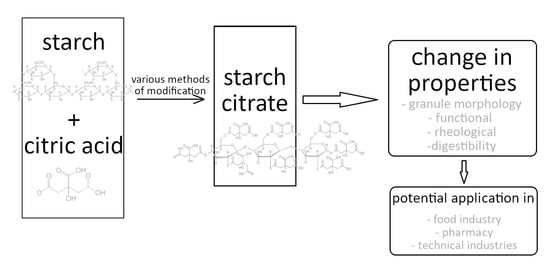
Production and Properties of Starch Citrates—Current Research
Abstract
1. Introduction
2. The Methods of Starch Citrate Preparation
3. Properties of Starch Citrate
4. Starch Citrates as Resistant Starch
5. The Application of Starch Citrate in Food Industry
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Tian, S.; Chen, Y.; Chen, Z.; Yang, Y.; Wang, Y. Preparation and characteristics of starch esters and its effects on dough physicochemical properties. J. Food Qual. 2018, 7. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, Z.; Lei, W.; Zhong, G. Synergistic effects of acetylated distarch adipate and sesbania gum on gelatinization and retrogradation of wheat starch. Int. J. Biol. Macromol. 2020, 156, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Golachowski, A.; Zięba, T.; Kapelko-Żeberska, M.; Drożdż, W.; Gryszkin, A.; Grzechac, M. Current research addressing starch acetylation. Food Chem. 2015, 176, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Colussi, R.; El Halal, S.L.M.; Pinto, V.Z.; Bartz, J.; Gutkoski, L.C.; Zavareze, E.R.; Dias, A.R.G. Acetylation of rice starch in an aqueous medium for use in food. LWT Food Sci. Technol. 2015, 62, 1076–1082. [Google Scholar] [CrossRef]
- Xie, X.S.; Liu, Q. Development and physicochemical characterization of new resistant citrate starch from different corn starches. Starch Stärke 2004, 56, 364–370. [Google Scholar] [CrossRef]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Citric acid: Emerging applications of key biotechnology industrial product. Chem. Cent. J. 2017, 11–22. [Google Scholar] [CrossRef]
- Zehra, N.; Ali, T.M.; Hasnain, A. Comparative study on citric acid modified instant starches (alcoholic alkaline treated) isolated from white sorghum and corn grains. Int. J. Biol. Macromol. 2020, 155, 1331–1341. [Google Scholar] [CrossRef]
- National Starch Products Inc. US-Patent 2.461.139, 1949. Available online: https://patentimages.storage.googleapis.com/b4/db/56/50b269ac7ba9ab/US2461139.pdf (accessed on 20 March 2020).
- National Starch and Chemical. Corp. US-Patent 2.935.510, 1960. Available online: https://patentimages.storage.googleapis.com/44/a7/cd/08cbecc4d8040f/US2935510.pdf (accessed on 20 March 2020).
- Niazi, B.K.M.; Broekhuis, A.A. Surface photo-crosslinking of plasticized thermoplastic starch films. Eur. Polym. J. 2015, 64, 229–243. [Google Scholar] [CrossRef]
- Ma, X.; Liu, X.; Anderson, D.P.; Chang, P.R. Modification of porous starch for the adsorption of heavy metal ions from aqueous solution. Food Chem. 2015, 181, 133–139. [Google Scholar] [CrossRef]
- Pornsuksomboon, K.; Holló, B.B.; Szécsényi, K.M.; Kaewtatip, K. Properties of baked foams from citric acid modified cassava starch and native cassava starch blends. Carbohydr. Polym. 2016, 136, 107–112. [Google Scholar] [CrossRef]
- Saliu, O.D.; Olatunji, G.A.; Olosho, A.I.; Adeniyi, A.G.; Azeh, Y.; Samo, F.T.; Adebayo, D.O.; Ajetomobi, O.O. Barrier property enhancement of starch citrate bioplastic film by an ammonium-thiourea complex modification. J. Saudi Chem. Soc. 2018, 23, 141–149. [Google Scholar] [CrossRef]
- Seligra, P.G.; Jaramillo, C.M.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch-glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Supriya, B.S.; Nagraja, P.; Byrappa, K. Hydrothermal synthesis and characterization of carbon spherees using citric-acid-catalyzed carbonization of starch. e-Polymers 2015, 15, 179–183. [Google Scholar] [CrossRef]
- Punia, S.; Siroha, A.K.; Sandhu, K.S.; Kaur, M. Rheological behavior of wheat starch and barley resistant starch (type IV) blends and their starch noodles making potential. Int. J. Biol. Macromol. 2019, 130, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Keenan, M.J.; Zhou, J.; Hegsted, M.; Pelkman, C.; Durham, H.A.; Coulon, D.B.; Martin, R.J. Role of resistant starch in improving gut health, adiposity, and insulin resistance. Adv. Nutr. 2015, 13, 198–205. [Google Scholar] [CrossRef] [PubMed]
- AACC. Approved Methods of the AACC, 10th ed.; Methods 46-13, 08-01, and 44-15; Cereals & Grace Association: St. Paul, MN, USA, 2000. [Google Scholar]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists International, 17th ed.; Method 991.43 Total Dietary Fiber. Enzymatic Gravimetric Method; Cereals & Grace Association: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Babu, A.S.; Parimalavalli, R.; Gaur, R.S. Effect of citric acid concentration and hydrolysis time on physicochemical properties of sweet potato starches. Int. J. Biol. Macromol. 2015, 80, 557–565. [Google Scholar] [CrossRef]
- Babu, A.S.; Parimalavalli, R.; Jagannadham, K.; Sudhakara Rao, J. Chemical and structural properties of sweet potato starch treated with organic and inorganic acid. J. Food Sci. Technol. 2015, 52, 5745–5753. [Google Scholar] [CrossRef]
- Huo, Y.; Zhang, B.; Niu, M.; Jia, C.; Zhao, S.; Huang, Q.; Du, H. An insight into the multi-scale structures and pasting behaviors of starch following citric acid treatment. Int. Biol. Macromol. 2018, 116, 793–800. [Google Scholar] [CrossRef]
- Martins, P.C.; Gutkoski, L.C.; Martins, V.G. Impact of acid hydrolysis and esterification process in rice and potato starch properties. Int. J. Biol. Macromol. 2018, 120, 959–965. [Google Scholar] [CrossRef]
- Hung, P.; Huong, N.T.M.; Phi, N.T.L.; Tien, N.N.T. Physicochemical characteristics and in vitro digestibility of potato and cassava starches under organic acid and heat-moisture treatments. Int. J. Biol. Macromol. 2017, 95, 299–305. [Google Scholar] [CrossRef]
- Hung, P.; Vien, N.L.; Phi, N.T.L. Resistant starch improvement of rice starches under a combination of acid and heat-moisture treatments. Food Chem. 2016, 191, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Fourati, Y.; Tarrés, Q.; Mutjé, P.; Boufi, S. PBAT/thermoplastic starch blends: Effect of compatiblizers on the rheological, mechanical and morphological properties. Carbohydr. Polym. 2018, 199, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Xu, H.; Kong, L.; Yang, Y. Non-toxic crosslinking of starch using polycarboxylic acids: Kinetic study and quantitative correlation of mechanical properties and crosslinking degrees. J. Polym. Environ. 2015, 23, 588–594. [Google Scholar] [CrossRef]
- Zhou, J.; Tong, J.; Su, X.; Ren, L. Hydrophobic starch nanocrystals preparations through crosslinking modification using citric acid. Int. J. Biol. Macromol. 2016, 91, 1186–1193. [Google Scholar] [CrossRef]
- Hong, J.S.; Chung, H.-J.; Lee, B.-H.; Kim, H.-S. Impact of static and dynamic modes of semi-dry heat reaction on thecharacteristics of starch citrates. Carbohyd. Polym. 2020, 233, 115853. [Google Scholar] [CrossRef]
- Kapelko-Żeberska, M.; Zięba, T.; Spychaj, R.; Gryszkin, A. Acetylated adipate of retrograded starch as RS3/4 type resistant strach. Food Chem. 2015, 188, 365–369. [Google Scholar] [CrossRef]
- Borries-Medrano, E.; Jaime-Fonseca, M.R.; Aguilar-Méndez, M.A.; García-Cruz, H.I. Addition of galactomannans and citric acid in corn starch processed by extrusion: Retrgradation and resistant starch studies. Food Hydrocoll. 2018, 83, 485–496. [Google Scholar] [CrossRef]
- Hedayati, S.; Niakousari, M. Microstructure, pasting and textural properties of wheat starch-corn starch citrate composites. Food Hydrocoll. 2018, 81, 1–5. [Google Scholar] [CrossRef]
- Hernandez-Jaimes, C.; Vernon-Carter, E.J.; Labato-Calleros, C.; Bello-Perez, L.A.; Alvarez-Ramirez, J. An electrochemical approach for citric acid treatment of corn starch granules. Starch Stärke 2016, 68, 558–567. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.S. Impact of reactive extrusion parameters on the resistant contents and pasting properties of starch citrates. Food Eng. Prog. 2015, 3, 193–200. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.S. Influence of semi-dry heating reaction conditions on resistant starch citrates. Food Eng. Prog. 2015, 4, 313–319. [Google Scholar] [CrossRef]
- Kim, S.H.; Min, S.C. Effects of microwave-discharged cold plasma on synthesis and characteristics of citrate derivatives of corn starch granules. Food Sci. Biotechnol. 2017, 26, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Li, M.N.; Xie, Y.; Chen, H.Q.; Zhang, B. Effects of heat-moisture treatment after citric acid esterification on structural properties and digestibility of wheat starch, A- and B- type starch granules. Food Chem. 2019, 272, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Butt, N.A.; Ali, T.M.; Hasnain, A. Rheological characterization of cold water soluble rice (Oryza sativa) starch lactates and citrates prepared via alcoholic-alkaline method. Int. J. Biol. Macromol. 2019, 123, 558–568. [Google Scholar] [CrossRef]
- Butt, N.A.; Ali, T.M.; Hasnain, A. Rice starch citrates and lactates: A comperative study on hot water and cold water swelling starches. Int. J. Biol. Macromol. 2019, 127, 107–117. [Google Scholar] [CrossRef]
- Harder, H.; Khol-Parisini, A.; Zebeli, Q. Treatments with organic acids and pullulanase differently affect resistant starch and fiber composition in flour of various barley genotypes (Hordeum vulgare L.). Starch Stärke 2015, 67, 512–520. [Google Scholar] [CrossRef]
- Kapelko-Żeberska, M.; Buksa, K.; Szumny, A.; Zięba, T.; Gryszkin, A. Analysis of molecular structure of starch citrate obtained by a well-stablished method. LWT Food Sci. Technol. 2016, 69, 334–341. [Google Scholar] [CrossRef]
- Kapelko-Żeberska, M.; Zięba, T.; Pietrzak, W.; Gryszkin, A. Effect of citric acid esterification conditions on the properties of the obtained resistant starch. Int. J. Food Sci. Technol. 2016, 51, 1647–1654. [Google Scholar] [CrossRef]
- Remya, R.; Jyothi, A.N.; Sreekumar, J. Effect of chemical modification with citric acid on the physicochemical properties and resistant starch formation in different starches. Carbohydr. Polym. 2018, 202, 29–38. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, Y.K.; Chang, Y.H. Structure and digestibility properties of resistant rice starch cross-linked with citric acid. Int. J. Food Proper. 2017, 52, 52166–52177. [Google Scholar] [CrossRef]
- Ye, J.; Luo, S.; Huang, A.; Chen, J.; Liu, C.; McClements, J. Synthesis and characterization of citric acid esterified rice starch by reactive extrusion: A new method of producing resistant starch. Food Hydrocoll. 2019, 92, 135–142. [Google Scholar] [CrossRef]
- Mei, J.Q.; Zhou, D.N.; Jin, Z.; Xu, X.; Chen, H. Effects of citric acid esterification on digestibility, structural and physicochemical properties of cassava starch. Food Chem. 2015, 187, 378–384. [Google Scholar] [CrossRef]
- Srikaeo, K.; Hao, P.T.; Lerdluksamee, C. Effects of heating temperatures and acid concentrations on physicochemical properties and starch digestibility of citric acid esterified tapioca starches. Starch Stärke 2019, 71. [Google Scholar] [CrossRef]
- Sánchez-Rivera, M.; Nūnez-Santiago, M.D.C.; Bello-Pérez, L.A. Citric acid esterification of unripe plantaion flour: Physicochemical properties and starch digestibility. Starch Stärke 2017, 69, 1700019. [Google Scholar] [CrossRef]
- Pachuau, L.; Dutta, R.S.; Devi, T.B.; Deka, D.; Hauzel, L. Taro starch (Colocasia esculenta) and citric acid modified taro starch ass tablet disintegrating agents. Int. J. Biol. Macromol. 2018, 118, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Falade, K.O.; Ayetigbo, E.O. Effects of annealing, acid hydrolysis and citric acid modifications on physical and functional properties of starches from four yam (Dioscorea spp.) cultivars. Food Hydrocoll. 2015, 43, 529–539. [Google Scholar] [CrossRef]
- Falade, K.O.; Ayetigbo, E.O. Effects of tempering (annealing), acid hydrolysis, low-citric acid substitution on chemical and physicochemical properites of starches of four yam (Dioscorea spp.) cultivars. J. Food Sci. Technol. 2017, 54, 1455–1466. [Google Scholar] [CrossRef]
- Xia, H.; Li, Y.; Gao, Q. Preparation and properties of RS4 citrate sweet potato starch by heat-moisture treatment. Food Hydrocoll. 2016, 53, 172–178. [Google Scholar] [CrossRef]
- Alimi, B.A.; Workneh, T.S. Structural and physicochemical properties of heat moisture treated and citric acid modified acha and iburu starches. Food Hydrocoll. 2018, 81, 449–455. [Google Scholar] [CrossRef]
- Gani, A.; Amreen, J.; Shah, A.; Masoodi, F.A.; Mudasir, A.; Ashwar, B.A.; Akhter, R.; Wani, I.A. Physico-chemical, functional and structural properties of RS3/RS4 from kidney bean (Phaseolus vulgaris) cultivars. Int. J. Biol. Macromol. 2016, 87, 514–521. [Google Scholar] [CrossRef]
- Wilpiszewska, K.; Antosik, A.K.; Spychaj, T. Novel hydrophilic carboxymethyl starch/montmorillonite nanocomposite films. Carbohydr. Polym. 2015, 128, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Saikia, C.; Das, M.K.; Ramteke, A.; Maji, T.K. Effect of cross linker on drug delivery properties of curcumin loaded starch coated iron oxide nanoparticles. Int. J. Biol. Macromol. 2016, 93, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Abhari, N.; Madadlou, A.; Dini, A.; Naveh, O.H. Textural and cargo release attributes of trisodium citrate cross-linked starch hydrogel. Food Chem. 2017, 214, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, K.Y.; Lee, H.G. Effect of different pH conditions on the in vitro digestibility and physicochemical properties of citric acid-treated potato starch. Int. J. Biol. Macromol. 2018, 107, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Agboola, S.O.; Akingbala, J.O.; Oguntimein, G.B. Production of low substituted cassava starch acetates and citrates. Starch Stärke 1991, 43, 13–15. [Google Scholar] [CrossRef]
- Oltramari, K.; Madrona, G.S.; Neto, A.M.; Morais, G.R.; Baesso, M.L.; Cássia Bergamasco, R.; Moraes, F.F. Citrate esterified cassava starch: Preparation, physicochemical characterisation, and application in dairy beverages. Starch Stärke 2017, 69, 1700044. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, L.; McCarthy, O.J. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications—A review. Food Hydrocol. 2007, 21, 1–22. [Google Scholar] [CrossRef]
- Kim, H.R.; Hong, J.S.; Ryu, A.-R.; Choi, H.-D. Combination of rice varieties and cooking methods resulting in a high content of resistant starch. Cereal Chem. 2020, 97, 149–157. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Kong, J.; Wang, R.; Liu, M.; Strappe, P.; Blanchard, C.; Zhou, Z. Citrate esterification of debranched waxy maize starch: Structural, physicochemical and amylolysis properties. Food Hydrocol. 2020, 104, 105704. [Google Scholar] [CrossRef]
- Zavareze, E.R.; Dias, A.R.G. Impact of heat-moisture treatment and annealing in starches: A review. Carbohydr. Polym. 2011, 83, 317–328. [Google Scholar] [CrossRef]
- Khalili, L.; Amini, A. Resistant starch in food industry. Polysacch. Bioact. Biotechnol. 2015, 663–673. [Google Scholar] [CrossRef]
- Shortt, C.; Hasselwander, O.; Meynier, A.; Nauta, A.; Fernández, E.N.; Putz, P.; Rowland, I.; Swann, J.; Türk, J.; Vermeiren, J.; et al. Systematic review of the effects of the intestinal microbiota on selected nutrients and non-nutrients. Eur. J. Nutr. 2018, 57, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.C.; Johansson-Boll, E.V.; Björck, I.M. Increased gut hormones and insulin sensitivity index following a 3-d intervention with a barley kernel-based product: A randomised cross-over study in healthy middleaged subjects. Br. J. Nutr. 2015, 114, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Suzuki, M.; Yamaguchi, Y.; Egashira, Y. Effects of resistant maltodextrin on bowel movements: A systematic review and meta-analysis. Clin. Exp. Gastroenterol. 2018, 11, 85–96. [Google Scholar] [CrossRef]
- Karimi, P.; Farhangi, M.A.; Sarmadi, B.; Gargari, B.P.; Javid, A.Z.; Pouraghaei, M.; Dehghan, P. The therapeutic potential of resistant starch in modulation of insulin resistance, endotoxemia, oxidative stress and antioxidant biomarkers in women with type 2 diabetes: A randomized controlled clinical trial. Ann. Nutr. Metab. 2016, 68, 85–93. [Google Scholar] [CrossRef]
- Messina, V. Nutritional and health benefits of dried beans. Am. J. Clin. Nutr. 2014, 100, 437S–442S. [Google Scholar] [CrossRef]
- Koh, G.Y.; Rowling, M.J. Resistant starch as a novel dietary strategy to maintain kidney health in diabetes mellitus. Nutr. Rev. 2017, 75, 350–360. [Google Scholar] [CrossRef]
- Bernstein, A.M.; Titgemeier, B.; Kirkpatrick, K.; Golubic, M.; Roizen, M.F. Major cereal grain fibers and psyllium in relation to cardiovascular health. Nutrients 2013, 5, 1471–1487. [Google Scholar] [CrossRef]
- Yang, X.; Darko, K.O.; Huang, Y.; He, C.; Yang, H.; He, S.; Li, J.; Li, J.; Hocher, B.; Yin, Y. Resistant starch regulates gut microbiota: Structure, biochemistry and cell signalling. Cell Physiol. Biochem. 2017, 42, 306–318. [Google Scholar] [CrossRef]
- Salonen, A.; Lahti, L.; Salojärvi, J.; Holtrop, G.; Korpela, K.; Duncan, S.H.; Date, P.; Farquharson, F.; Johnstone, A.M.; Lobley, G.E.; et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014, 8, 2218–2230. [Google Scholar] [CrossRef]
- Upadhyaya, B.; McCormack, L.; Fardin-Kia, A.R.; Juenemann, R.; Nichenametla, S.; Clapper, J.; Specker, B.; Dey, M. Impact of dietary resistant starch type 4 on human gut microbiota and immunometabolic functions. Sci. Rep. 2016, 6, 28797. [Google Scholar] [CrossRef] [PubMed]
- Sorndech, W.; Rodtong, S.; Blennow, A.; Tonga, S. Impact of resistant maltodextrins and resistant starch on human gut microbiota and organic acid production. Starch Stärke 2019, 71, 1800231. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Tsai, S.-J.; Sun, N.-N.; Dai, F.-J.; Yu, P.-H.; Chen, Y.-C.; Chau, C.-F. Enhanced thermal stability of green banana starch by heat-moisture treatment and its ability to reduce body fat accumulation and modulate gut microbiota. Int. J. Biol. Macromol. 2020, 160, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Seo, J.-M.; Kim, H.-S.; Seo, D.-H.; Kim, J.; Choi, H.; Lee, B.-H. Citric-acid treatment during rice processing increases the level of slowly digestible starch with a potential to regulate the post-prandial blood glucose level. J. Cereal Sci. 2019, 89, 102821. [Google Scholar] [CrossRef]
- Shaikh, F.; Ali, T.-M.; Mustafa, G.; Hasnain, A. Comparative study on effects of citric and lactic acid treatment on morphological, functional, resistant starch fraction and glycemic index of corn and sorghum starches. Int. J. Biol. Macromol. 2019, 15, 314–327. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Chang, Y.H. Structural and in vitro digestibility properties of esterified maca starch with citric acid and its application as an oil-in-water (O/W) pickering emulsion stabilizer. Macromolecules 2019, 134, 798–806. [Google Scholar] [CrossRef]
- Zdybel, E.; Zięba, T.; Tomaszewska-Ciosk, E.; Rymowicz, W. Effect of the esterification of starch with a mixture of carboxylic acids from Yarrowia lipolitica fermentation broth on its selected properties. Polymers 2020, 12, 1383. [Google Scholar] [CrossRef]
- Jarosz, M. Normy żYwienia Dla Populacji Polskiej; Instytut Żywności i Żywienia: Warsaw, Poland, 2017. [Google Scholar]
- Zhou, X.H.; Dong, Y.; Zhao, Y.S.; Xiao, X.; Wang, Y.; He, Y.Q.; Liu, Q.Q. A three generation reproduction study with Sprague–Dawley rats consuming high-amylose transgenic rice. Food Chem. Toxicol. 2014, 74, 20–27. [Google Scholar] [CrossRef]
- Ruiz, M.S.A.; Espinosa, M.D.B.; Fernández, C.J.C.; Rubia, A.J.L.; Ayllón, F.S.; García, M.A.; Santamaría, C.G.; Román, F.J.L. Digestion-resistant maltodextrin effects on colonic transit time and stool weight: A randomized controlled clinical study. Eur. J. Nutr. 2016, 55, 2389–2397. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, 33–50. [Google Scholar]
- Zięba, T.; Szumny, A.; Kapelko, M. Properties of retrograded and acetylated starch preparations: Part 1. Structure, susceptibility to amylase, and pasting characteristics. LWT Food Sci. Technol. 2011, 44, 1314–1320. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Wepner, B.; Berghofer, E.; Miesenberger, E. Citrate starch—Application as resistant starch in different food systems. Starch Stärke 1999, 51, 354–361. [Google Scholar] [CrossRef]
| Method | Type | Degree of Substitution | References |
|---|---|---|---|
| Dry | Oven | 0.01–0.42 | [11,35,37,38,39,41,42,43,46,47,52,58] |
| Extrusion | 0.01–0.03 | [31,45] | |
| Microwave with plasma | 0.012–0.015 | [36] | |
| Wet | Water suspension | 0.002–0.023 | [50,51,53,54,57,59] |
| Electrochemical | 0.02–0.12 | [55,56] |
| Starch Source | Parameters Studied | Observed Results | Ref. |
|---|---|---|---|
| waxy maize | acid concentration 0–2.5%, seven days storage in 4 °C 10% concentration of guar gum and tara gum screw compression ratio 1:1, extrussion temperature 70 °C, 85 °C, 140 °C | freeze–thaw stability decrease of digestion | [31] |
| acid concentration 1.0 M open circuit potential 1–1.2 V number of 0.5 Hz triangular-shaped potential cycle (50, 100, 200, 400) | reduced crystallinity freeze–thaw stability decrease of digestion | [33] | |
| acid concentration 10–40% screw compression ratio 1:1, temperature 130–190 °C, twin screw | lower swelling power decrease of viscosity decrease of digestion | [34] | |
| acid concentration 10–20%, MCP time 20 min, power 900 W oven temperature 135 °C, oven time 1 h, | higher swelling power and solubility increase of viscosity no changes in crystallinity | [36] | |
| wheat | acid concentration 40% temperature of oven 130 °C, time of oven 5 h HMT temperature 100 °C, HMT time 3 h | change the relative crystallinity absent of gelatinization peak | [37] |
| acid concentration 0–40%, temperature 140 °C, time 7 h | lower swelling power and solubility decrease of viscosity | [32] | |
| potato | acid concentration 10%, 20%, 40% temperature 100 °C, 130 °C, 160 °C, time 3 h | decrease of solubility decrease in phase transition absent of gelatinization peak | [42] |
| acid concentration 20%, 40%, 60% temperature 130 °C, time 2 h | lower swelling power change the relative crystallinity increase water sorption | [43] | |
| acid concentration 10%, 20%, 30% temperature 150 °C, time 5 h pH = 3.5, 4.5, 5.5 | lower swelling power change the relative crystallinity incapable of paste formation | [58] | |
| sweet potato | acid concentration 10–60% temperature 140 °C, time 4 h | lower swelling power change the relative crystallinity decrease enthalpy of gelatinization decrease of viscosity | [52] |
| rice | acid concentration 1%, 10%, 30% temperature 140 °C, time 4 h | lower swelling power change the relative crystallinity decrease of phase transition absent of gelatinization peak decrease of viscosity clarity of starch paste | [44] |
| cassava | acid concentration 10–40% temperature 130 °C, time 5 h | lower swelling power and solubility decrease of phase transition absent of gelatinization peak | [46] |
| acid concentration 10% 30%, 50% temperature 100 °C, 120 °C, 140 °C, time 5 h | decrease of phase transition absent of gelatinization peak lower digestibility | [47] | |
| acid concentration 40% temperature 135 °C, time 5 h | lower swelling power and solubility change the relative crystallinity incapable of paste formation | [60] | |
| taro | acid concentration 40% temperature 100 °C, time 0.5 h | decrease of phase transition absent of gelatinization peak decrease of viscosity | [49] |
| yam | acid concentration 15% water:starch suspension ratio 400:300 (v/w) temperature 27 ± 2 °C, time 5 h | increase of water sorption decrease in phase transition foam stability | [50] |
| acid concentration 15%, water:starch suspension ratio 400:300 (v/w) temperature 28 ± 2 °C, time 5 h | lower swelling power higher solubility decrease of viscosity clarity of starch paste | [51] | |
| acha and iburu | acid concentration 15%, water:starch suspension ratio 400:300 (v/w) temperature room (25 °C), time 5 h | increase of water sorption decrease in phase transition temperature decrease of viscosity decrease enthalpy of gelatinization | [53] |
| kidney bean | acid concentration 15% water:starch suspension ratio 400:300 (v/w) temperature 27 ± 2 °C, time 5 h | lower swelling power and solubility decrease in phase transition temperature decrease of viscosity | [54] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golachowski, A.; Drożdż, W.; Golachowska, M.; Kapelko-Żeberska, M.; Raszewski, B. Production and Properties of Starch Citrates—Current Research. Foods 2020, 9, 1311. https://doi.org/10.3390/foods9091311
Golachowski A, Drożdż W, Golachowska M, Kapelko-Żeberska M, Raszewski B. Production and Properties of Starch Citrates—Current Research. Foods. 2020; 9(9):1311. https://doi.org/10.3390/foods9091311
Chicago/Turabian StyleGolachowski, Antoni, Wioletta Drożdż, Magdalena Golachowska, Małgorzata Kapelko-Żeberska, and Bartosz Raszewski. 2020. "Production and Properties of Starch Citrates—Current Research" Foods 9, no. 9: 1311. https://doi.org/10.3390/foods9091311
APA StyleGolachowski, A., Drożdż, W., Golachowska, M., Kapelko-Żeberska, M., & Raszewski, B. (2020). Production and Properties of Starch Citrates—Current Research. Foods, 9(9), 1311. https://doi.org/10.3390/foods9091311