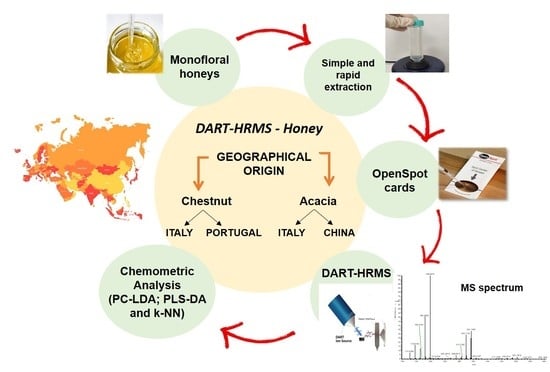
Geographical Origin Discrimination of Monofloral Honeys by Direct Analysis in Real Time Ionization-High Resolution Mass Spectrometry (DART-HRMS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Honey Samples
2.3. Sample Preparation
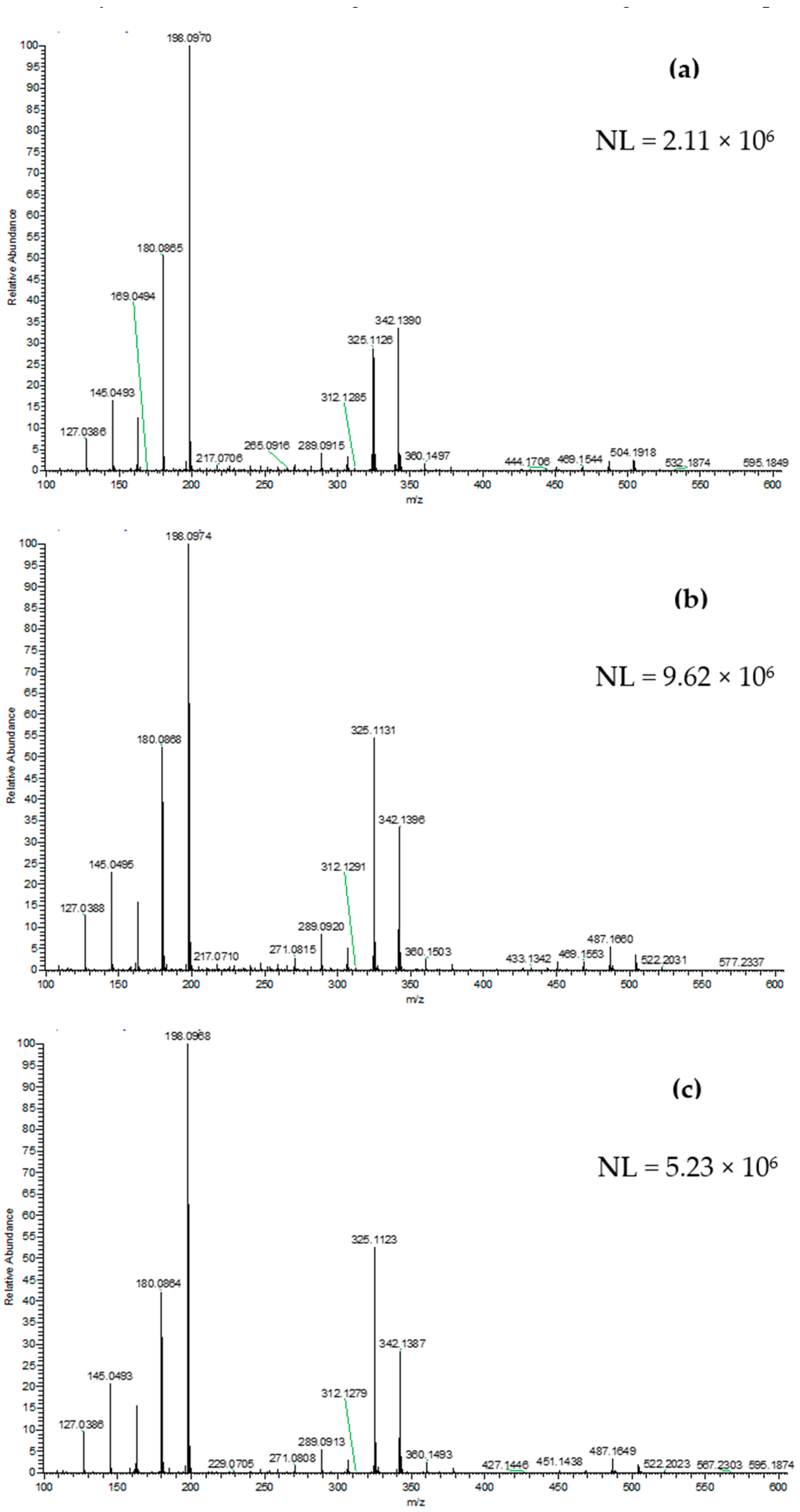
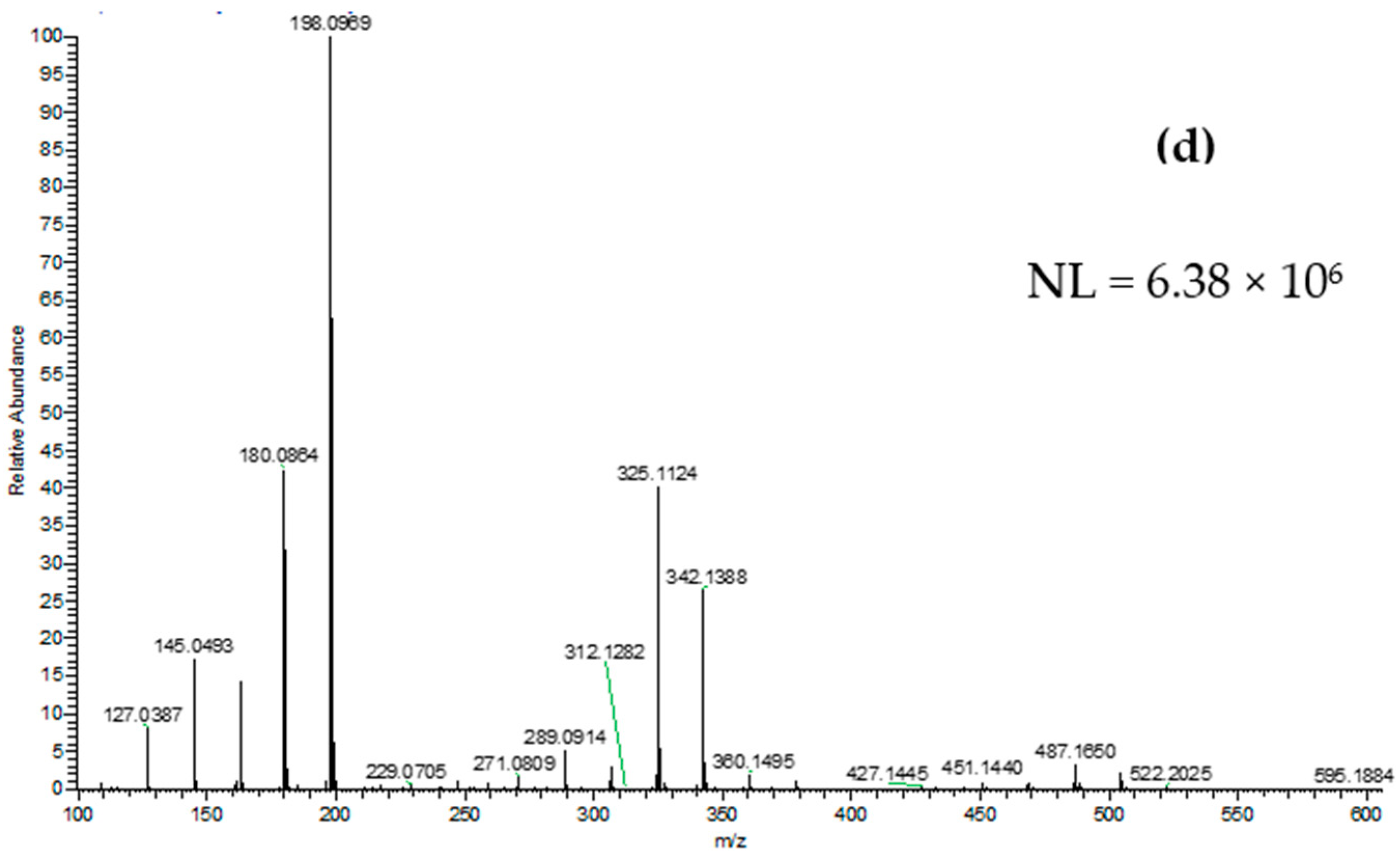
2.4. DART-HRMS Analysis
2.5. Data Processing and Statistical Analysis
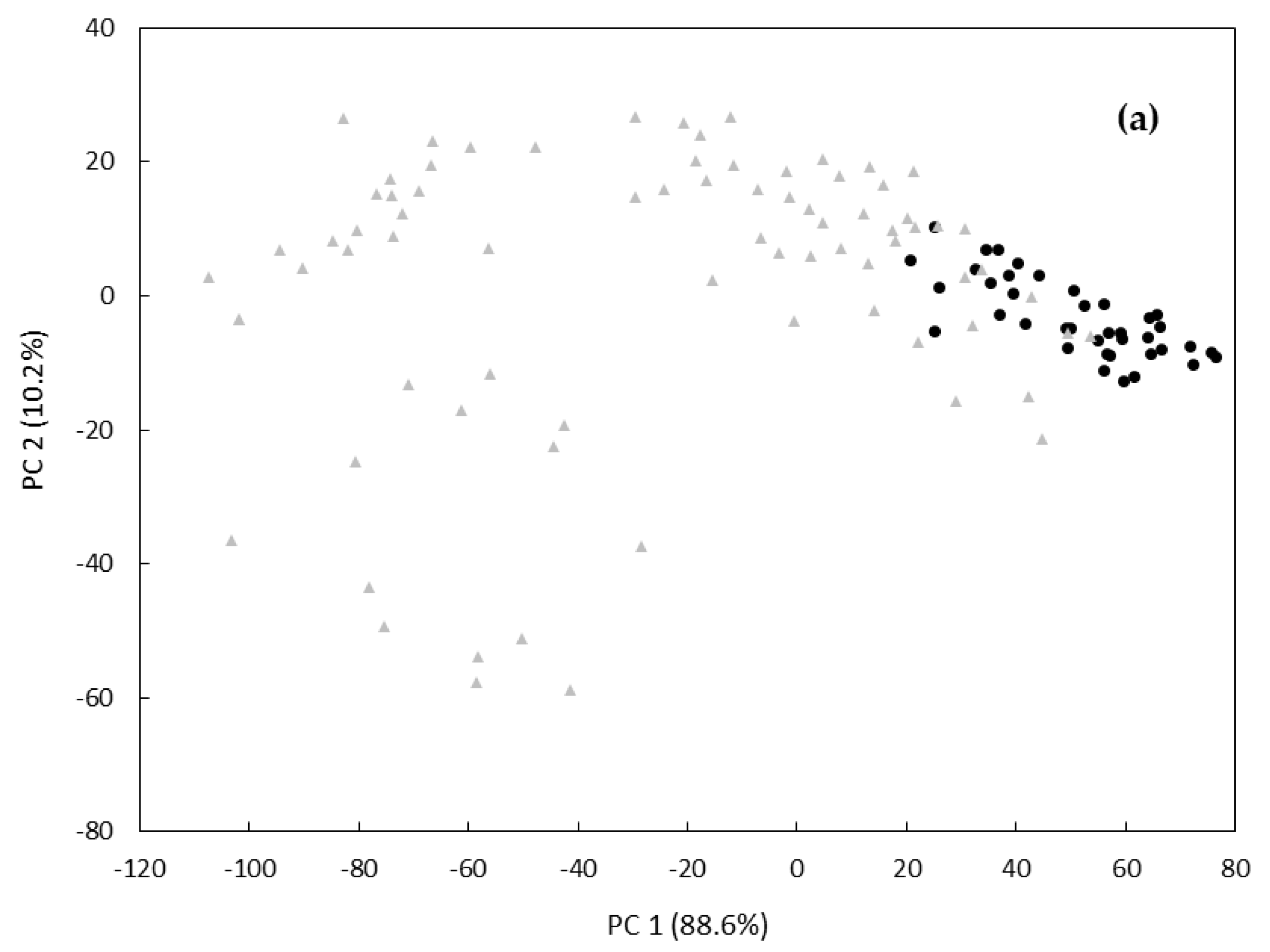
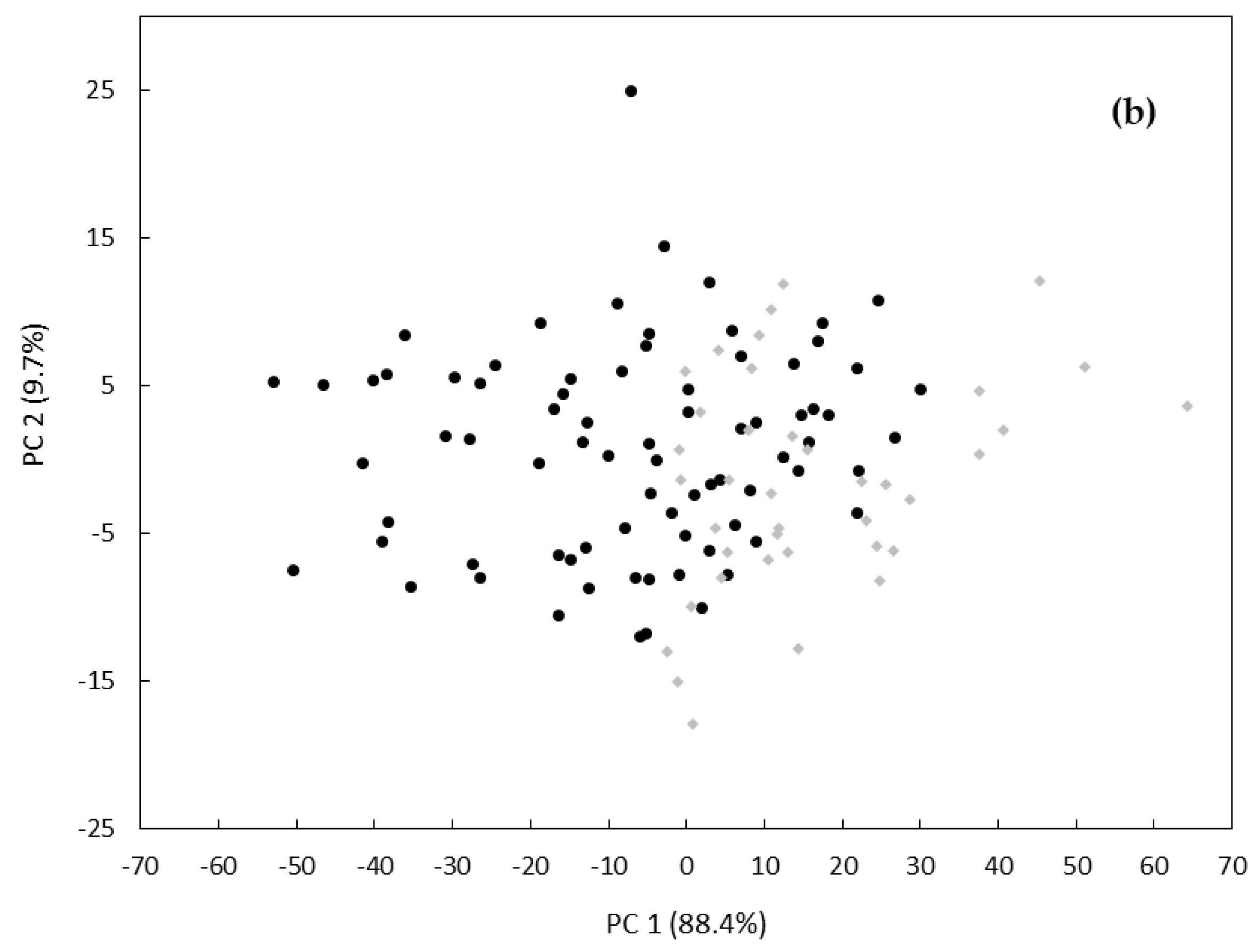
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. Directive 2014/63/EU of The European Parliament and of the Council of 15 May 2014 amending Council Directive 2001/110/EC relating to honey. Off. J. Eur. Comm. 2014, L 164, 1–5. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Revised Codex Standard for Honey (No. CODEX STAN 12–1981). 2001. Available online: www.fao.org/input/download/standards/310/cxs_012e.pdf (accessed on 25 July 2020).
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Amaral, J.S.; Oliveira, M.B.P.P.; Mafra, I. A comprehensive review on the main honey authentication issues: Production and origin. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1072–1100. [Google Scholar] [CrossRef]
- Schievano, E.; Stocchero, M.; Zuccato, V.; Conti, I.; Piana, L. NMR assessment of European acacia honey origin and composition of EU-blend based on geographical floral markers. Food Chem. 2019, 288, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; de Torres, C.; Pérez-Coello, M.S. Effect of geographical origin on the chemical and sensory characteristics of chestnut honeys. Food Res. Int. 2010, 43, 2335–2340. [Google Scholar] [CrossRef]
- Persano Oddo, L.; Piro, R. Main European unifloral honeys: Descriptive sheets. Apidologie 2004, 35, S38–S81. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations, FAOSTAT. Livestock Primary Data. 2018. Available online: http://www.fao.org/faostat/en/#data/QL/visualize (accessed on 25 July 2020).
- European Commission. Agriculture and Rural Development DOOR. 2017. Available online: http://ec.europa.eu/agriculture/quality/door/list.html (accessed on 25 July 2020).
- European Commission. Report from the Commission to the European Parliament and the Council on the Implementation of Apiculture Programmes. 2019. Available online: https://ec.europa.eu/info/food-farming-fisheries/animals-and-animal-products/animal-products/honey_en (accessed on 25 July 2020).
- Aries, E.; Burton, J.; Carrasco, L.; De Rudder, O.; Maquet, A. Scientific Support to the Implementation of a Coordinated Control Plan with a View to Establishing the Prevalence of Fraudulent Practices in the Marketing of Honey; JRC Technical Report JRC104749; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- Von Der Ohe, W.; Oddo, L.P.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized methods of melissopalynology. Apidologie 2004, 35, S18–S25. [Google Scholar] [CrossRef]
- Pita-Calvo, C.; Guerra-Rodríguez, M.E.; Vázquez, M. Analytical methods used in the quality control of honey. J. Agric. Food Chem. 2017, 65, 690–703. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Musharraf, S.G.; Choudhary, M.I.; Rahman, A. Application of analytical methods in authentication and adulteration of honey. Food Chem. 2017, 217, 687–698. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, Y.; Wu, H.; Dong, J.; Feng, J. Origin Identification and Quantitative Analysis of Honeys by Nuclear Magnetic Resonance and Chemometric Techniques. Food Anal. Methods 2016, 9, 1470–1479. [Google Scholar] [CrossRef]
- Corvucci, F.; Nobili, L.; Melucci, D.; Grillenzoni, F. The discrimination of honey origin using melissopalynology and Raman spectroscopy techniques coupled with multivariate analysis. Food Chem. 2015, 169, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Ruoff, K.; Luginbühl, W.; Künzli, R.; Iglesias, M.T.; Bogdanov, S.; Bosset, J.O.; Amadò, R. Authentication of the botanical and geographical origin of honey by midinfrared spectroscopy. J. Agric. Food Chem. 2006, 54, 6873–6880. [Google Scholar] [CrossRef] [PubMed]
- Ozcan-Sinir, G.; Copur, O.U.; Barringer, S.A. Botanical and geographical origin of Turkish honeys by selected-ion flow-tube mass spectrometry and chemometrics. J. Sci. Food Agric. 2020, 100, 2198–2207. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Papastephanou, C.; Karabagias, V.K. Geographical differentiation of Cypriot multifloral honeys through specific volatile compounds and the use of DFA. AIMS Agric. Food 2019, 4, 149–162. [Google Scholar] [CrossRef]
- Jandrić, Z.; Frew, R.D.; Fernandez-Cedi, L.N.; Cannavan, A. An investigative study on discrimination of honey of various floral and geographical origins using UPLC QToF MS and multivariate data analysis. Food Control 2017, 72, 189–197. [Google Scholar] [CrossRef]
- El Hassani, N.E.A.; Tahri, K.; Llobet, E.; Bouchikhi, B.; Errachid, A.; Zine, N.; El Bari, N. Emerging approach for analytical characterization and geographical classification of Moroccan and French honeys by means of a voltammetric electronic tongue. Food Chem. 2018, 243, 36–42. [Google Scholar] [CrossRef]
- Bougrini, M.; Tahri, K.; Saidi, T.; El Hassani, N.E.A.; Bouchikhi, B.; El Bari, N. Classification of Honey According to Geographical and Botanical Origins and Detection of Its Adulteration Using Voltammetric Electronic Tongue. Food Anal. Methods 2016, 9, 2161–2173. [Google Scholar] [CrossRef]
- Benedetti, S.; Mannino, S.; Sabatini, A.G.; Marcazzan, G.L. Electronic nose and neural network use for the classification of honey. Apidologie 2004, 35, 397–402. [Google Scholar] [CrossRef]
- Čačić, F.; Primorac, L.; Kenjerić, D.; Benedetti, S.; Mandić, M.L. Application of electronic nose in honey geographical origin characterisation. J. Cent. Eur. Agric. 2009, 10, 19–26. [Google Scholar]
- Black, C.; Chevallier, O.P.; Elliott, C.T. The current and potential applications of Ambient Mass Spectrometry in detecting food fraud. Trends Anal. Chem. 2016, 82, 268–278. [Google Scholar] [CrossRef]
- Guo, T.; Yong, W.; Jin, Y.; Liu, J.; Wang, S.; Chen, Q.; Dong, Y.; Su, H.; Tan, T. Application of DART-MS for food quality and safety assurance in food supply chain. Mass Spectrom. Rev. 2017, 36, 161–187. [Google Scholar] [CrossRef] [PubMed]
- Vaclavik, L.; Hrbek, V.; Cajka, T.; Rohlik, B.A.; Pipek, P.; Hajslova, J. Authentication of animal fats using direct analysis in real time (DART) ionization-mass spectrometry and chemometric tools. J. Agric. Food Chem. 2011, 59, 5919–5926. [Google Scholar] [CrossRef] [PubMed]
- Vaclavik, L.; Hrbek, V.; Cajka, T.; Hajslova, J. Ambient mass spectrometry employing direct analysis in real time ion source for olive oil quality and authenticity assessment. Anal. Chim. Acta 2009, 645, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Cajka, T.; Riddellova, K.; Tomaniova, M.; Hajslova, J. Ambient mass spectrometry employing a DART ion source for metabolomic fingerprinting/profiling: A powerful tool for beer origin recognition. Metabolomics 2011, 7, 500–508. [Google Scholar] [CrossRef]
- Rubert, J.; Lacina, O.; Fauhl-Hassek, C.; Hajslova, J. Metabolic fingerprinting based on high-resolution tandem mass spectrometry: A reliable tool for wine authentication? Anal. Bioanal. Chem. 2014, 406, 6791–6803. [Google Scholar] [CrossRef] [PubMed]
- Hrbek, V.; Vaclavik, L.; Elich, O.; Hajslova, J. Authentication of milk and milk-based foods by direct analysis in real time ionization-high resolution mass spectrometry (DART-HRMS) technique: A critical assessment. Food Control 2014, 36, 138–145. [Google Scholar] [CrossRef]
- Fiorino, G.M.; Losito, I.; De Angelis, E.; Arlorio, M.; Logrieco, A.F.; Monaci, L. Assessing fish authenticity by direct analysis in real time-high resolution mass spectrometry and multivariate analysis: Discrimination between wildtype and farmed salmon. Food Res. Int. 2019, 116, 1258–1265. [Google Scholar] [CrossRef]
- Hrbek, V.; Rektorisova, M.; Chmelarova, H.; Ovesna, J.; Hajslova, J. Authenticity assessment of garlic using a metabolomic approach based on high resolution mass spectrometry. J. Food Compos. Anal. 2018, 67, 19–28. [Google Scholar] [CrossRef]
- Chernetsova, E.S.; Morlock, G.E. Assessing the capabilities of direct analysis in real time mass spectrometry for 5-hydroxymethylfurfural quantitation in honey. Int. J Mass Spectrom. 2012, 314, 22–32. [Google Scholar] [CrossRef]
- Rajchl, A.; Drgová, L.; Grégrová, A.; Čížková, H.; Ševčík, R.; Voldřich, M. Rapid determination of 5-hydroxymethylfurfural by DART ionization with time-of-flight mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 4737–4745. [Google Scholar] [CrossRef]
- AOAC Official Method. AOAC 920.180. Honey (Liquid, Strained, or Comb). Preparation of Test Sample; 2020 Edition; AOAC: Rockville, MD, USA, 1920.
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Oliveri, P.; Downey, G. Multivariate class modelling for the verification of food-authenticity claims. TrAC Trend Anal. Chem. 2012, 35, 74–86. [Google Scholar] [CrossRef]
- Casale, M.; Casolino, C.; Oliveri, P.; Forina, M. The potential of coupling information using three analytical techniques for identifying the geographical origin of Liguria extra virgin olive oil. Food Chem. 2010, 118, 163–170. [Google Scholar] [CrossRef]
- Granato, D.; Putnik, P.; Kovačević, D.B.; Santos, J.S.; Calado, V.; Rocha, R.S.; Da Cruz, A.G.; Jarvis, B.; Rodionova, O.E.; Pomerantsev, A. Trends in chemometrics: Food authentication, microbiology, and effects of processing. Compr. Rev. Food Sci. Food Saf. 2018, 17, 663–677. [Google Scholar] [CrossRef]
| Model Performance (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Recognition Ability (Modelling) | Prediction Ability (CV c 10) | External Prediction | |||||||
| ITA a | POR b | Mean | ITA | POR | Mean | ITA | POR | Mean | |
| PCA/LDA d (7 Principal Components) | 100.0 (30/30) | 96.7 (29/30) | 98.4 | 100.0 (30/30) | 96.7 (29/30) | 98.4 | 88.9 (8/9) | 91.7 (44/48) | 90.3 |
| PLS-DA e (10 Latent Variables) | 100.0 (30/30) | 96.7 (29/30) | 98.4 | 100.0 (30/30) | 93.3 (28/30) | 96.7 | 88.9 (8/9) | 89.6 (43/48) | 89.2 |
| k-NN f (k value of 3) | 96.7 (29/30) | 100.0 (30/30) | 98.4 | 96.7 (29/30) | 100.0 (30/30) | 98.4 | 88.9 (8/9) | 93.8 (45/48) | 91.4 |
| Model Performance (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Recognition Ability (Modelling) | Prediction Ability (CV c 10) | External Prediction | |||||||
| ITA a | CHI b | Mean | ITA | CHI | Mean | ITA | CHI | Mean | |
| PCA/LDA d (9 Principal Components) | 96.7 (29/30) | 93.3 (28/30) | 95.0 | 96.7 (29/30) | 90.0 (27/30) | 93.4 | 89.6 (43/48) | 88.9 (8/9) | 89.2 |
| PLS-DA e (12 Latent Variables) | 100.0 (30/30) | 93.3 (28/30) | 96.7 | 96.7 (29/30) | 93.3 (28/30) | 95.0 | 93.8 (45/48) | 77.8 (7/9) | 85.8 |
| k-NN f (k value of 3) | 100.0 (30/30) | 90.0 (27/30) | 95.0 | 93.3 (28/30) | 90.0 (27/30) | 91.7 | 91.7 (44/48) | 88.9 (8/9) | 90.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lippolis, V.; De Angelis, E.; Fiorino, G.M.; Di Gioia, A.; Arlorio, M.; Logrieco, A.F.; Monaci, L. Geographical Origin Discrimination of Monofloral Honeys by Direct Analysis in Real Time Ionization-High Resolution Mass Spectrometry (DART-HRMS). Foods 2020, 9, 1205. https://doi.org/10.3390/foods9091205
Lippolis V, De Angelis E, Fiorino GM, Di Gioia A, Arlorio M, Logrieco AF, Monaci L. Geographical Origin Discrimination of Monofloral Honeys by Direct Analysis in Real Time Ionization-High Resolution Mass Spectrometry (DART-HRMS). Foods. 2020; 9(9):1205. https://doi.org/10.3390/foods9091205
Chicago/Turabian StyleLippolis, Vincenzo, Elisabetta De Angelis, Giuseppina Maria Fiorino, Annalisa Di Gioia, Marco Arlorio, Antonio Francesco Logrieco, and Linda Monaci. 2020. "Geographical Origin Discrimination of Monofloral Honeys by Direct Analysis in Real Time Ionization-High Resolution Mass Spectrometry (DART-HRMS)" Foods 9, no. 9: 1205. https://doi.org/10.3390/foods9091205
APA StyleLippolis, V., De Angelis, E., Fiorino, G. M., Di Gioia, A., Arlorio, M., Logrieco, A. F., & Monaci, L. (2020). Geographical Origin Discrimination of Monofloral Honeys by Direct Analysis in Real Time Ionization-High Resolution Mass Spectrometry (DART-HRMS). Foods, 9(9), 1205. https://doi.org/10.3390/foods9091205