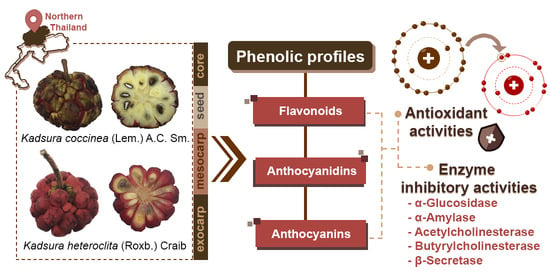
Phenolic Profiles, Antioxidant, and Inhibitory Activities of Kadsura heteroclita (Roxb.) Craib and Kadsura coccinea (Lem.) A.C. Sm.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection, Preparation, and Extraction
2.2. Determination of Antioxidant Activity
2.3. Determination of Total Phenolic Contents, Total Flavonoid Contents (TFCs), Total Anthocyanin Contents (TACs), and Phenolic Profiles
2.4. Determination of Enzyme Inhibitory Activities
2.5. Statistical Analysis
3. Results
3.1. Total Phenolic Contents (TPCs), Total Flavonoid Contents (TFCs), Total Anthocyanin Contents (TACs), and Phenolic Profiles
3.2. Antioxidant Activities
3.3. In Vitro Enzyme Inhibitory Activities
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trisonthi, C.; Trisonthi, P. A new description of Kadsura ananosma Kerr (Schisandraceae). In Thai Forest Bulletin (Botany); Forest Herbarium: Bangkok, Thailand, 1999; Volume 27, pp. 31–35. [Google Scholar]
- Tepkaew, V.; Sangkaew, W.; Mahawan, P.; Sanguanpong, V.; Thainurak, H.; Ittiponjun, B.; Klongklaew, B.; Worapratheep, K.; Suwanthada, C.; Trisonthi, P.; et al. Surveying of RSPG Conserved Plant Genus Kadsura (Schisandraceae) in Northern Thailand. In Proceedings of the 7th Conference of RGSP Committee, Khon Haen University, Khon Kaen, Thailand, 24–26 March 2016; pp. 315–318. [Google Scholar]
- Mulyaningsih, S.; Youns, M.; El-Readi, M.Z.; Ashour, M.L.; Nibret, E.; Sporer, F.; Herrmann, F.; Reichling, J.; Wink, M. Biological activity of the essential oil of Kadsura longipedunculata (Schisandraceae) and its major components. J. Pharm. Pharmacol. 2010, 62, 1037–1044. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, X.; Tian, J.; Liu, C.; Cheng, X.; Ren, G. Antioxidant and antidiabetic activities of black mung bean (Vigna radiata L.). J. Agric. Food Chem. 2013, 61, 8104–8109. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhang, T.; Yin, C.; Zhou, J.; Huang, R.; Gao, S.; Zheng, L.; Wang, X.; Manyande, A.; Tian, X.; et al. Effects of the stem extracts of Schisandra glaucescens Diels on collagen-induced arthritis in Balb/c mice. J. Ethnopharmacol. 2016, 194, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Xu, L.J.; Peng, Y.; Li, R.T.; Xiao, P.G. Chemical study on ethyl acetate soluble portion of Kadsura oblongifolia. China J. Chin. Mater. Med. 2009, 34, 864–866. [Google Scholar]
- Sun, J.Y.; Jiang, I.; Amin, Z.; Li, K.N.; Prasad, X.; Duan, B.Y.; Xu, L. An exotic fruit with high nutritional value: Kadsura coccinea fruit. Int. Food Res. 2011, 18, 651–657. [Google Scholar]
- Banaszczak, E.W.; Radzikowska, D.; Ratajczak, K. Chemical profile and antioxidant activity of Trollius europaeus under the influence of feeding aphids. Open Life Sci. 2018, 13, 312–318. [Google Scholar] [CrossRef]
- Kowalczewski, P.L.; Radzikowska, D.; Ivanišová, E.; Szwengiel, A.; Kačániová, M.; Sawinska, Z. Influence of Abiotic Stress Factors on the Antioxidant Properties and Polyphenols Profile Composition of Green Barley (Hordeum vulgare L.). Int. J. Mol. Sci. 2020, 21, 397. [Google Scholar] [CrossRef] [Green Version]
- Vanacker, H.; Guichard, M.; Bohrer, A.S.; Issakidis-Bourguet, E. Redox regulation of monodehydroascorbate reductase by thioredoxin y in plastids revealed in the context of water stress. Antioxidants 2018, 7, 183. [Google Scholar] [CrossRef] [Green Version]
- Kowalczewski, P.L.; Olejnik, A.; Białas, W.; Kubiak, P.; Siger, A.; Nowicki, M.; Lewandowicz, G. Effect of thermal processing on antioxidant activity and cytotoxicity of waste potato juice. Open Life Sci. 2019, 14, 150–157. [Google Scholar] [CrossRef]
- Keng, H. Schisandraceae. In Flora of Thailand; Smitinand, T., Larsen, K., Eds.; ASRCT Press: Bangkok, Thailand, 1972; Volume 2, pp. 112–114. [Google Scholar]
- Plant Genetic Conservation Project Under The Royal Initiative of Her Royal Highness Princess Maha Chakri Sirindhorn (RSPG) Webmaster. Available online: www.rspg.or.th (accessed on 30 December 2017).
- Qi, X.Z.; Liu, J.B.; Chen, J.B.; Li, S. Lignans and triterpenoids from roots of Kadsura longipedunculata. China Trad. Herb. Drug 2017, 48, 2164–2171. [Google Scholar]
- Chen, D.F.; Zhang, S.X.; Chen, K.; Zhou, B.N.; Wang, P.; Cosentino, L.M.; Lee, K.H. Two new lignans, interiotherins A and B, as anti-HIV principles from Kadsura interior. China J. Nat. Prod. 1996, 59, 1066–1068. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukumoto, L.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reduction ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4926. [Google Scholar] [CrossRef] [PubMed]
- Atukeren, P.; Cengiz, M.; Yavuzer, H.; Gelisgen, R.; Altunoglu, E.; Oner, S.; Erdenen, F.; Yuceakın, D.; Derici, H.; Cakatay, U.; et al. The efficacy of donepezil administration on acetylcholinesterase activity and altered redox homeostasis in Alzheimer’s disease. Biomed. Pharmacother. 2017, 90, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other antioxidant substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Sripum, C.; Kukreja, R.K.; Charoenkiatkul, S.; Kriengsinyos, W.; Suttisansanee, U. The effect of extraction conditions on antioxidant activities and total phenolic contents of different processed Thai Jasmine rice. Int. Food Res. J. 2017, 24, 1644–1650. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Lee, J. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Thuphairo, K.; Sornchan, P.; Suttisansanee, U. Bioactive compounds, antioxidant activity and inhibition of key enzymes relevant to Alzheimer’s disease from sweet pepper (Capsicum annuum) extracts. Prev. Nutr. Food Sci. 2019, 24, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Temviriyakul, P.; Sritalahareuthai, V.; Na Jom, K.; Jongruaysup, B.; Tabtimsri, S.; Pruesapan, K.; Thangsiri, S.; Inthachat, W.; Siriwan, D.; Charoenkiatku, S.; et al. Comparison of phytochemicals, antioxidant, and in vitro anti-Alzheimer properties of twenty-seven Morus spp. cultivated in Thailand. Molecules 2020, 25, 2600. [Google Scholar] [CrossRef] [PubMed]
- Suttisansanee, U.; Charoenkiatkul, S.; Jongruaysup, B.; Tabtimsri, S.; Siriwan, D.; Temviriyanukul, P. Mulberry fruit cultivar ‘Chiang Mai’ prevents beta-amyloid toxicity in PC12 neuronal cells and in a Drosophila model of Alzheimer’s disease. Molecules 2020, 25, 1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Promyos, N.; Temviriyanukul, P.; Suttisansanee, U. Evaluation of α-glucosidase inhibitory assay using different sub-classes of flavonoids. Curr. Appl. Sci. Technol. J. 2017, 17, 172–180. [Google Scholar]
- Pongkunakorn, T.; Watcharachaisoponsiri, T.; Chupeerach, C.; On-nom, N.; Suttisansanee, U. Inhibitions of key enzymes relevant to obesity and diabetes of Thai local mushroom extracts. Curr. Appl. Sci. Technol. J. 2017, 17, 181–190. [Google Scholar]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Quantitation of flavonoid constituents in citrus fruits. J. Agric. Food Chem. 1999, 47, 3565–3571. [Google Scholar] [CrossRef]
- Hakkinen, S.H.; Karenlampi, S.O.; Heinonen, M.; Mykkanen, H.M.; Torronen, A.R. Content of the flavonols quercetin, myricetin and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; de Vos, C.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. J. Agric. Food Chem. 2008, 56, 696–704. [Google Scholar] [CrossRef]
- Mertz, C.; Cheynier, V.; Gunata, Z.; Brat, P. Analysis of phenolic compounds in two blackberry species (Rubus glaucus and Rubus adenotrichus) by high-performance liquid chromatographywith diode array detection and electrospray ion trap mass spectrometry. J. Agric. Food Chem. 2007, 55, 8616–8624. [Google Scholar] [CrossRef]
- Borges, G.; Degeneve, A.; Mullen, W.; Crozier, A. Identification of flavonoid and phenolic antioxidants in black currants, blueberries, raspberries, red currants, and cranberries. J. Agric. Food Chem. 2010, 58, 3901–3909. [Google Scholar] [CrossRef] [PubMed]
- Fredericks, C.H.; Fanning, K.J.; Gidley, M.J.; Netzel, G.; Zabaras, D.; Herrington, M.; Netzel, M. High-anthocyanin strawberries through cultivar selection. J. Sci. Food Agric. 2013, 93, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Yina, Y.; Xuc, C.; Liu, J. Isolation of high-purity anthocyanin mixtures and monomersfrom blueberries using combined chromatographic techniques. J. Chromatogr. A 2014, 1327, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yao, J.Y.; Huang, S.X.; Long, X.; Wang, J.B.; Garcia-Garcia, E. Antioxidant activity of polyphenol and anthocyanin extracts from fruits of Kadsura coccinea (Lem.) A.C. Smith. Food Chem. 2009, 117, 276–281. [Google Scholar] [CrossRef]
- USDA Database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods. Available online: www.orac-info-portal.de/download/ORAC_R2.pdf (accessed on 23 March 2018).
- Lodovici, M.; Guglielmi, F.; Meoni, M.; Dolara, P. Effect of natural phenolic acids on DNA oxidation in vitro. Food Chem. Toxicol. 2001, 39, 1205–1210. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Manchope, M.F.; Campos, C.C.; Silva, L.C.; Zarpelon, A.C.; Ribeiro, F.A.P.; Georgetti, S.R.; Baracat, M.M.; Casagrande, R.; Verri, W.A., Jr. Naringenin inhibits superoxide anion-induced inflammatory pain: Role of oxidative stress, cytokines, Nrf-2 and the NO−cGMP−PKG−KATP channel signaling pathway. PLoS ONE 2016, 11, e0153015. [Google Scholar] [CrossRef]
- Raza, S.S.; Khan, M.M.; Ahmad, A.; Ashafaq, M.; Islam, F.; Wagner, A.P.; Safhi, M.M.; Islam, F. Neuroprotective effect of naringenin is mediated through suppression of NF-kB signaling pathway in experimental stroke. Neuroscience 2013, 230, 157–171. [Google Scholar] [CrossRef]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muniz, P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: A comparative study. J. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Deng, G.; Guo, R.; Chen, D.F.; Fu, Z.M. DFT studies on the antioxidant activity of naringenin and its derivatives: Effects of the substituents at C3. Int. J. Mol. Sci. 2019, 20, 1450. [Google Scholar] [CrossRef] [Green Version]
- Adisakwattana, S.; Jiphimai, P.; Prutanopajai, P.; Chanathong, B.; Sapwarobol, S.; Ariyapitipan, T. Evaluation of α-glucosidase, α-amylase and protein glycation inhibitory activities of edible plants. Int. J. Food. Sci. Nutr. 2010, 61, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J. Nutr. Sci. Vitaminol. (Tokyo) 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priscilla, D.H.; Roy, D.; Suresh, A.; Kumar, V.; Thirumurugan, K. Naringenin inhibits α-glucosidase activity: A promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem. Biol. Interact. 2014, 210, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Ingkaninan, K.; de Best, C.M.; van der Heijden, R.; Hofte, A.J.P.; Karabatak, B.; Irth, H.; Tjaden, U.R.; van der Greef, J.; Verpoorte, R. High-performance liquid chromatography with on-line coupled UV, mass spectrometric and biochemical detection for identification of acetylcholinesterase inhibitors from natural products. J. Chromatogr. A 2000, 872, 61–73. [Google Scholar] [CrossRef]
- Singhai, A.K.; Naithani, V.; Bangar, O.P. Medicinal plants with a potential to treat Alzheimer and associated symptoms. Int. J. Nutr. Pharmacol. Dis. 2012, 2, 84–91. [Google Scholar] [CrossRef]
- Lee, S.; Youn, K.; Lim, G.; Lee, J.; Jun, M. In silico docking and in vitro approaches towards BACE1 and cholinesterases inhibitory effect of citrus flavanones. Molecules 2018, 23, 1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liaquat, L.; Batool, Z.; Sadir, S.; Rafiq, S.; Shahzad, S.; Perveen, T.; Haider, S. Naringenin-induced enhanced antioxidant defence system meliorates cholinergic neurotransmission and consolidates memory in male rats. Life Sci. 2018, 194, 213–223. [Google Scholar] [CrossRef]
- Querfurth, H.W.; Laferla, F.M. Mechanisms of disease: Alzheimers disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [Green Version]
- Ghofrani, S.; Joghataei, M.-T.; Mohseni, S.; Baluchnejadmojarad, T.; Bagheri, M.; Khamse, S.; Roghani, M. Naringenin improves learning and memory in an Alzheimer’s disease rat model: Insights into the underlying mechanisms. Eur. J. Pharmacol. 2015, 764, 195–201. [Google Scholar] [CrossRef] [Green Version]
| Time (min) | Flow Rate (mL/min) | Solvent A (%) | Solvent B (%) | Solvent C (%) |
|---|---|---|---|---|
| 0 | 0.6 | 90 | 6 | 4 |
| 5 | 0.6 | 85 | 9 | 6 |
| 30 | 0.6 | 71 | 17.4 | 11.6 |
| 60 | 0.6 | 0 | 85 | 15 |
| 61 | 0.6 | 90 | 6 | 4 |
| 66 | 0.6 | 90 | 6 | 4 |
| Time (min) | Solvent A | Solvent B |
|---|---|---|
| 0 | 88 | 12 |
| 6 | 88 | 12 |
| 8 | 85 | 15 |
| 25 | 85 | 15 |
| 25 | 88 | 12 |
| 30 | 88 | 12 |
| Kadsura spp. | Total Phenolic Contents (mg GAE/g DW) | Total Flavonoid Contents (mg QE/g DW) | Total Anthocyanin Contents (mg C3GE/g DW) |
|---|---|---|---|
| KCE | |||
| Exocarp | 43.61 ± 0.65 a,* | 60.52 ± 5.51 a,* | 0.02 ± 0.00 b,* |
| Mesocarp | 6.20 ± 0.15 c,* | 1.45 ± 0.52 c,* | 0.27 ± 0.02 a |
| Seed | 0.83 ± 0.08 d,* | 3.48 ± 1.06 c,* | ND |
| Core | 17.71 ± 0.07 b,* | 43.23 ± 1.18 b,* | ND |
| KHE | |||
| Exocarp | 54.00 ± 2.11 B | 113.36 ± 4.87 B | 0.28 ± 0.02 A |
| Mesocarp | 21.20 ± 0.54 C | 33.02 ± 1.55 C | 0.26 ± 0.01 A |
| Seed | 1.13 ± 0.14 D | 1.40 ± 0.10 D | ND |
| Core | 103.72 ± 1.14 A | 206.92 ± 4.08 A | 0.14 ± 0.01 B |
| Kadsura spp. | Flavonoids (mg/100 g DW) | Anthocyanidins (µg/100 g DW) | Anthocyanins (µg/100 g DW) | |||||
|---|---|---|---|---|---|---|---|---|
| Quercetin | Naringenin | Cyanidin | Delphinidin | Cyanin | Ideain | Kuromanin | Keracyanin | |
| KCE | ||||||||
| Exocarp | 17.94 ± 0.96 * | 1972.65 ± 135.07 a | 1.03 ± 0.13 a,* | 4.02 ± 0.91 a | ND | ND | ND | ND |
| Mesocarp | ND | 1395.22 ± 50.23 c,* | 0.99 ± 0.38 b | 0.42 ± 0.19 a | 9.94 ± 0.20 | 10.16 ± 0.19 * | ND | ND |
| Seed | ND | 1861.15 ± 31.35 ab | ND | ND | ND | ND | ND | ND |
| Core | ND | 1752.54 ± 0.70 b | 0.34 ± 0.05 b | 0.60 ± 0.29 b,* | ND | ND | ND | ND |
| KHE | ||||||||
| Exocarp | 51.19 ± 1.92 A | 1811.02 ± 15.28 A | 1.00 ± 0.07 A | 0.50 ± 0.02 A | ND | 53.36 ± 2.40 A | 26.06 ± 4.08 A | 112.54 ± 9.65 A |
| Mesocarp | 12.59 ± 1.17 B | 1812.07 ± 88.25 A | 0.41 ± 0.07 B | 0.43 ± 0.11 AB | ND | 6.83 ± 0.49 B | 2.68 ± 0.98 B | 12.56 ± 0.65 B |
| Seed | ND | 1835.46 ± 54.82 A | ND | ND | ND | ND | ND | ND |
| Core | ND | 1739.91 ± 55.90 A | 0.03 ± 0.01 C | 0.31 ± 0.09 B | ND | 2.11 ± 0.08 C | 0.27 ± 0.07 B | 4.16 ± 0.56 B |
| Kadsura spp. | Antioxidant Activities (µmol TE/g DW) | ||
|---|---|---|---|
| DPPH Radical Scavenging Assay | FRAP Assay | ORAC Assay | |
| KCE | |||
| Exocarp | 2.73 ± 0.08 a | 300.44 ± 12.09 a,* | 957.80 ± 77.76 a,* |
| Mesocarp | 0.23 ± 0.00 c,* | 25.80 ± 1.04 c,* | 120.20 ± 10.88 c,* |
| Seed | 0.04 ± 0.00 d | 3.14 ± 0.18 d,* | 41.93 ± 4.17 d,* |
| Core | 1.00 ± 0.03 b,* | 100.19 ± 1.69 b,* | 440.35 ± 33.60 b,* |
| KHE | |||
| Exocarp | 2.75 ± 0.10 B | 351.48 ± 18.79 B | 812.66 ± 77.67 B |
| Mesocarp | 1.02 ± 0.03 C | 143.23 ± 12.29 C | 260.91 ± 23.48 C |
| Seed | 0.04 ± 0.00 D | 2.91 ± 0.24 D | 15.84 ± 1.41 D |
| Core | 6.48 ± 0.22 A | 900.60 ± 6.22 A | 1330.23 ± 49.67 A |
| Flavonoids | |||
| Naringenin | 0.02 ± 0.00 | 30.98 ± 0.69 | 25,592.45 ± 495.92 |
| Quercetin | 8.07 ± 0.25 | 13,519.54 ± 242.20 | 30,112.47 ± 1631.24 |
| Kadsura spp. | α-Glucosidase | α-Amylase | AChE | BChE | BACE1 | |||
|---|---|---|---|---|---|---|---|---|
| %Inhibition 1 | IC50 (mg/mL) | %Inhibition 2 | %Inhibition 3 | IC50 (mg/mL) | %Inhibition 3 | IC50 (mg/mL) | %Inhibition 3 | |
| KCE | ||||||||
| Exocarp | 92.32 ± 7.04 a | 0.13 ± 0.01 a,* | 51.58 ± 3.52 a,* | 87.44 ± 8.67 a | 0.88 ± 0.01 a,* | 96.81 ± 1.26 a,* | 0.49 ± 0.02 a,* | 41.39 ± 2.56 b,* |
| Mesocarp | 60.04 ± 5.17 b,* | 0.56 ± 0.03 c | 40.17 ± 3.31 c,* | 20.40 ± 1.60 c,* | N/A | 41.27 ± 1.97 c,* | N/A | 56.34 ± 2.12 a,* |
| Seed | ND | N/A | 12.18 ± 0.71 d | ND | N/A | ND | N/A | 4.72 ± 2.85 d,* |
| Core | 98.23 ± 1.49 a,* | 0.45 ± 0.00 b,* | 44.78 ± 2.72 b | 62.86 ± 3.21 b,* | 1.54 ± 0.10 b,* | 88.86 ± 0.65 b,* | 0.67 ± 0.04 b,* | 26.36 ± 1.59 c |
| KHE | ||||||||
| Exocarp | 91.84 ± 3.76 A | 0.06 ± 0.01 A | 44.75 ± 3.52 A | 88.64 ± 2.34 A | 0.52 ± 0.05 A | 99.15 ± 1.53 A | 0.20 ± 0.04 A | 16.29 ± 1.16 B |
| Mesocarp | 90.11 ± 2.60 A | 0.64 ± 0.03 B | 33.93 ± 2.00 B | 29.98 ± 3.01 B | N/A | 63.67 ± 5.56 B | 1.32 ± 0.08 B | 35.26 ± 2.61 A |
| Seed | ND | N/A | ND | ND | N/A | ND | N/A | 12.70 ± 0.93 C |
| Core | 89.72 ± 3.68 A | 0.06 ± 0.00 A | 44.12 ± 3.20 A | 91.74 ± 0.71 A | 0.41 ± 0.00 A | 97.99 ± 0.22 A | 0.16 ± 0.00 A | ND |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sritalahareuthai, V.; Temviriyanukul, P.; On-nom, N.; Charoenkiatkul, S.; Suttisansanee, U. Phenolic Profiles, Antioxidant, and Inhibitory Activities of Kadsura heteroclita (Roxb.) Craib and Kadsura coccinea (Lem.) A.C. Sm. Foods 2020, 9, 1222. https://doi.org/10.3390/foods9091222
Sritalahareuthai V, Temviriyanukul P, On-nom N, Charoenkiatkul S, Suttisansanee U. Phenolic Profiles, Antioxidant, and Inhibitory Activities of Kadsura heteroclita (Roxb.) Craib and Kadsura coccinea (Lem.) A.C. Sm. Foods. 2020; 9(9):1222. https://doi.org/10.3390/foods9091222
Chicago/Turabian StyleSritalahareuthai, Varittha, Piya Temviriyanukul, Nattira On-nom, Somsri Charoenkiatkul, and Uthaiwan Suttisansanee. 2020. "Phenolic Profiles, Antioxidant, and Inhibitory Activities of Kadsura heteroclita (Roxb.) Craib and Kadsura coccinea (Lem.) A.C. Sm." Foods 9, no. 9: 1222. https://doi.org/10.3390/foods9091222