A Real-Time Quantitative PCR Method Specific for Detection and Quantification of the First Commercialized Genome-Edited Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Canola Germplasm
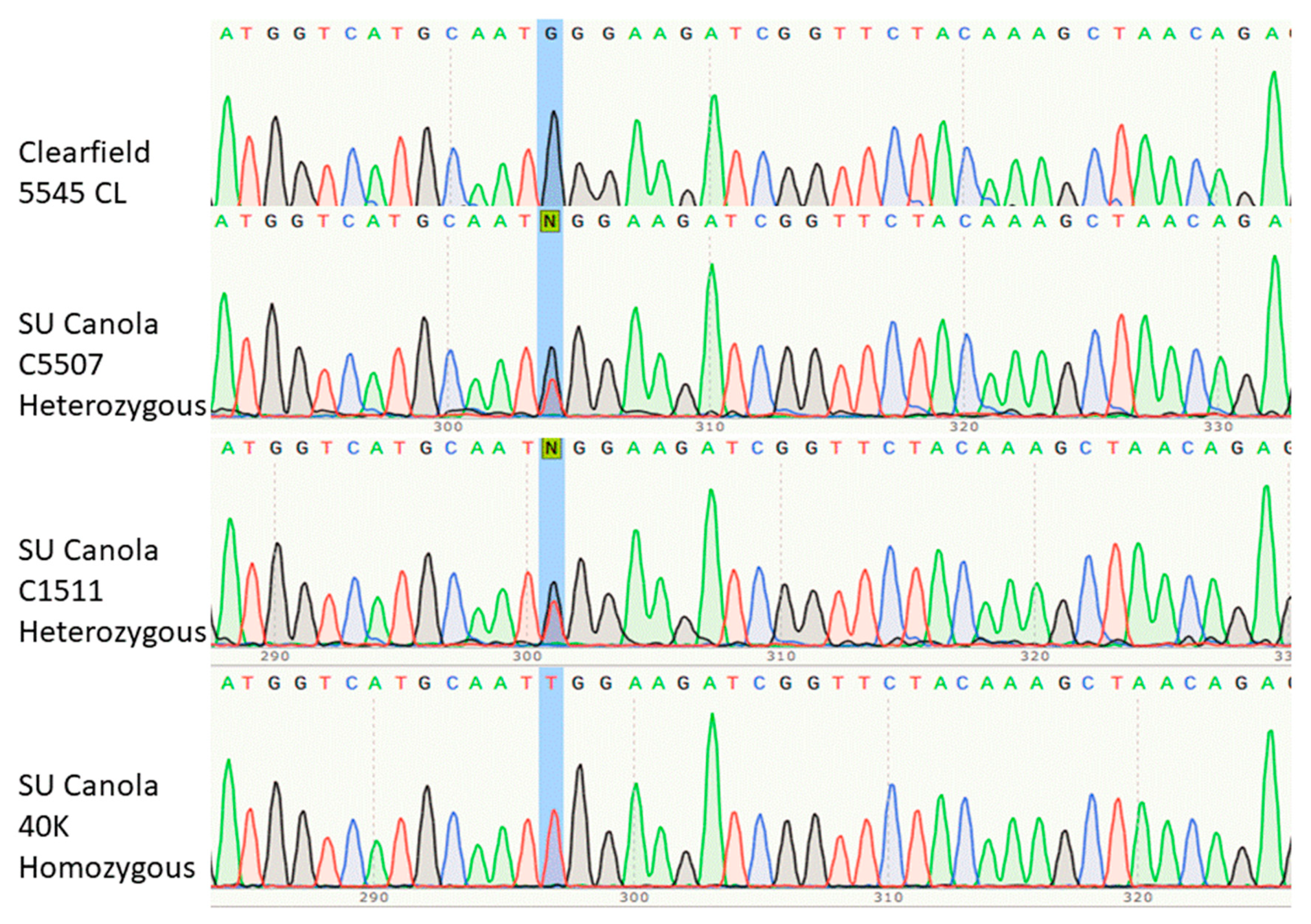
2.2. Sanger Sequencing
2.3. DNA Extraction
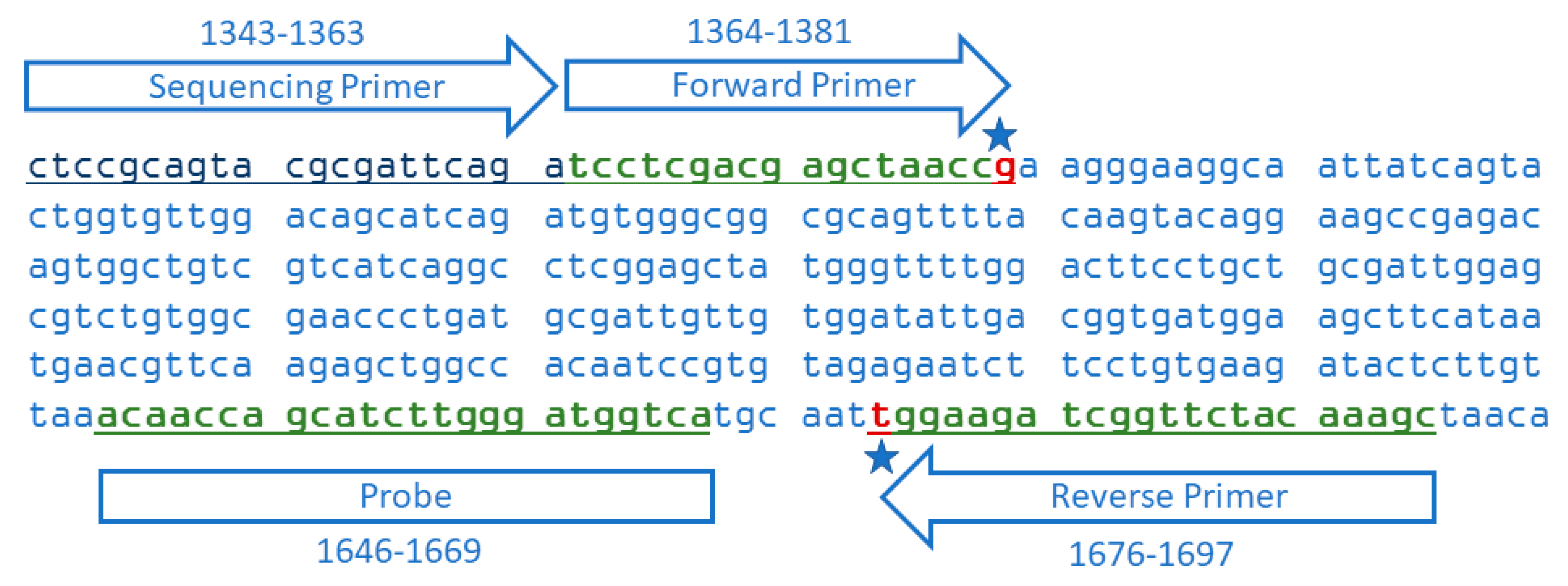
2.4. Oligonucleotide Primers and Probes
2.5. Real-Time Quantitative PCR
2.6. Independent Method Validation
2.7. Intellectual Property
3. Results
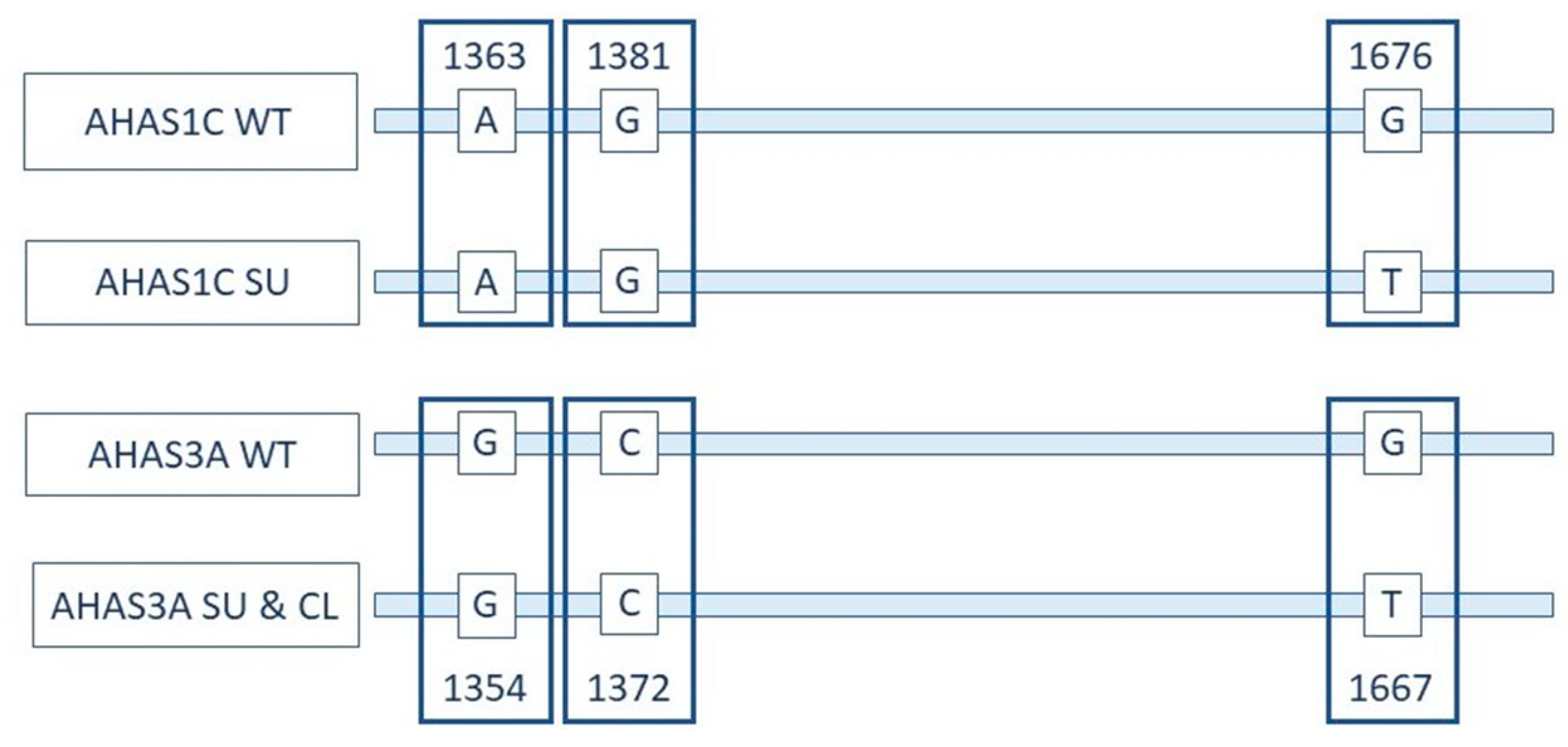
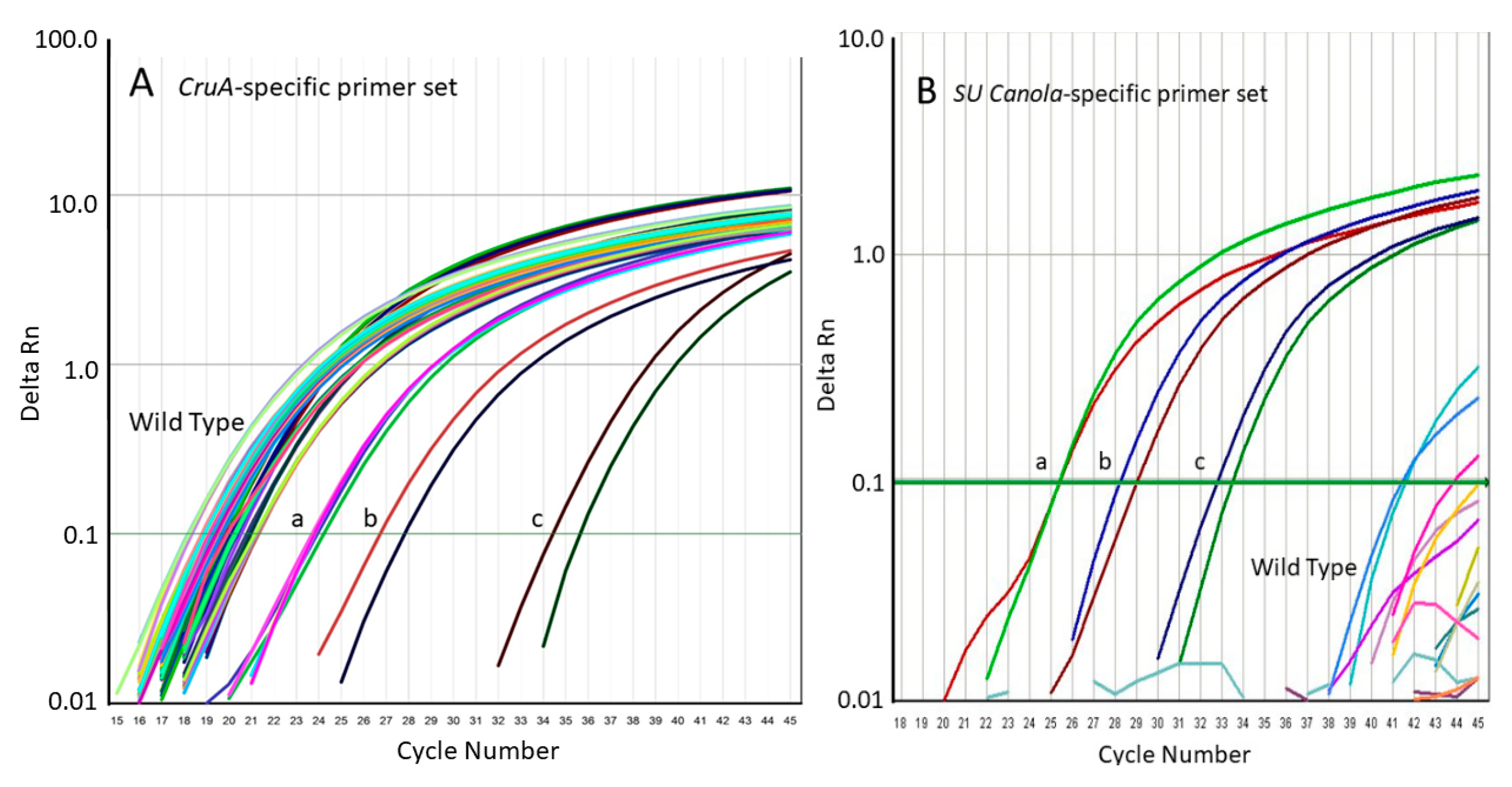
3.1. Development of a qPCR Method Specific for SU Canola
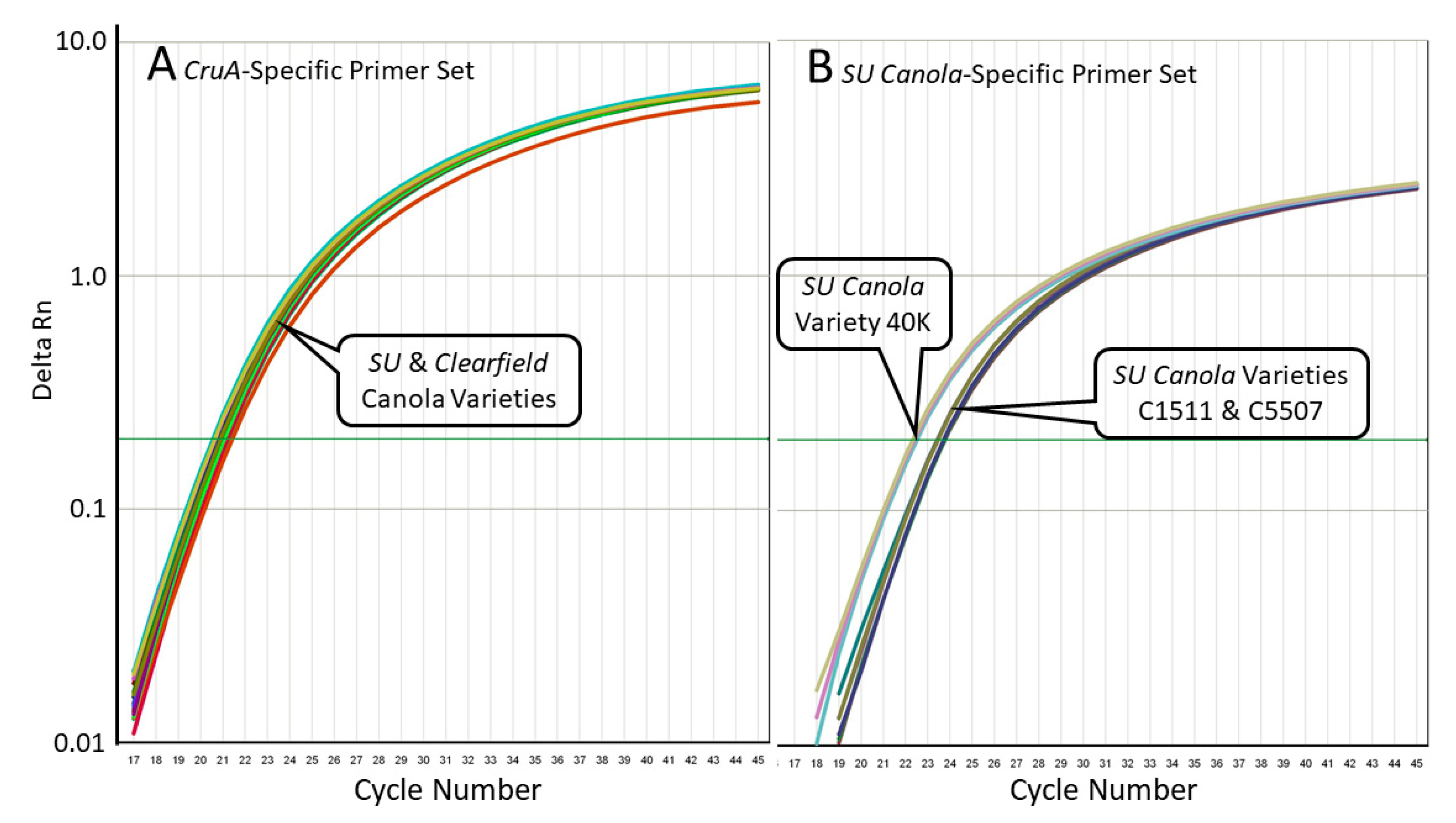
3.2. Specificity of the SU Canola-Specific qPCR Method
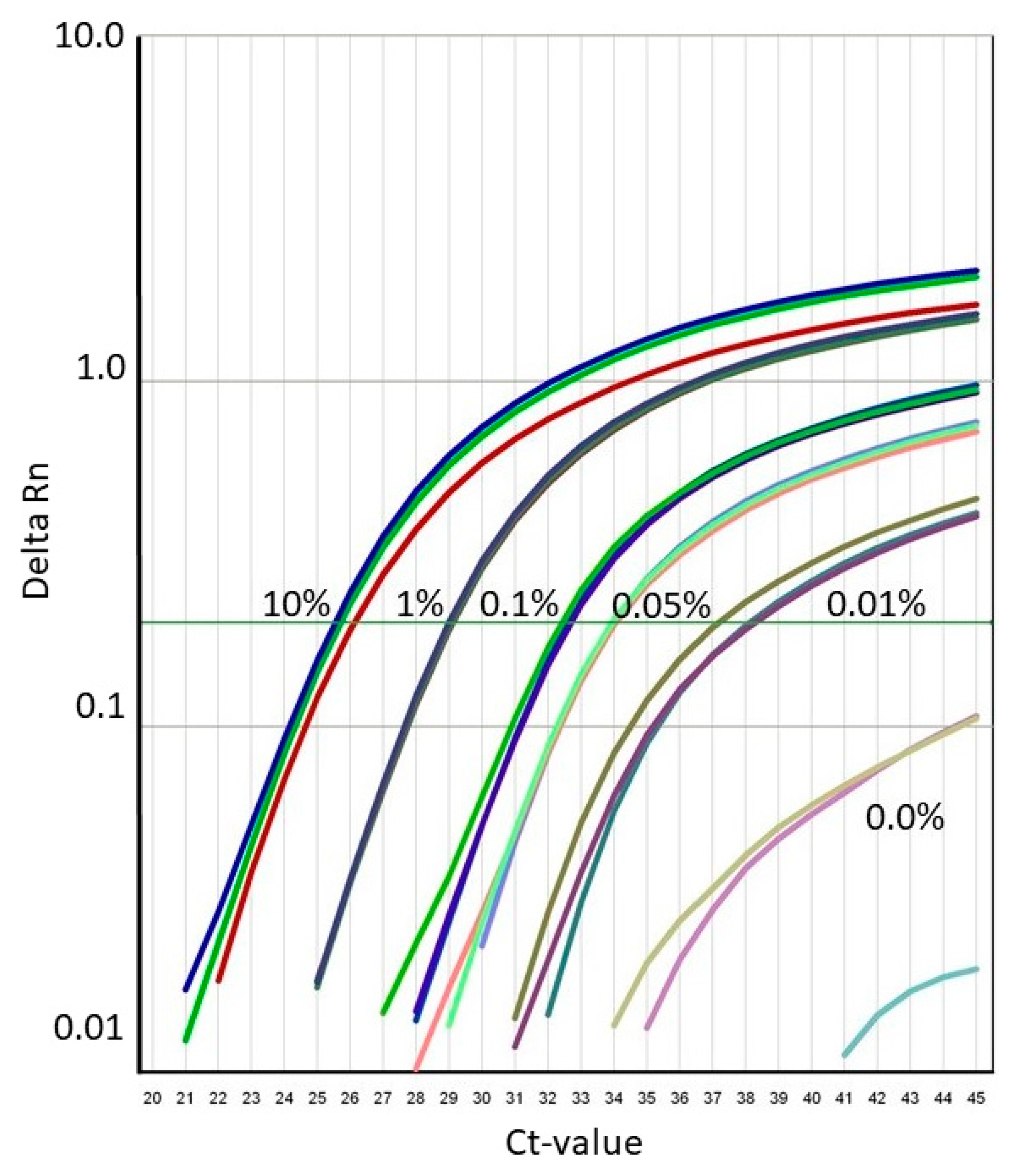
3.3. Precision, Trueness, Limit of Quantitation and Limit of Detection of the SU Canola Method
3.4. Independent Validation of the SU Canola-Specific qPCR Method
4. Discussion
“The method(s) shall be specific to the transformation event (hereafter referred to as ‘event-specific’) and thus shall only be functional with the genetically modified organism or genetically modified based product considered and shall not be functional if applied to other transformation events already authorized; otherwise the method cannot be applied for unequivocal detection/identification/quantification.”—Section 3.1.C.1 of Annex III to Regulation (EU) No 503/2013
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anon Cibus—Value-Enhancing Traits for Globally Accepted Crops. Available online: https://cibus.com/crops.php (accessed on 16 May 2020).
- Anon Products Calyxt. Available online: https://calyxt.com/our-products/ (accessed on 16 May 2020).
- Duensing, N.; Sprink, T.; Parrott, W.A.; Fedorova, M.; Lema, M.A.; Wolt, J.D.; Bartsch, D. Novel Features and Considerations for ERA and Regulation of Crops Produced by Genome Editing. Front. Bioeng. Biotechnol. 2018, 6, 79. [Google Scholar] [CrossRef] [Green Version]
- European Court of Justice C-528/16-Judgement of 25 July 2018 on New Mutagenesis Techniques. Available online: http://curia.europa.eu/juris/document/document.jsf?text=&docid=204387&pageIndex=0&doclang=EN&mode=lst&dir=&occ=first&part=1&cid=138460 (accessed on 16 May 2020).
- Commission Declaration Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC. Off. J. Eur. Communities 2001, L106, 39.
- Scientific Advice Mechanism of the European Commission. New Techniques in Agricultural Biotechnology—Explanatory Note; Publications Office of the European Union: Brussels, Belgium, 2017; ISBN 978-92-79-66222-5. [Google Scholar]
- Scientific Advice Mechanism of the European Commission. A Scientific Perspective on the Regulatory Status of Products Derived from Gene Editing and the Implications for the GMO Directive. Available online: https://op.europa.eu/en/publication-detail/-/publication/a9100d3c-4930-11e9-a8ed-01aa75ed71a1/language-en/format-PDF/source-94584603 (accessed on 19 January 2020).
- Lusser, M.; Parisi, C.; Plan, D.; Rodriguez-Cerezo, E. New Plant Breeding Techniques: State-of-the-Art and Prospects for Commercial Development; Joint Research Centre of the European Union: Brussels, Belgium, 2011; ISBN 978-92-79-19715-4. [Google Scholar]
- COGEM. The Status of Oligonucleotides within the Context of Site-Directed Mutagenesis. Available online: https://cogem.net/en/publication/the-status-of-oligonucleotides-within-the-context-of-site-directed-mutagenesis/ (accessed on 16 May 2020).
- Advisory Committee on Releases to the Environment. Advice on a Plant Breeding Technique Involving Oligo-Directed Mutagenesis: RTDSTM. Available online: https://www1.health.gov.au/internet/ogtr/publishing.nsf/Content/8884A10B0BA5CF42CA2580B10016087D/$File/Cibus%20-%203.pdf (accessed on 16 May 2020).
- Advisory Committee on Releases to the Environment. Advice on An Application for Deliberate Release of a GMO for Research and Development Purposes. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/302572/ACRE_advice_FINAL.pdf (accessed on 16 May 2020).
- Angevin, F.; Bagnis, C.; Collonnier, C.; Félix, A.-M.; Garric, J. Scientific Opinion on New Plant Breeding Techniques. Available online: http://www.hautconseildesbiotechnologies.fr/en (accessed on 16 May 2020).
- Convention on Biological Diversity Report of the Ad Hoc Technical Expert Group on Synthetic Biology, Montreal, Canada, 5–8 December 2017. Available online: https://www.cbd.int/doc/c/aa10/9160/6c3fcedf265dbee686715016/synbio-ahteg-2017-01-03-en.pdf (accessed on 16 May 2020).
- Convention on Biological Diversity Report of the Ad Hoc Technical Expert Group on Synthetic Biology, Montreal Canada, 4–7 June 2019. Available online: https://www.cbd.int/doc/c/b2bb/cf58/b09729bb00be6abf72325a1a/synbio-ahteg-2019-01-03-en.pdf (accessed on 16 May 2020).
- CropLife. International Technical Summaries of New Plant Breeding Techniques-NBTs. Available online: https://croplife.org/wp-content/uploads/pdf_files/Technical-Summary-of-NBTs_final.pdf (accessed on 16 May 2020).
- European Seed Association. Plant Breeding Innovation Applying the Latest Plant Breeding Methods for the Benefit of Sustainable Agriculture, Consumers and Society. Available online: https://www.euroseeds.eu/app/uploads/2019/07/18.1010-Euroseeds-PBI-Position-1.pdf (accessed on 16 May 2020).
- Bioökonomierat Genome Editing: Europe Needs New Genetic Engineering Legislation. Available online: https://biooekonomierat.de/fileadmin/Publikationen/berichte/BOER-Memo_Genome-Editing_ENG.pdf (accessed on 16 May 2020).
- Birner, R.; Bock, R.; Dederer, H.-G.; Friedrich, B.; Fritsch, J. Towards a Scientifically Justified, Differentiated Regulation of Genome Edited Plants in the EU; Nationale Akademie der Wissenschaften Leopoldina: Halle, Germany; Deutsche Forschungsgemeinschaft: Bonn, Germany; Union der Deutschen Akademien der Wissenschaften: Mainz, Germany, 2019; ISBN 978-3-8047-4064-8. [Google Scholar]
- European Academies Science Advisory Council New Breeding Techniques—Scientific Potential and Regulation. Available online: https://easac.eu/fileadmin/PDF_s/reports_statements/Planting_the_Future/New_breeding_techniques_-_EASAC_statement_July12.pdf (accessed on 16 May 2020).
- Purnhagen, K.P.; Kok, E.; Kleter, G.A.; Schebesta, H.; Visser, R.G.F.; Wesseler, J. EU court casts new plant breeding techniques into regulatory limbo. Nat. Biotechnol. 2018, 36, 799–800. [Google Scholar] [CrossRef]
- Genome Editing: Scientific Opportunities, Public Interests and Policy Options in the European Union; European Academies Science Advisory Council; Deutsche Akademie der Naturforscher Leopoldina (Eds.) EASAC Policy Report; EASAC Secretariat, Deutsche Akademie der Naturforscher Leopoldina, German National Academy of Sciences: Halle, Germany, 2017; ISBN 978-3-8047-3727-3. [Google Scholar]
- Eckerstorfer, M.; Dolezel, M.; Heissenberger, A.; Miklau, M.; Reichenbecher, W.; Steinbrecher, R.A.; Waßmann, F. An EU Perspective on Biosafety Considerations for Plants Developed by Genome Editing and Other New Genetic Modification Techniques (nGMs). Front. Bioeng. Biotechnol. 2019, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Eckerstorfer, M.F.; Engelhard, M.; Heissenberger, A.; Simon, S.; Teichmann, H. Plants Developed by New Genetic Modification Techniques—Comparison of Existing Regulatory Frameworks in the EU and Non-EU Countries. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaart, J.G.; Visser, R.G.F. Novel Plant Breeding Techniques Consequences of New Genetic Modification-Based Plant Breeding Techniques in Comparison to Conventional Plant Breeding. Available online: https://library.wur.nl/WebQuery/wurpubs/fulltext/137009 (accessed on 19 January 2020).
- Advisory Committee on Releases to the Environment ACRE Advice: New Techniques Used in Plant Breeding. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/239542/new-techniques-used-in-plant-breeding.pdf (accessed on 10 May 2020).
- Broeders, S.R.M.; Keersmaecker, S.C.; Roosens, N.H.C. How to Deal with the Upcoming Challenges in GMO Detection in Food and Feed. J. Biomed. Biotechnol. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Bertheau, Y. New Breeding Techniques: Detection and Identification of the Techniques and Derived Products. In Reference Module in Food Science, Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 320–336. [Google Scholar] [CrossRef]
- European Network of GMO Laboratories Detection of Food and Feed Plant Products Obtained by New Mutagenesis Techniques. Available online: https://gmo-crl.jrc.ec.europa.eu/doc/JRC116289-GE-report-ENGL.pdf (accessed on 16 May 2020).
- Grohmann, L.; Keilwagen, J.; Duensing, N.; Dagand, E.; Hartung, F.; Wilhelm, R.; Bendiek, J.; Sprink, T. Detection and Identification of Genome Editing in Plants: Challenges and Opportunities. Front. Plant Sci. 2019, 10, 236. [Google Scholar] [CrossRef] [Green Version]
- Health Canada Novel Food Information—Cibus Canola Event 5715 (Imidazolinone and Sulfonylurea Herbicide Tolerant). Available online: http://www.hc-sc.gc.ca/fn-an/gmf-agm/appro/canola-5715-eng.php (accessed on 23 May 2016).
- European Commission. Commission Implementing Regulation (EU) No 503/2013 of 3 April 2013 on applications for authorisation of genetically modified food and feed in accordance with Regulation (EC) No 1829/2003 of the European Parliament and of the Council and amending Commission Regulations (EC) No 641/2004 and (EC) No 1981/2006Text with EEA relevance. Off. J. Eur. Union 2013, L157(46), 1–48. [Google Scholar]
- European Parliament and The Council Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 Concerning the Traceability and Labelling of Genetically Modified Organisms and the Traceability of Food and Feed Products Produced from Genetically Modified Organisms and Amending Directive 2001/18/EC. Off. J. Eur. Union 2003, L268(46), 1–24.
- Grohmann, L.; Broll, H.; Dagand, E.; Hildebrandt, S.; Hubert, P.; Kiesecker, H.; Lieske, K.; Made, D.; Mankertz, J.; Reiting, R.; et al. Guidelines for the Single-Laboratory Validation of Qualitative Real-Time PCR Methods; Federal Office of Consumer Protection and Food Safety [Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (BVL)]: Braunschweig, Germany, 2016. [Google Scholar]
- Hougs, L.; Gatto, F.; Goerlich, O.; Grohmann, L.; Lieske, K.; Mazzara, M.; Narendja, F.; Ovesna, J.; Papazova, N.; Scholtens, I.M.J.; et al. Verification of Analytical Methods. In Testing and Analysis of GMO-Containing Foods and Feed; Mahgoub, S.E.O., Nollet, L.M.L., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 245–266. ISBN 978-1-315-17859-2. [Google Scholar]
- Hattori, J.; Rutledge, R.G.; Miki, B.L.; Baum, B.R. DNA sequence relationships and origins of acetohydroxy acid synthase genes of Brassica napus. Can. J. Bot. 1992, 70, 1957–1963. [Google Scholar] [CrossRef]
- Hattori, J.; Brown, D.; Mourad, G.; Labbé, H.; Ouellet, T.; Sunohara, G.; Rutledge, R.; King, J.; Miki, B. An acetohydroxy acid synthase mutant reveals a single site involved in multiple herbicide resistance. Mol. Genet. Genom. 1995, 246, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Anon, S.U. Canola Entry in EUginius Database. Available online: https://euginius.eu/euginius/pages/gmo_detail.jsf?gmoname=5715 (accessed on 10 May 2020).
- Convention on Biological Diversity Modified Organism-Herbicide Tolerant SU Canola. Available online: http://bch.cbd.int/database/record.shtml?documentid=110268 (accessed on 16 May 2020).
- Wu, G.; Zhang, L.; Wu, Y.; Cao, Y.; Lu, C. Comparison of Five Endogenous Reference Genes for Specific PCR Detection and Quantification of Brassica napus. J. Agric. Food Chem. 2010, 58, 2812–2817. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Abdel Nour, A.M.; Pfaffl, M.W. MIQE & QPCR Ibook How to Apply the MIQE Guidelines—A Visual, Interactive and Practical QPCR Guide; bioMCC: Freising, Germany, 2015; ISBN 978-3-00-048806-1. [Google Scholar]
- European Network of GMO Laboratories. ENGL-Definition of Minimum Performance Requirements for Analytical Methods of GMO Testing. Available online: https://gmo-crl.jrc.ec.europa.eu/doc/MPR%20Report%20Application%2020_10_2015.pdf (accessed on 16 May 2020).
- Demeke, T.; Jenkins, G.R. Influence of DNA extraction methods, PCR inhibitors and quantification methods on real-time PCR assay of biotechnology-derived traits. Anal. Bioanal. Chem. 2010, 396, 1977–1990. [Google Scholar] [CrossRef] [PubMed]
- Holst-Jensen, A.; Bertheau, Y.; Alnutt, T.; Broll, H.; de Loose, M.; Grohmann, L.; Henry, C.; Hougs, H.; Moens, W.; Morisset, D.; et al. Overview on the Detection, Interpretation and Reporting on the Presence of Unauthorised Genetically Modified Materials: Guidance Document from the European Network of GMO Laboratories (ENGL); Publications Office of the European Union: Luxembourg, 2011; ISBN 978-92-79-21800-. [Google Scholar]
- Holst-Jensen, A.; Bertheau, Y.; De Loose, M.; Grohmann, L.; Hamels, S.; Hougs, L.; Morisset, D.; Pecoraro, S.; Pla, M.; Bulcke, M.V.D.; et al. Detecting un-authorized genetically modified organisms (GMOs) and derived materials. Biotechnol. Adv. 2012, 30, 1318–1335. [Google Scholar] [CrossRef] [PubMed]
- Arulandhu, A.J.; Van Dijk, J.P.; Staats, M.; Hagelaar, R.; Voorhuijzen, M.; Molenaar, B.; Van Hoof, R.A.; Li, R.; Yang, L.; Shi, J.; et al. NGS-based amplicon sequencing approach; towards a new era in GMO screening and detection. Food Control 2018, 93, 201–210. [Google Scholar] [CrossRef]
- Herbold, C.W.; Pelikan, C.; Kuzyk, O.; Hausmann, B.; Angel, R.; Berry, D.; Loy, A. A flexible and economical barcoding approach for highly multiplexed amplicon sequencing of diverse target genes. Front. Microbiol. 2015, 6, 731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demorest, Z.L.; Coffman, A.; Baltes, N.J.; Stoddard, T.J.; Clasen, B.M.; Luo, S.; Retterath, A.; Yabandith, A.; Gamo, M.E.; Bissen, J.; et al. Direct stacking of sequence-specific nuclease-induced mutations to produce high oleic and low linolenic soybean oil. BMC Plant Biol. 2016, 16, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haun, W.; Coffman, A.; Clasen, B.M.; Demorest, Z.L.; Lowy, A.; Ray, E.; Retterath, A.; Stoddard, T.; Juillerat, A.; Cédrone, F.; et al. Improved soybean oil quality by targeted mutagenesis of the fatty acid desaturase 2 gene family. Plant Biotechnol. J. 2014, 12, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Chen, K.; Li, T.; Zhang, Y.; Wang, Y.; Zhao, Q.; Liu, J.; Zhang, H.; Liu, C.; Ran, Y.; et al. Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat. Commun. 2017, 8, 14261. [Google Scholar] [CrossRef] [PubMed]
| Name | Primer Sequence (5′ to 3′) | Position | Amplicon Length | Reference |
|---|---|---|---|---|
| SU Canola-Specific Primers and Probe | ||||
| SU-Forward Primer | TCC TCG ACG AGC TAA CCG | 1364–1381 | 334 | This Study |
| SU-Reverse Primer | GCT TTG TAG AAC CGA TCT TCC +A | 1676–1697 | ||
| SU-Probe | FAM-ACA ACC AGC ATC TTG GGA TGG TCA-BHQ | 1646–1669 | ||
| Endogenous Reference Primers and Probe | ||||
| CruA-Forward Primer | GGC CAG GGT TTC CGT GAT | 1408–1425 | 101 | [39] |
| CruA-Reverse Primer | CCG TCG TTG TAG AAC CAT TGG | 1488–1508 | ||
| CruA-Probe | FAM- AGT CCT TAT GTG CTC CAC TTT CTG GTG CA-BHQ | 1427–1455 |
| PCR Specificity | AHAS1C-SU | CruA | ||
|---|---|---|---|---|
| Canola Variety | Mean Ct | Ct% CV | Mean Ct | Ct% CV |
| 5545 CL | ND | NA | 20.81 | 0.16% |
| CS2200 CL | ND | NA | 21.24 | 0.48% |
| 2022 CL | ND | NA | 21.02 | 0.16% |
| C5507 | 23.71 | 0.15% | 20.93 | 0.09% |
| C1511 | 23.40 | 0.08% | 20.87 | 0.10% |
| 40K | 22.37 | 0.41% | 20.55 | 0.29% |
| Water | ND | NA | ND | NA |
| PCR Specificity | AHAS1C-SU | CruA | ||
|---|---|---|---|---|
| Canola Variety | Mean Ct | Ct% CV | Mean Ct | Ct% CV |
| 40K-10 | 25.36 | 0.08% | 19.88 | 0.20% |
| 40K-1 | 28.19 | 2.09% | 20.10 | 0.72% |
| 40K-0.1 | 33.43 | 1.44% | 20.22 | 0.12% |
| 5545 CL | ND | NA | 20.88 | 0.46% |
| Variety 1 | ND | NA | 21.25 | 0.20% |
| Variety 2 | ND | NA | 19.64 | 0.75% |
| Variety 3 | ND | NA | 20.15 | 0.35% |
| Variety 4 | ND | NA | 19.45 | 0.11% |
| Variety 5 | ND | NA | 19.37 | 0.52% |
| Variety 6 | ND | NA | 18.74 | 0.49% |
| Variety 7 | ND | NA | 19.27 | 0.19% |
| Variety 8 | ND | NA | 19.19 | 0.48% |
| Variety 9 | ND | NA | 19.22 | 0.55% |
| Variety 10 | ND | NA | 19.67 | 0.73% |
| Variety 11 | ND | NA | 19.56 | 0.78% |
| Variety 12 | ND | NA | 19.12 | 0.38% |
| Variety 13 | ND | NA | 19.45 | 0.19% |
| Variety 14 | ND | NA | 19.08 | 0.49% |
| Variety 15 | ND | NA | 19.62 | 0.28% |
| Variety 16 | ND | NA | 20.47 | 1.12% |
| Variety 17 | ND | NA | 21.23 | 0.20% |
| Variety 18 | ND | NA | 19.06 | 0.50% |
| Variety 19 | ND | NA | 23.65 | 0.26% |
| Variety 20 | ND | NA | 18.29 | 0.39% |
| No DNA | ND | NA | 34.36 | 2.48% |
| Declared SU Canola DNA Conc. | Average Measured SU Canola DNA Conc. | Std Dev of DNA Conc. | % CV of DNA Conc. | Percent Trueness of Measured DNA Conc. | Average Ct | Std Dev of Ct | % CV of Ct |
|---|---|---|---|---|---|---|---|
| 10.00% | 8.631% | 0.758 | 8.8 | 13.69% | 25.6490 | 0.1545 | 0.60 |
| 1.00% | 1.011% | 0.050 | 5.0 | 1.10% | 29.0010 | 0.0762 | 0.26 |
| 0.10% | 0.103% | 0.012 | 11.1 | 3.38% | 32.5780 | 0.1798 | 0.55 |
| 0.05% | 0.045% | 0.006 | 16.8 | 6.93% | 33.8381 | 0.2587 | 0.76 |
| 0.01% | 0.004% | 0.003 | 69.0 | 56.68% | 37.8368 | 0.9926 | 2.62 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chhalliyil, P.; Ilves, H.; Kazakov, S.A.; Howard, S.J.; Johnston, B.H.; Fagan, J. A Real-Time Quantitative PCR Method Specific for Detection and Quantification of the First Commercialized Genome-Edited Plant. Foods 2020, 9, 1245. https://doi.org/10.3390/foods9091245
Chhalliyil P, Ilves H, Kazakov SA, Howard SJ, Johnston BH, Fagan J. A Real-Time Quantitative PCR Method Specific for Detection and Quantification of the First Commercialized Genome-Edited Plant. Foods. 2020; 9(9):1245. https://doi.org/10.3390/foods9091245
Chicago/Turabian StyleChhalliyil, Pradheep, Heini Ilves, Sergei A. Kazakov, Stephanie J. Howard, Brian H. Johnston, and John Fagan. 2020. "A Real-Time Quantitative PCR Method Specific for Detection and Quantification of the First Commercialized Genome-Edited Plant" Foods 9, no. 9: 1245. https://doi.org/10.3390/foods9091245
APA StyleChhalliyil, P., Ilves, H., Kazakov, S. A., Howard, S. J., Johnston, B. H., & Fagan, J. (2020). A Real-Time Quantitative PCR Method Specific for Detection and Quantification of the First Commercialized Genome-Edited Plant. Foods, 9(9), 1245. https://doi.org/10.3390/foods9091245





