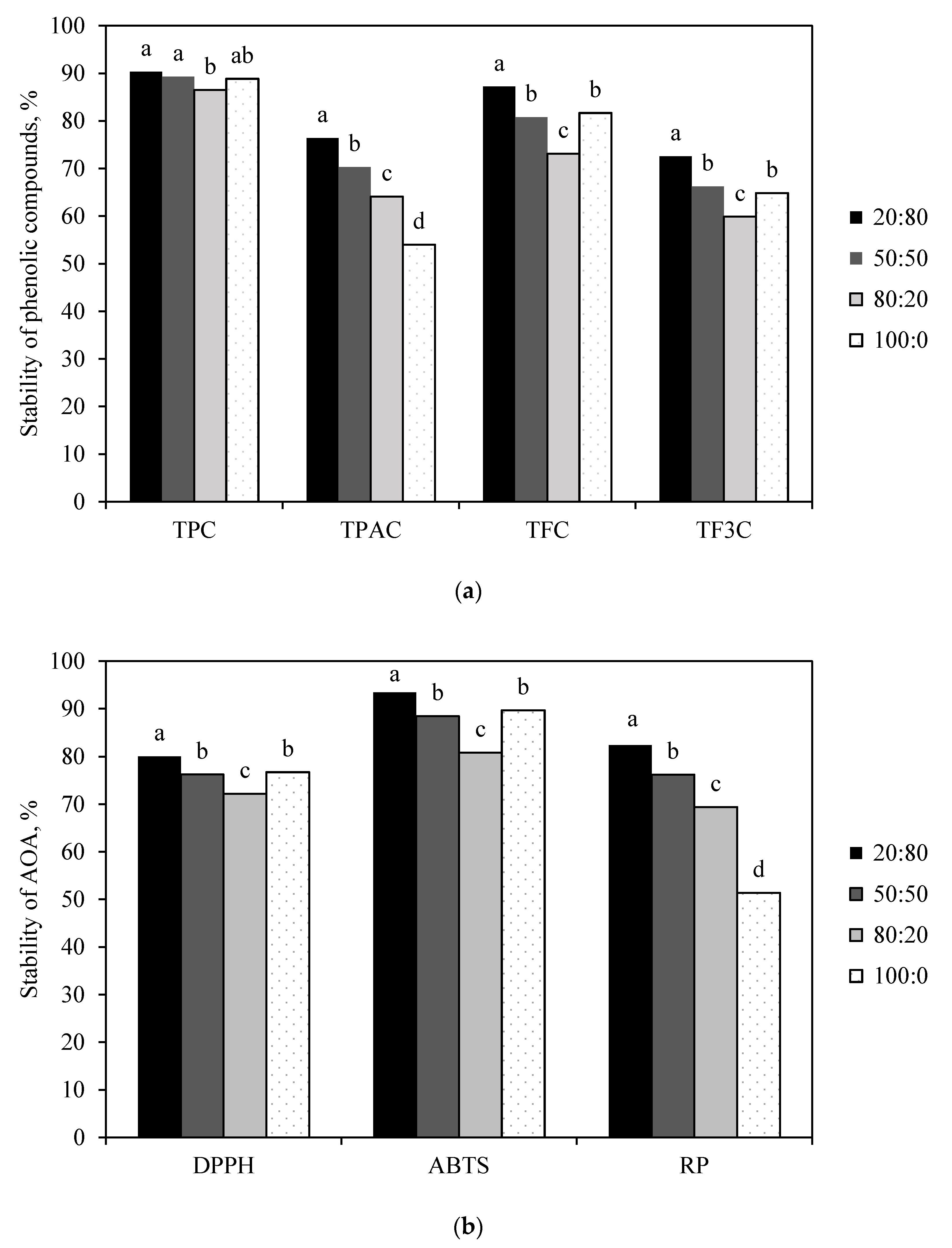
3.2.3. Effect of Core-to-Wall Ratio on the Stability of Horseradish-Juice Microcapsules
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dekić, M.S.; Radulović, N.S.; Stojanović, N.M.; Randjelović, P.J.; Stojanović-Radić, Z.Z.; Najman, S.; Stojanović, S. Spasmolytic, Antimicrobial and Cytotoxic Activities of 5-Phenylpentyl Isothiocyanate, a New Glucosinolate Autolysis Product from Horseradish (Armoracia rusticana P. Gaertn., B. Mey. & Scherb., Brassicaceae). Food Chem. 2017, 232, 329–339. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Devahastin, S.; Liu, Y. Influence of Low-Temperature Ball Milling Time on Physicochemical Properties, Flavor, Bioactive Compounds Contents and Antioxidant Activity of Horseradish Powder. Adv. Powder Technol. 2019, 31, 914–921. [Google Scholar] [CrossRef]
- Bertóti, R.; Böszörményi, A.; Alberti, Á.; Béni, S.; M.-Hamvas, M.; Szőke, É.; Vasas, G.; Gonda, S. Variability of Bioactive Glucosinolates, Isothiocyanates and Enzyme Patterns in Horseradish Hairy Root Cultures Initiated from Different Organs. Molecules 2019, 24, 2828. [Google Scholar] [CrossRef]
- Medjakovic, S.; Zoechling, A.; Vollmer, G.; Zierau, O.; Kretzschmar, G.; Möller, F.; Kolba, S.; Papke, A.; Opietnik, M.; Kosma, P.; et al. Indolylfuran, a Potent Aryl Hydrocarbon Receptor Agonist from Sauerkraut, Interacts with the Oestrogen Pathway. Food Chem. 2011, 127, 1764–1772. [Google Scholar] [CrossRef]
- Su, D.; Wang, Z.; Dong, L.; Huang, F.; Zhang, R.; Jia, X.; Wu, G.; Zhang, M. Impact of Thermal Processing and Storage Temperature on the Phenolic Profile and Antioxidant Activity of Different Varieties of Lychee Juice. LWT 2019, 116, 1–9. [Google Scholar] [CrossRef]
- Labuschagne, P. Impact of Wall Material Physicochemical Characteristics on the Stability of Encapsulated Phytochemicals: A Review. Food Res. Int. 2018, 107, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.; Grace, M.H.; Esposito, D.; Lila, M.A. Wild Blueberry Polyphenol-Protein Food Ingredients Produced by Three Drying Methods: Comparative Physico-Chemical Properties, Phytochemical Content, and Stability during Storage. Food Chem. 2017, 235, 76–85. [Google Scholar] [CrossRef]
- Ramakrishnan, Y.; Adzahan, N.M.; Yusof, Y.A.; Muhammad, K. Effect of Wall Materials on the Spray Drying Efficiency, Powder Properties and Stability of Bioactive Compounds in Tamarillo Juice Microencapsulation. Powder Technol. 2018, 328, 406–414. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Chen, X.; Quek, S.Y. Effect of Spray Drying on Phenolic Compounds of Cranberry Juice and Their Stability during Storage. J. Food Eng. 2020, 269, 109744. [Google Scholar] [CrossRef]
- Idham, Z.; Muhamad, I.I.; Sarmidi, M.R. Degradation Kinetics and Color Stability of Spray-Dried Encapsulated Anthocyanins from Hibiscus Sabdariffa L. J. Food Process Eng. 2012, 35, 522–542. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of Antioxidant Phenolic Compounds Extracted from Spent Coffee Grounds by Freeze-Drying and Spray-Drying Using Different Coating Materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A Review of Microencapsulation Methods for Food Antioxidants: Principles, Advantages, Drawbacks and Applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef]
- Dalmoro, A.; Barba, A.A.; Lamberti, G.; D’Amore, M. Intensifying the Microencapsulation Process: Ultrasonic Atomization as an Innovative Approach. Eur. J. Pharm. Biopharm. 2012, 80, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.; Noreña, C.P.Z. Encapsulation of Garlic Extract Using Complex Coacervation with Whey Protein Isolate and Chitosan as Wall Materials Followed by Spray Drying. Food Hydrocoll. 2019, 89, 360–369. [Google Scholar] [CrossRef]
- Santhalakshmy, S.; Don Bosco, S.J.; Francis, S.; Sabeena, M. Effect of Inlet Temperature on Physicochemical Properties of Spray-Dried Jamun Fruit Juice Powder. Powder Technol. 2015. [Google Scholar] [CrossRef]
- Cano-Chauca, M.; Stringheta, P.C.; Ramos, A.M.; Cal-Vidal, J. Effect of the Carriers on the Microstructure of Mango Powder Obtained by Spray Drying and Its Functional Characterization. Innov. Food Sci. Emerg. Technol. 2005, 6, 420–428. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 119, 152–178. [Google Scholar]
- Gawlik-Dziki, U. Dietary Spices as a Natural Effectors of Lipoxygenase, Xanthine Oxidase, Peroxidase and Antioxidant Agents. LWT Food Sci. Technol. 2012, 47, 138–146. [Google Scholar] [CrossRef]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant Capacity of Phenolic Phytochemicals from Various Cultivars of Plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Blasco, B.; Rios, J.J.; Cervilla, L.M.; Sánchez-Rodrigez, E.; Ruiz, J.M.; Romero, L. Iodine Biofortification and Antioxidant Capacity of Lettuce: Potential Benefits for Cultivation and Human Health. Ann. Appl. Biol. 2008, 152, 289–299. [Google Scholar] [CrossRef]
- Zam, W.; Bashour, G.; Abdelwahed, W.; Khayata, W. Separation and Purification of Proanthocyanidins Extracted from Pomegranate’s Peels (Punica granatum). Int. J. Pharm. Sci. Nanotechnol. 2012, 5, 1808–1813. [Google Scholar]
- Yu, L.; Perret, J.; Harris, M.; Wilson, J.; Haley, S. Antioxidant Properties of Bran Extracts from “Akron” Wheat Grown at Different Locations. J. Agric. Food Chem. 2003, 51, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Athukorala, Y.; Kim, K.N.; Jeon, Y.J. Antiproliferative and Antioxidant Properties of an Enzymatic Hydrolysate from Brown Alga, Ecklonia Cava. Food Chem. Toxicol. 2006, 44, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Priecina, L.; Karklina, D.; Kince, T. The Impact of Steam-Blanching and Dehydration on Phenolic, Organic Acid Composition, and Total Carotenoids in Celery Roots. Innov. Food Sci. Emerg. Technol. 2018, 49, 192–201. [Google Scholar] [CrossRef]
- Nesterenko, A.; Alric, I.; Silvestre, F.; Durrieu, V. Vegetable Proteins in Microencapsulation: A Review of Recent Interventions and Their Effectiveness. Ind. Crops Prod. 2013. [Google Scholar] [CrossRef]
- Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of Grape (Vitis labrusca Var. Bordo) Skin Phenolic Extract Using Gum Arabic, Polydextrose, and Partially Hydrolyzed Guar Gum as Encapsulating Agents. Food Chem. 2016, 194, 569–576. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Pallet, D.; Brat, P.; Hubinger, M.D. Physicochemical and Morphological Characterisation of Açai (Euterpe Oleraceae Mart.) Powder Produced with Different Carrier Agents. Int. J. Food Sci. Technol. 2009, 44, 1950–1958. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Microencapsulation of Thai Rice Grass (O. Sativa Cv. Khao Dawk Mali 105) Extract Incorporated to Form Bioactive Carboxymethyl Cellulose Edible Film. Food Chem. 2018, 242, 239–246. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Germer, S.P.M.; de Aguirre, J.M. Effects of Spray-Drying Conditions on the Physicochemical Properties of Blackberry Powder. Dry. Technol. 2011, 30, 154–163. [Google Scholar] [CrossRef]
- Akhavan Mahdavi, S.; Jafari, S.M.; Assadpoor, E.; Dehnad, D. Microencapsulation Optimization of Natural Anthocyanins with Maltodextrin, Gum Arabic and Gelatin. Int. J. Biol. Macromol. 2016, 85, 379–385. [Google Scholar] [CrossRef]
- De Souza, V.B.; Thomazini, M.; Balieiro, J.C.D.C.; Fávaro-Trindade, C.S. Effect of Spray Drying on the Physicochemical Properties and Color Stability of the Powdered Pigment Obtained from Vinification Byproducts of the Bordo Grape (Vitis labrusca). Food Bioprod. Process. 2015, 93, 39–50. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of Process Conditions on the Physicochemical Properties of Açai (Euterpe oleraceae Mart.) Powder Produced by Spray Drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Microencapsulation of Extracts of Bioactive Compounds Obtained from Acerola (Malpighia emarginata DC) Pulp and Residue by Spray and Freeze Drying: Chemical, Morphological and Chemometric Characterization. Food Chem. 2018, 254, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, E.C.Q.; Calado, V.M.D.A.; Monteiro, M.; Finotelli, P.V.; Torres, A.G.; Perrone, D. Starch, Inulin and Maltodextrin as Encapsulating Agents Affect the Quality and Stability of Jussara Pulp Microparticles. Carbohydr. Polym. 2016, 151, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Daza, L.D.; Fujita, A.; Fávaro-Trindade, C.S.; Rodrigues-Ract, J.N.; Granato, D.; Genovese, M.I. Effect of Spray Drying Conditions on the Physical Properties of Cagaita (Eugenia dysenterica DC.) Fruit Extracts. Food Bioprod. Process. 2016, 97, 20–29. [Google Scholar] [CrossRef]
- Molina Ortiz, S.E.; Mauri, A.; Monterrey-Quintero, E.S.; Trindade, M.A.; Santana, A.S.; Favaro-Trindade, C.S. Production and Properties of Casein Hydrolysate Microencapsulated by Spray Drying with Soybean Protein Isolate. LWT Food Sci. Technol. 2009, 42, 919–923. [Google Scholar] [CrossRef]
- Yousefi, S.; Emam-Djomeh, Z.; Mousavi, S.M. Effect of Carrier Type and Spray Drying on the Physicochemical Properties of Powdered and Reconstituted Pomegranate Juice (Punica granatum L.). J. Food Sci. Technol. 2011, 48, 677–684. [Google Scholar] [CrossRef]
- Paim, D.R.S.F.; Costa, S.D.O.; Walter, E.H.M.; Tonon, R.V. Microencapsulation of Probiotic Jussara (Euterpe edulis M.) Juice by Spray Drying. LWT Food Sci. Technol. 2016, 74, 21–25. [Google Scholar] [CrossRef]
- Tomsone, L. Investigation of Biologically Active Substances in Horseradish and Lovage. Ph.D. Thesis, Latvia University of Life Sciences and Technologies, Jelgava, Latvia, 2015. [Google Scholar]
- Naczk, M.; Shahidi, F. Phenolics in Cereals, Fruits and Vegetables: Occurrence, Extraction and Analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Marrelli, M.; Menichini, F.; Statti, G.A.; Bonesi, M.; Duez, P.; Menichini, F.; Conforti, F. Changes in the Phenolic and Lipophilic Composition, in the Enzyme Inhibition and Antiproliferative Activity of Ficus carica L. Cultivar Dottato Fruits during Maturation. Food Chem. Toxicol. 2012, 50, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.S.; Lister, C. Flavonoid Functions in Plants. In Flavonoids Chemistry, Biochemistry and Applications; Andersen, Ø.M., Markham, K.R., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 397–441. [Google Scholar]
- Incandela, L.; Cesarone, M.R.; DeSanctis, M.T.; Belcaro, G.; Dugall, M.; Acerbi, G. Treatment of Diabetic Microangiopathy and Edema with HR (Paroven, Venoruton; 0-(β-Hydroxyethyl)-Rutosides): A Prospective, Placebo-Controlled, Randomized Study. J. Cardiovasc. Pharmacol. Ther. 2002, 7 (Suppl. 1), S11–S15. [Google Scholar] [CrossRef] [PubMed]
- Moser, P.; Telis, V.R.N.; de Andrade Neves, N.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Storage Stability of Phenolic Compounds in Powdered BRS Violeta Grape Juice Microencapsulated with Protein and Maltodextrin Blends. Food Chem. 2017, 214, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Boyano-Orozco, L.C.; Gallardo-Velázquez, T.; Meza-Márquez, G.; Osorio-Revilla, G. Microencapsulation of Rambutan Peel Extract by Spray Drying. Foods 2020, 9, 899. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.F.; Yusoff, M.M.; Gimbun, J. Assessment of Phenolic Compounds Stability and Retention during Spray Drying of Orthosiphon stamineus Extracts. Food Hydrocoll. 2014, 37, 159–165. [Google Scholar] [CrossRef]
- Sablania, V.; Bosco, S.J.D.; Rohilla, S.; Shah, M.A. Microencapsulation of Murraya Koenigii L. Leaf Extract Using Spray Drying. J. Food Meas. Charact. 2018, 12, 892–901. [Google Scholar] [CrossRef]
- Osamede Airouyuwa, J.; Kaewmanee, T. Microencapsulation of Moringa oleifera Leaf Extracts with Vegetable Protein as Wall Materials. Food Sci. Technol. Int. 2019, 25, 533–543. [Google Scholar] [CrossRef]
| Wall Materials | Core to Wall Ratios * | |||||
|---|---|---|---|---|---|---|
| Horseradish Leaf Juice | Horseradish Root Juice | |||||
| 20:80 | 50:50 | 80:20 | 20:80 | 50:50 | 80:20 | |
| Maltodextrin (MD) | L20M80 | L50M50 | L80M20 | R20M80 | R50M50 | R80M20 |
| Maltodextrin/gum Arabic (3:2) (MG) | L20MG80 | L50MG50 | L80MG20 | R20MG80 | R50MG50 | R80MG20 |
| Soy protein isolate (SPI) | L20P80 | L50P50 | L80P20 | R20P80 | R50P50 | R80P20 |
| Starch (S) | L20S80 | L50S50 | L80S20 | R20S80 | R50S50 | R80S20 |
| Materials | Sample Abbreviation | Parameters | ||
|---|---|---|---|---|
| Particle Size (μm) | Hygroscopicity (g/100 g) | Solubility (%) | ||
| Horseradish leaf (L) juice | ||||
| Juice (blank) | LB | n.a. | 20.99 ± 1.08 a | 79.06 ± 0.89 g,h |
| Maltodextrin (MD) | L20M80 | 4.24 ± 0.17 i | 14.97 ± 0.75 d,e,f | 91.02 ± 0.21 a |
| L50M50 | 3.98 ± 0.03 j | 16.92 ± 0.82 c,d | 88.82 ± 0.47 a,b | |
| L80M20 | 3.84 ± 0.07 j | 19.25 ± 0.98 a,b | 82.53 ± 0.38 e,f | |
| Maltodextrin/gum Arabic (MG) | L20MG80 | 4.34 ± 0.12 i | 12.05 ± 0.60 h,i,j | 89.71 ± 1.22 a |
| L50MG50 | 3.77 ± 0.07 j,k | 13.40 ± 0.67 f,g,h | 84.70 ± 0.63 d,e | |
| L80MG20 | 4.20 ± 0.10 i | 14.71 ± 0.71 e,f,g | 80.74 ± 1.94 f,g | |
| Soy protein isolate (SPI) | L20P80 | 14.00 ± 1.37 b | 8.45 ± 0.39 l,m | 59.94 ± 1.23 n |
| L50P50 | 11.50 ± 0.38 c | 9.20 ± 0.43 k,l,m | 63.44 ± 1.32 m | |
| L80P20 | 7.46 ± 0.10 f | 10.82 ± 0.57 i,j,k | 72.80 ± 0.10 i | |
| Starch (S) | L20S80 | 9.88 ± 0.84 d | 7.46 ± 0.30 m | 46.57 ± 0.25 r |
| L50S50 | 14.10 ± 0.50 b | 7.93 ± 0.42 m | 53.50 ± 0.58 p | |
| L80S20 | 9.48 ± 0.25 d | 8.29 ± 0.39 l,m | 66.60 ± 0.28 l | |
| Horseradish root (R) juice | ||||
| Juice (blank) | RB | n.a. | 19.73 ± 1.03 a,b | 71.87 ± 0.95 i,j |
| Maltodextrin (MD) | R20M80 | 3.74 ± 0.02 k | 16.01 ± 0.81 d,e | 87.05 ± 0.06 b,c |
| R50M50 | 4.01 ± 0.26 i,j | 18.89 ± 0.92 b,c | 78.52 ± 0.39 g,h | |
| R80M20 | 3.89 ± 0.28 i,j | 20.89 ± 0.95 a,b | 70.30 ± 0.31 j,k | |
| Maltodextrin/gum Arabic (MG) | R20MG80 | 3.68 ± 0.02 k,l | 10.06 ± 0.51 j,k,l | 89.56 ± 0.57 a |
| R50MG50 | 3.64 ± 0.12 k,l | 12.82 ± 0.64 g,h,i | 85.18 ± 0.45 c,d | |
| R80MG20 | 3.73 ± 0.08 j,k | 14.99 ± 0.60 d,e,f | 78.08 ± 0.49 h | |
| Soy protein isolate (SPI) | R20P80 | 6.57 ± 0.27 g,h | 8.19 ± 0.42 l,m | 57.03 ± 0.87 o |
| R50P50 | 6.47 ± 0.16 h | 9.37 ± 0.47 k,l,m | 68.53 ± 0.56 k,l | |
| R80P20 | 6.86 ± 0.20 g | 10.57 ± 0.53 j,k | 67.60 ± 0.55 l | |
| Starch (S) | R20S80 | 18.40 ± 1.98 a | 7.54 ± 0.35 m | 28.80 ± 0.56 s |
| R50S50 | 10.30 ± 0.26 d | 7.87 ± 0.39 m | 46.64 ± 0.04 r | |
| R80S20 | 8.84 ± 0.46 d,e | 8.04 ± 0.34 l,m | 59.80 ± 0.06 n | |
| Materials | Sample Abbreviation | Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| TPC | TPAC | TFC | TF3C | DPPH• | ABTS•+ | RP | ||
| Horseradish leaf (L) juice | ||||||||
| Juice (blank) | LB | 7232 ± 143 a | 3629 ± 92 a | 12006 ± 195 c | 4550 ± 111 i | 148 ± 2 a | 1100 ± 20 a | 1885 ± 25 a |
| Maltodextrin (MD) | L20M80 | 1915 ± 26 h | 1664 ± 128 g | 2870 ± 186 j | 11073 ± 154 a | 88 ± 1 k | 320 ± 11 k,l | 967 ± 2 t |
| L50M50 | 3969 ± 63 e | 2357 ± 132 d,e | 6686 ± 159 g | 9664 ± 177 c | 121 ± 2 d | 653 ± 20 g | 126 ± 1 s | |
| L80M20 | 6129 ± 152 b | 2925 ± 146 b | 11054 ± 192 d,e | 4175 ± 191 j | 129 ± 2 c | 895 ± 14 c | 238 ± 5 o | |
| Maltodextrin/gum Arabic (MG) | L20MG80 | 1867 ± 14 i | 2191 ± 127 e | 2675 ± 127 j | 1823 ± 112 r | 98 ± 1 h | 305 ± 6 l | 291 ± 6 k |
| L50MG50 | 3872 ± 116 e | 2960 ± 135 b | 6303 ± 254 g,h | 2709 ± 28 n | 112 ± 2 e | 630 ± 8 g,h | 459 ± 6 f | |
| L80MG20 | 5842 ± 61 c | 3602 ± 120 a | 10615 ± 153 e | 3970 ± 129 j | 136 ± 2 b | 873 ± 13 c,d | 599 ± 6 d | |
| Soy protein isolate (SPI) | L20P80 | 3103 ± 105 f | 2129 ± 151 e | 6049 ± 18 h | 8334 ± 107 e | 107 ± 1 f | 544 ± 10 i | 70 ± 2 w |
| L50P50 | 4524 ± 75 d | 2413 ± 141 d | 12851 ± 19 b | 9528 ± 124 d | 119 ± 1 d | 759 ± 14 e | 88 ± 3 u | |
| L80P20 | 6150 ± 85 b | 2927 ± 133 b | 18213 ± 19 a | 10259 ± 149 b | 138 ± 2 b | 949 ± 19 b | 144 ± 3 r | |
| Starch (S) | L20S80 | 2282 ± 81 g | 2683 ± 125 c | 3667 ± 79 i | 2374 ± 42 o | 109 ± 1 f | 377 ± 7 j | 324 ± 6 j |
| L50S50 | 4592 ± 47 d | 3204 ± 169 b | 8079 ± 121f | 2943 ± 55 m | 120 ± 3 d | 742 ± 5 e,f | 370 ± 7 h | |
| L80S20 | 6059 ± 41 b | 3729 ± 162 a | 10866 ± 265 d,e | 3620 ± 135 k | 137 ± 2 b | 963 ± 19 b | 396 ± 8 g | |
| Horseradish root (R) juice | ||||||||
| Juice (blank) | RB | 1008 ± 40 k,l | 2461 ± 68 d | 620 ± 69 m | 2173 ± 74 p | 96 ± 2 h,i | 157 ± 4 o | 230 ± 4 o,p |
| Maltodextrin (MD) | R20M80 | 321 ± 25 p | 1141 ± 107 j | 259 ± 10 s | 9046 ± 139 d | 64 ± 1 n | 36 ± 1 v | 269 ± 3 m |
| R50M50 | 469 ± 11 o | 1257 ± 48 h | 307 ± 24 r | 6354 ± 75 f | 90 ± 1 j | 90 ± 2 s | 281 ± 5 l | |
| R80M20 | 732 ± 18 m | 1603 ± 65 g | 381 ± 28 o,p | 1821 ± 30 r | 95 ± 1 h,i | 114 ± 1 p | 479 ± 6 e | |
| Maltodextrin/gum Arabic (MG) | R20MG80 | 304 ± 17 p | 1542 ± 108 g | 208 ± 22 t | 1222 ± 50 v | 86 ± 2 l | 44 ± 1 u | 258 ± 3 n |
| R50MG50 | 599 ± 11 n | 1930 ± 121 f | 420 ± 39 o | 1392 ± 62 u | 95 ± 2 h,i | 90 ± 1 s | 603 ± 15 d | |
| R80MG20 | 935 ± 75 l | 2252 ± 164 e | 551 ± 37 n | 1714 ± 27 s | 102 ± 2 g | 108 ± 3 r | 838 ± 9 b | |
| Soy protein isolate (SPI) | R20P80 | 1840 ± 31 i | 3127 ± 141 b | 568 ± 58 m,n | 3194 ± 121 l | 95 ± 2 h,i | 339 ± 13 j | 41 ± 2 y |
| R50P50 | 1407 ± 21 j | 2585 ± 138 c,d | 834 ± 64 l | 4978 ± 73 h | 99 ± 1 g,h | 223 ± 5 m | 55 ± 2 x | |
| R80P20 | 1014 ± 32 k | 1911 ± 150 f | 958 ± 19 k | 6050 ± 81 g | 101 ± 2 g | 195 ± 7 n | 75 ± 3 v | |
| Starch (S) | R20S80 | 325 ± 13 p | 1575 ± 115 g | 366 ± 34 p | 1372 ± 35 u | 74 ± 1 m | 44 ± 1 u | 649 ± 7 c |
| R50S50 | 623 ± 23 n | 1619 ± 112 g | 490 ± 46 n,o | 1514 ± 22 t | 94 ± 2 i | 73 ± 4 t | 352 ± 4 i | |
| R80S20 | 852 ± 32 l | 2049 ± 116 e,f | 521 ± 37 n | 1764 ± 19 r | 103 ± 2 g | 92 ± 4 s | 98 ± 2 t | |
| Parameters | Plant Part | Wall Material | |||||
|---|---|---|---|---|---|---|---|
| Roots | Leaves | MD | MG | SPI | S | Blank | |
| TPC | 81.58 A | 62.77 B | 74.93 b | 79.94 a | 56.40 c | 77.43 a,b | 75.48 b |
| TPAC | 75.91 A | 56.30 | 72.57 a | 74.35 a | 47.72 c | 69.78 b | 67.73 b |
| TFC | 65.99 B | 87.38 A | 73.71 c | 78.84 a | 76.23 a | 77.97 a,b | 75.25 b,c |
| TF3C | 61.08 A | 68.90 A | 83.53 a | 49.22 d | 71.27 b | 55.93 c | 48.62 d |
| DPPH• | 78.90 A | 49.28 B | 69.07 a | 67.15 b | 59.76 c | 60.37 c | 61.50 c |
| ABTS•+ | 79.27 A | 56.89 B | 76.38 a | 63.02 c | 66.07 b | 66.86 b | 66.30 b |
| RP | 73.96 A | 44.04 B | 51.98 d | 68.37 a | 60.43 b | 55.23 c | 35.65 e |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomsone, L.; Galoburda, R.; Kruma, Z.; Durrieu, V.; Cinkmanis, I. Microencapsulation of Horseradish (Armoracia rusticana L.) Juice Using Spray-Drying. Foods 2020, 9, 1332. https://doi.org/10.3390/foods9091332
Tomsone L, Galoburda R, Kruma Z, Durrieu V, Cinkmanis I. Microencapsulation of Horseradish (Armoracia rusticana L.) Juice Using Spray-Drying. Foods. 2020; 9(9):1332. https://doi.org/10.3390/foods9091332
Chicago/Turabian StyleTomsone, Lolita, Ruta Galoburda, Zanda Kruma, Vanessa Durrieu, and Ingmars Cinkmanis. 2020. "Microencapsulation of Horseradish (Armoracia rusticana L.) Juice Using Spray-Drying" Foods 9, no. 9: 1332. https://doi.org/10.3390/foods9091332
APA StyleTomsone, L., Galoburda, R., Kruma, Z., Durrieu, V., & Cinkmanis, I. (2020). Microencapsulation of Horseradish (Armoracia rusticana L.) Juice Using Spray-Drying. Foods, 9(9), 1332. https://doi.org/10.3390/foods9091332