Changes of the Aroma Composition and Other Quality Traits of Blueberry ‘Garden Blue’ during the Cold Storage and Subsequent Shelf Life
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Postharvest Storage
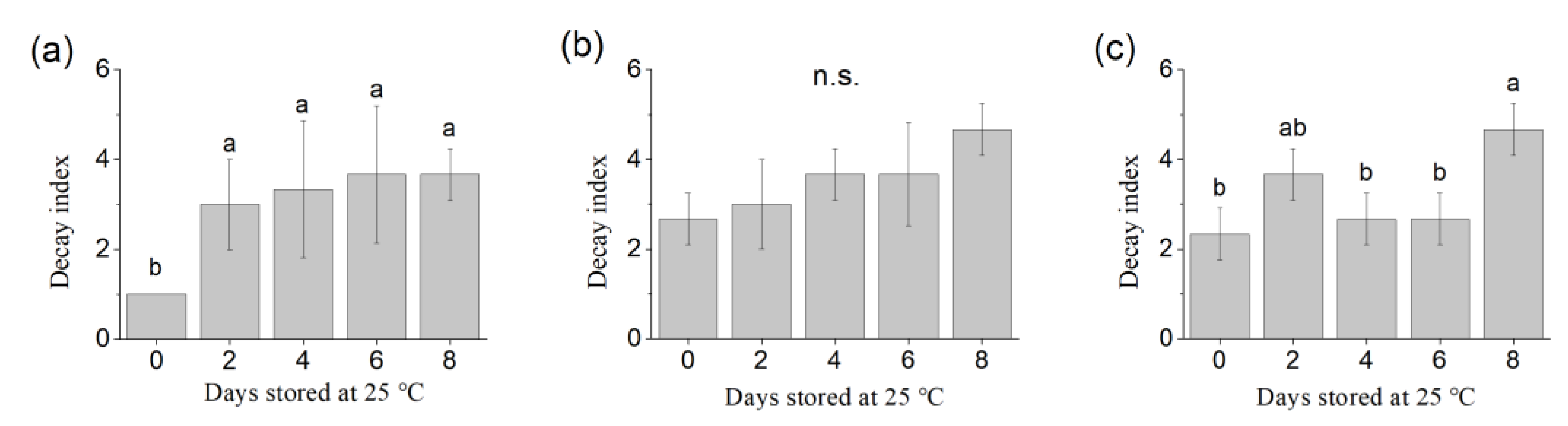
2.2. Measurements of Weight Loss and Decay Index
2.3. Measurements of Total Soluble Solids, pH and Titratable Acidity
2.4. Measurement of Firmness
2.5. Analysis of Volatile Compounds
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results
3.1. Weight Loss and Decay Index
3.2. TSS, pH and TA
3.3. Berry Firmness
3.4. Aroma Compounds
3.5. Off-Odor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Howard, L.R.; Prior, R.L.; Liyanage, R.; Lay, J.O. Processing and Storage Effect on Berry Polyphenols: Challenges and Implications for Bioactive Properties. J. Agric. Food Chem. 2012, 60, 6678–6693. [Google Scholar] [CrossRef]
- Sater, H.M.; Bizzio, L.N.; Tieman, D.M.; Muñoz, P.D. A Review of the Fruit Volatiles Found in Blueberry and Other Vaccinium Species. J. Agric. Food Chem. 2020, 68, 5777–5786. [Google Scholar] [CrossRef] [PubMed]
- Caleb, O.J.; Mahajan, P.V.; Al-Said, A.J.; Opara, U.L. Modified Atmosphere Packaging Technology of Fresh and Fresh-cut Produce and the Microbial Consequences—A Review. Food Bioprocess Technol. 2013, 6, 303–329. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Y.; Tao, Y.; Wu, X. Influence of γ-irradiation on the reactive-oxygen metabolism of blueberry fruit during cold storage. Innov. Food Sci. Emerg. 2017, 41, 397–403. [Google Scholar] [CrossRef]
- Bounous, G.; Giacalone, G.; Guarinoni, A.; Peano, C. Modified atmosphere storage of high bush blueberry. Acta Hortic. 1997, 446, 197–203. [Google Scholar] [CrossRef]
- Almenar, E.; Samsudin, H.; Auras, R.; Harte, B.; Rubino, M. Postharvest shelf life extension of blueberries using a biodegradable package. Food Chem. 2008, 110, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Reque, P.M.; Steffens, R.S.; Jablonski, A.; Flôres, S.H.; Rios, A.D.O.; De Jong, E.V. Cold storage of blueberry (Vaccinium spp.) fruits and juice: Anthocyanin stability and antioxidant activity. J. Food Compos. Anal. 2014, 33, 111–116. [Google Scholar] [CrossRef]
- Villarreal, N.M.; Bustamante, C.A.; Civello, P.M.; Martinez, G.A. Effect of ethylene and 1-MCP treatments on strawberry fruit ripening. J. Sci. Food Agric. 2010, 90, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Sun, P.; Chen, L.; Gao, S.; Shao, W.; Li, J. Comparative analysis of fruit volatiles and related gene expression between the wild strawberry Fragaria pentaphylla and cultivated Fragaria × ananassa. Eur. Food Res. Technol. 2018, 244, 57–72. [Google Scholar] [CrossRef]
- Maclean, D.; Nesmith, D.S. Rabbiteye Blueberry Postharvest Fruit Quality and Stimulation of Ethylene Production by 1-Methylcyclopropene. Hortscience 2011, 46, 1278–1281. [Google Scholar] [CrossRef]
- Fu, X.M.; Cheng, S.H.; Zhang, Y.Q.; Du, B.; Feng, C.; Zhou, Y.; Mei, X.; Jiang, Y.M.; Duan, X.W.; Yang, Z.Y. Differential responses of four biosynthetic pathways of aroma compounds in postharvest strawberry (Fragaria × ananassa Duch.) under interaction of light and temperature. Food Chem. 2017, 221, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Forney, C.F. Horticultural and other Factors Affecting Aroma Volatile Composition of Small Fruit. HortTechnology 2001, 11, 529–538. [Google Scholar] [CrossRef]
- Dymerski, T.; Namieśnik, J.; Vearasilp, K.; Arancibia-Avila, P.; Toledo, F.; Weisz, M.; Katrich, E.; Gorinstein, S. Comprehensive two-dimensional gas chromatography and three-dimensional fluorometry for detection of volatile and bioactive substances in some berries. Talanta 2015, 134, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Horvat, R.J.; Senter, S.D.; Dekazos, E.D. GLC-MS Analysis of Volatile Constituents in Rabbiteye Blueberries. J. Food Sci. 1983, 48, 278–279. [Google Scholar] [CrossRef]
- Horvat, R.J.; Schlotzhauer, W.S.; Chortyk, O.T.; Nottingham, S.F.; Payne, J.A. Comparison of Volatile Compounds from Rabbiteye Blueberry (Vaccinium ashei) and Deerberry (V. stamineum) during Maturation. J. Essent. Oil Res. 1996, 8, 645–648. [Google Scholar] [CrossRef]
- Farneti, B.; Khomenko, I.; Grisenti, M.; Ajelli, M.; Betta, E.; Algarra, A.A.; Cappellin, L.; Aprea, E.; Gasperi, F.; Biasioli, F. Exploring blueberry aroma complexity by chromatographic and direct-injection spectrometric techniques. Front. Plant Sci. 2017, 8, 617. [Google Scholar] [CrossRef]
- Liu, F.; Li, S.; Gao, J.; Cheng, K.; Yuan, F. Changes of terpenoids and other volatiles during alcoholic fermentation of blueberry wines made from two southern highbush cultivars. LWT Food Sci. Technol. 2019, 109, 233–240. [Google Scholar] [CrossRef]
- Yuan, F.; Cheng, K.; Gao, J.; Pan, S. Characterization of Cultivar Differences of Blueberry Wines Using GC-QTOF-MS and Metabolic Profiling Methods. Molecules 2018, 23, 2376. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Zhu, Y.; Yu, N.; Wei, Y.; Zhang, J.; Hou, Y.; Sun, A.D. Evaluation of microbial, physicochemical parameters and flavor of blueberry juice after microchip-pulsed electric field. Food Chem. 2018, 274, 146–155. [Google Scholar] [CrossRef]
- Gilbert, J.L.; Schwieterman, M.L.; Colquhoun, T.A.; Clark, D.G.; Olmstead, J.W. Potential for Increasing Southern Highbush Blueberry Flavor Acceptance by Breeding for Major Volatile Components. Hortscience 2013, 48, 835–843. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Rouseff, R. Aroma active volatiles in four southern highbush blueberry cultivars determined by gas chromatography–olfactometry (GC-O) and gas chromatography–mass spectrometry (GC-MS). J. Agric. Food Chem. 2014, 62, 4537–4543. [Google Scholar] [CrossRef] [PubMed]
- Ferrao, L.F.V.; Johnson, T.S.; Benevenuto, J.; Edger, P.P.; Colquhoun, T.A.; Munoz, P.R. Genome-wide association of volatiles reveals candidate loci for blueberry flavor. New Phytol. 2020, 226, 1725–1737. [Google Scholar] [CrossRef]
- Cheng, K.; Peng, B.Z.; Yuan, F. Volatile composition of eight blueberry cultivars and their relationship with sensory attributes. Flavour Fragr. J. 2020, 35, 443–453. [Google Scholar] [CrossRef]
- Yang, J.; Shi, W.; Li, B.; Bai, Y.; Hou, Z. Preharvest and postharvest uv radiation affected flavonoid metabolism and antioxidant capacity differently in developing blueberries (Vaccinium corymbosum L.). Food Chem. 2019, 301, 125248. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jin, L.; Pan, X.; Yang, L.; Guo, W. Proteins expression and metabolite profile insight into phenolic biosynthesis during highbush blueberry fruit maturation. Food Chem. 2019, 290, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Eichholz, I.; Huyskens-Keil, S.; Keller, A.; Ulrich, D.; Kroh, L.W.; Rohn, S. UV-B-induced changes of volatile metabolites and phenolic compounds in blueberries (Vaccinium corymbosum L.). Food Chem. 2011, 126, 60–64. [Google Scholar] [CrossRef]
- Cao, S.; Hu, Z.; Pang, B.; Wang, H.; Xie, H.; Wu, F. Effect of ultrasound treatment on fruit decay and quality maintenance in strawberry after harvest. Food Control 2010, 21, 529–532. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, G.; Zhang, Q.; Wang, Y.; Meng, X. Ripening affects the physicochemical properties, phytochemicals and antioxidant capacities of two blueberry cultivars. Postharvest Biol. Technol. 2020, 162, 111097. [Google Scholar] [CrossRef]
- Paniagua, A.C.; East, A.R.; Heyes, J.A. Effects of delays in cooling on blueberry quality outcomes. Acta Hortic. 2013, 1012, 1493–1498. [Google Scholar] [CrossRef]
- Sanford, K.A.; Lidster, P.D.; Mcrae, K.B.; Jackson, E.D.; Lawrence, R.A.; Stark, R.; Prange, R.K. Lowbush blueberry quality changes in response to mechanical damage and storage temperature. J. Am. Soc. Hortic. Sci. 1991, 116, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Ribereau-Gayon, P.; Boidron, J.N.; Terrier, A. Aroma of Muscat grape varieties. J. Agric. Food Chem. 1975, 23, 1042–1047. [Google Scholar] [CrossRef]
- Capone, D.L.; Van Leeuwen, K.; Taylor, D.K.; Jeffery, D.W.; Pardon, K.H.; Elsey, G.M.; Sefton, M.A. Evolution and occurrence of 1,8-cineole (eucalyptol) in australian wine. J. Agric. Food Chem. 2011, 59, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Kilcast, D. Taints and Off-Flavours in Foods; Baigrie, B., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2003. [Google Scholar]
- Dozier, W.A.; Caylor, A.W.; Himelrick, D.G.; Powell, A.A.; Akridge, J.R. Rabbiteye blueberry cultivar performance. Fruit Var. J. 1991, 45, 128–134. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G.; Rolle, L. Mechanical behaviour and quality traits of highbush blueberry during postharvest storage. J. Sci. Food Agric. 2009, 89, 989–992. [Google Scholar] [CrossRef]
- Paniagua, A.C.; East, A.R.; Hindmarsh, J.P.; Heyes, J. Moisture loss is the major cause of firmness change during postharvest storage of blueberry. Postharvest Biol. Technol. 2013, 79, 13–19. [Google Scholar] [CrossRef]
- Allanwojtas, P.M.; Forney, C.F.; Carbyn, S.E.; Nicholas, K.U. Microstructural Indicators of Quality-related Characteristics of Blueberries—An Integrated Approach. LWT Food Sci. Technol. 2001, 34, 23–32. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ponce, A.G.; Moreira, M.R. Influence of polysaccharide-based edible coatings as carriers of prebiotic fibers on quality attributes of ready-to-eat fresh blueberries. J. Sci. Food Agric. 2018, 98, 2587–2597. [Google Scholar] [CrossRef]
- Klee, H.J. Improving the flavor of fresh fruits: Genomics, biochemistry, and biotechnology. New Phytol. 2010, 187, 44–56. [Google Scholar] [CrossRef]
- van Gemert, L.J. Odour Thresholds. Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans, Punter & Partners BV: Utrecht, The Netherlands, 2011. [Google Scholar]
- Dixon, J.; Hewett, E.W. Factors affecting apple aroma/flavour volatile concentration: A review. N. Z. J. Crop Hortic. Sci. 2000, 28, 155–173. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Luo, J.; Li, Q.; Li, J.; Liu, T.; Wang, R.; Chen, W.; Li, X. Low temperature storage reduces aroma-related volatiles production during shelf-life of banana fruit mainly by regulating key genes involved in volatile biosynthetic pathways. Postharvest Biol. Technol. 2018, 146, 68–78. [Google Scholar] [CrossRef]
- Yuan, F.; Yan, J.; Yan, X.; Liu, H.; Pan, S. Comparative transcriptome analysis of genes involved in volatile compound synthesis in blueberries (Vaccinium virgatum) during postharvest storage. Postharvest Biol. Technol. 2020, 170, 111327. [Google Scholar] [CrossRef]
- Francis, I.; Newton, J. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ikoma, Y. Effect of postharvest temperature on the muscat flavor and aroma volatile content in the berries of ‘Shine Muscat’ (Vitis labruscana Baily × V. vinifera L.). Postharvest Biol. Technol. 2016, 112, 256–265. [Google Scholar] [CrossRef]
- Siegmund, B. Flavour Development Analysis & Perception in Food & Beverages; Woodhead Publishing: Cambridge, UK, 2015; pp. 127–149. [Google Scholar]
- Beaulieu, J.C.; Stein-Chisholm, R.E.; Boykin, D.L. Qualitative Analysis of Volatiles in Rabbiteye Blueberry Cultivars at Various Maturities Using Rapid Solid-phase Microextraction. J. Am. Soc. Hortic. Sci. 2014, 139, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Krumbein, A.; Peters, P.; Bruckner, B. Flavour compounds and a quantitative descriptive analysis of tomatoes (Lycopersicon esculentum Mill.) of different cultivars in short-term storage. Postharvest Biol. Technol. 2004, 32, 15–28. [Google Scholar] [CrossRef]
- Brovelli, E.A.; Brecht, J.K.; Sherman, W.B.; Sims, C.A. Quality of Fresh-Market Melting- and Nonmelting-Flesh Peach Genotypes as Affected by Postharvest Chilling. J. Food Sci. 1998, 63, 730–733. [Google Scholar] [CrossRef]



| 0 °C Storage Time | Subsequent 25 °C Storage Time | pH | Total Soluble Solids (Brix) | TA (meq/L) | Firmness (N) |
|---|---|---|---|---|---|
| 0 d | 0 d | 2.94 ± 0.2 b | 14.3 ± 0.6 | 6.52 ± 0.10 a | 4.08 ± 1.16 b |
| 2 d | 3.01 ± 0.1 ab | 13.9 ± 0.6 | 6.40 ± 0.22 ab | 4.45 ± 0.72 b | |
| 4 d | 3.06 ± 0.2 ab | 14.0 ± 0.3 | 6.35 ± 0.16 ab | 4.68 ± 1.22 ab | |
| 6 d | 3.07 ± 0.2 ab | 13.2 ± 0.8 | 6.37 ± 0.35 ab | 5.06 ± 1.13 ab | |
| 8 d | 3.17 ± 0.1 a | 13.7 ± 0.3 | 6.21 ± 0.12 b | 5.58 ± 1.26 a | |
| 15 d | 0 d | 3.04 ± 0.09 c | 13.5 ± 1.0 ab | 6.44 ± 0.23 | 4.00 ± 1.00 c |
| 2 d | 3.14 ± 0.04 abc | 14.5 ± 0.3 a | 6.47 ± 0.19 | 4.86 ± 1.42 b | |
| 4 d | 3.20 ± 0.03 ab | 14.6 ± 0.3 a | 6.30 ± 0.22 | 7.06 ± 1.34 a | |
| 6 d | 3.21 ± 0.05 a | 13.0 ± 0.8 b | 6.33 ± 0.14 | 5.96 ± 1.82 ab | |
| 8 d | 3.14 ± 0.03 bc | 12.5 ± 0.4 b | 6.21 ± 0.54 | 6.95 ± 0.99 a | |
| 60 d | 0 d | 3.21 ± 0.09 bc | 15.7 ± 0.5 a | 5.34 ± 0.24 | 5.83 ± 2.31 a |
| 2 d | 3.27 ± 0.03 abc | 15.0 ± 0.6 ab | 5.26 ± 0.33 | 3.79 ± 1.53 b | |
| 4 d | 3.31 ± 0.05 ab | 13.7 ± 0.3 c | 5.39 ± 0.45 | 6.53 ± 2.82 a | |
| 6 d | 3.36 ± 0.03 a | 13.6 ± 0.6 c | 5.45 ± 0.39 | 5.35 ± 1.88 ab | |
| 8 d | 3.19 ± 0.03 c | 14.2 ± 0.3 bc | 5.54 ± 0.47 | 6.10 ± 1.30 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Yan, J.; Pan, S.; Yuan, F. Changes of the Aroma Composition and Other Quality Traits of Blueberry ‘Garden Blue’ during the Cold Storage and Subsequent Shelf Life. Foods 2020, 9, 1223. https://doi.org/10.3390/foods9091223
Yan X, Yan J, Pan S, Yuan F. Changes of the Aroma Composition and Other Quality Traits of Blueberry ‘Garden Blue’ during the Cold Storage and Subsequent Shelf Life. Foods. 2020; 9(9):1223. https://doi.org/10.3390/foods9091223
Chicago/Turabian StyleYan, Xiaoxue, Jun Yan, Siyi Pan, and Fang Yuan. 2020. "Changes of the Aroma Composition and Other Quality Traits of Blueberry ‘Garden Blue’ during the Cold Storage and Subsequent Shelf Life" Foods 9, no. 9: 1223. https://doi.org/10.3390/foods9091223
APA StyleYan, X., Yan, J., Pan, S., & Yuan, F. (2020). Changes of the Aroma Composition and Other Quality Traits of Blueberry ‘Garden Blue’ during the Cold Storage and Subsequent Shelf Life. Foods, 9(9), 1223. https://doi.org/10.3390/foods9091223






