Effect of Breed on the Volatile Compound Precursors and Odor Profile Attributes of Lamb Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Management and Sample Collection
2.2. Chemical Analysis
2.3. Fatty Acid Analysis
2.4. Amino Acid Analysis
2.5. Volatile Compounds Analysis
2.5.1. Volatile Compound Extraction
2.5.2. Volatile Compound Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition
3.2. Fatty Acid Composition
3.3. Amino Acid Composition
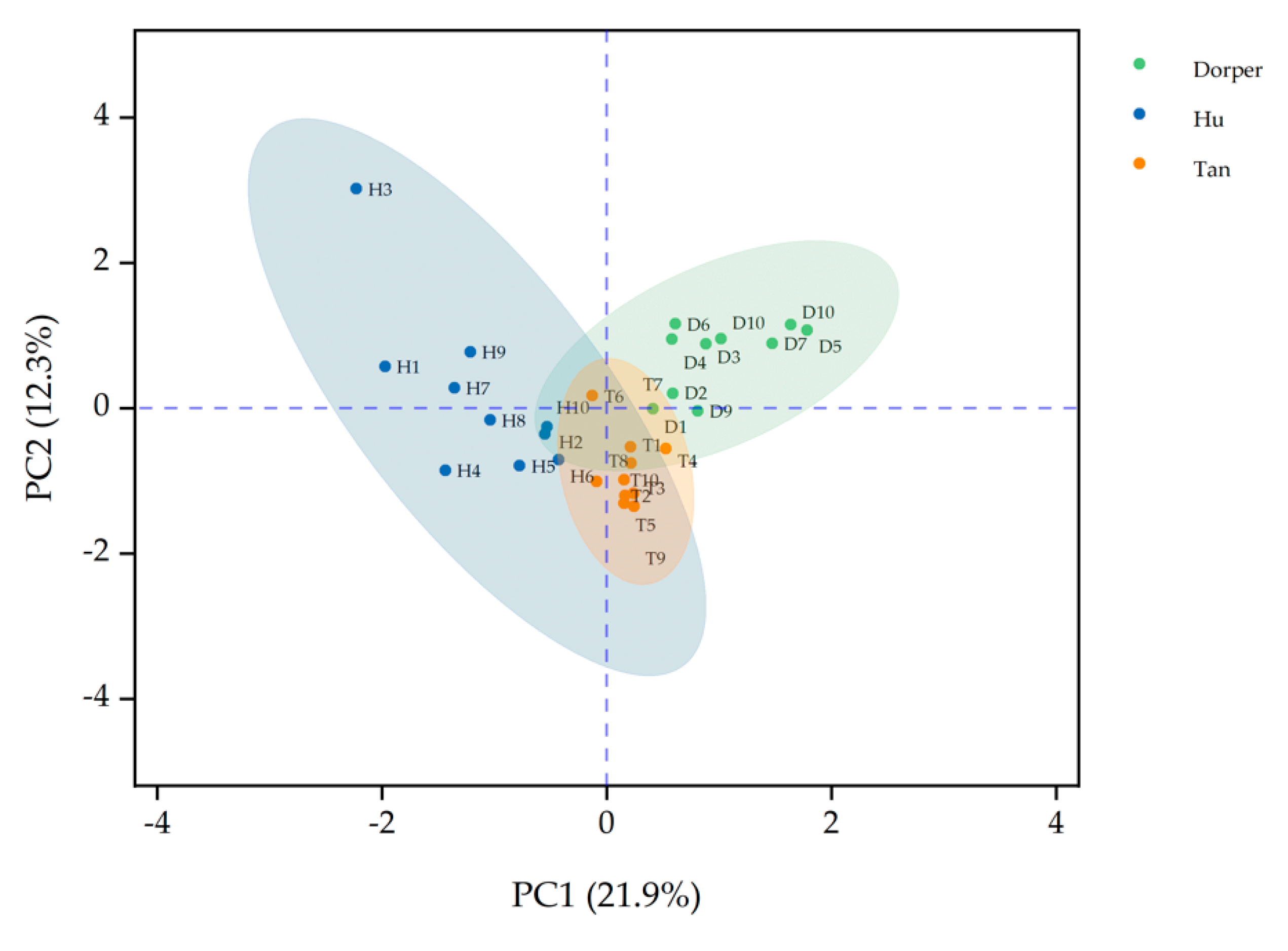
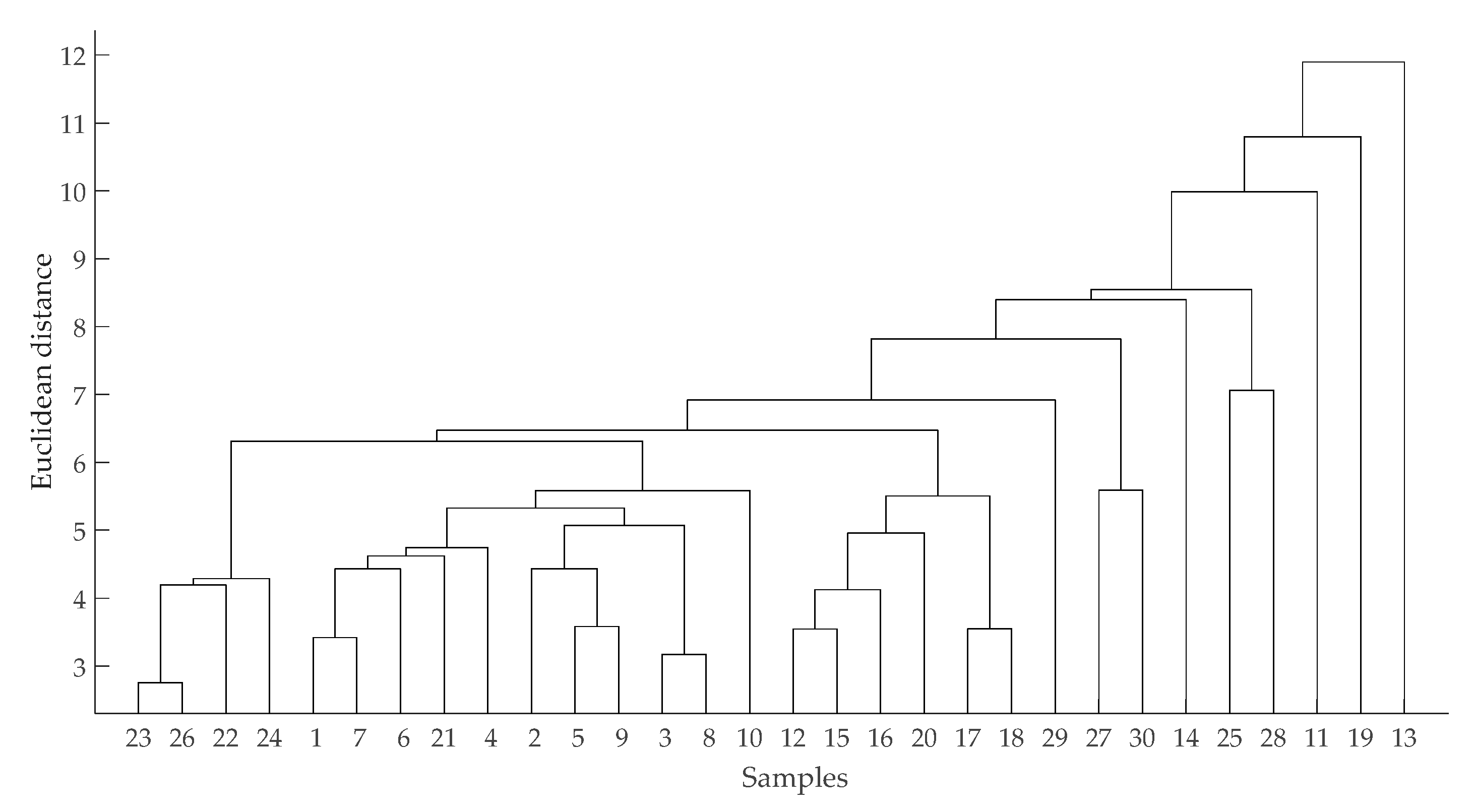
3.4. Volatile Compound Composition
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mapiye, C.; Aldai, N.; Turner, T.D.; Aalhus, J.L.; Rolland, D.C.; Kramer, J.K.G.; Dugan, M.E.R. The labile lipid fraction of meat: From perceived disease and waste to health and opportunity. Meat Sci. 2012, 92, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Oltra, O.R.; Farmer, L.J.; Gordon, A.W.; Moss, B.W.; Birnie, J.; Devlin, D.J.; Tolland, E.L.C.; Tollerton, I.J.; Beattie, A.M.; Kennedy, J.T. Identification of sensory attributes, instrumental and chemical measurements important for consumer acceptability of grilled lamb Longissimus lumborum. Meat Sci. 2015, 100, 97–109. [Google Scholar] [CrossRef]
- Oliver, M.A.; Nute, G.R.; Furnols, M.F.I.; Julian, R.S.; Campo, M.M.; Sanudo, C.; Caneque, V.; Guerrero, L.; Alvarez, I.; Diaz, M.T. Eating quality of beef, from different production systems, assessed by German, Spanish and British consumers. Meat Sci. 2006, 74, 435–442. [Google Scholar] [CrossRef]
- Frank, D.; Watkins, P.; Ball, A.; Krishnamurthy, R.; Piyasiri, U.; Sewell, J.; Ortu, O.J.; Stark, J.; Warner, R. Impact of Brassica and Lucerne Finishing Feeds and Intramuscular Fat on Lamb Eating Quality and Flavor. A Cross-Cultural Study Using Chinese and Non-Chinese Australian Consumers. J. Agric. Food Chem. 2016, 64, 6856–6868. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.; Resconi, V.C.; Campo, M.M.; Cacho, J.; Ferreira, V.; Escudero, A. Effect of freezing method and frozen storage duration on odor-active compounds and sensory perception of lamb. Food Res. Int. 2013, 54, 772–780. [Google Scholar] [CrossRef]
- Gkarane, V.; Brunton, N.P.; Allen, P.; Gravador, R.S.; Claffey, N.A.; Diskin, M.G.; Fahey, A.G.; Farmer, L.J.; Moloney, A.P.; Alcalde, M.J. Effect of finishing diet and duration on the sensory quality and volatile profile of lamb meat. Food Res. Int. 2019, 115, 54–64. [Google Scholar] [CrossRef]
- Young, O.A.; Lane, G.A.; Priolo, A.; Fraser, K. Pastoral and species flavour in lambs raised on pasture, lucerne or maize. J. Sci. Food Agric. 2003, 83, 93–104. [Google Scholar] [CrossRef]
- Francisco, A.; Dentinho, M.T.; Alves, S.P.; Portugal, P.V.; Fernandes, F. Growth performance, carcass and meat quality of lambs supplemented with increasing levels of a tanniferous bush (Cistus ladanifer L.) and vegetable oils. Meat Sci. 2015, 100, 275–282. [Google Scholar] [CrossRef]
- Gkarane, V.; Allen, P.; Gravador, R.S.; Diskin, M.G.; Claffey, N.A.; Fahey, A.G.; Brunton, N.P.; Farmer, L.J.; Moloney, A.P.; Monahan, F.J. Effect of castration and age at slaughter on sensory perception of lamb meat. Small Rumin. Res. 2017, 157, 65–74. [Google Scholar] [CrossRef]
- Sanudo, C.; Muela, E.; Del, M.; Campo, M. Key Factors Involved in Lamb Quality from Farm to Fork in Europe. J. Integr. Agric. 2013, 12, 1919–1930. [Google Scholar] [CrossRef]
- Gkarane, V.; Brunton, N.P.; Harrison, S.M.; Gravador, R.S.; Allen, P.; Claffey, N.A.; Diskin, M.G.; Fahey, A.G.; Farmer, L.J.; Moloney, A.P. Volatile Profile of Grilled Lamb as Affected by Castration and Age at Slaughter in Two Breeds. J. Food Sci. 2018, 83, 2466–2477. [Google Scholar] [CrossRef] [PubMed]
- Boughalmi, A.; Araba, A. Effect of feeding management from grass to concentrate feed on growth, carcass characteristics, meat quality and fatty acid profile of Timahdite lamb breed. Small Rumin. Res. 2016, 144, 158–163. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Chen, Y.; Luo, H.L.; Liu, X.; Liu, K. Influence of Restricted Grazing Time Systems on Productive Performance and Fatty Acid Composition of Longissimus dorsi in Growing Lambs. Asian Australas. J. Anim. Sci. 2015, 28, 1105–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Wang, Z.; Miao, J.; Xie, L.; Dai, Y.; Li, X.; Chen, Y.; Luo, H.; Dai, R. Influence of different production strategies on the stability of color oxygen consumption and metmyoglobin reducing activity of meat from Ningxia Tan sheep. Meat Sci. 2014, 96, 769–774. [Google Scholar] [CrossRef]
- Ahmed, E.M.; Dennison, R.A.; Dougherty, R.H.; Shaw, P.E. Flavor and odor thresholds in water of selected orange juice components. J. Agric. Food Chem. 1978, 26, 187–191. [Google Scholar] [CrossRef]
- Hajji, H.; Joy, M.; Ripoll, G.; Smeti, S.; Mekki, I.; Gahete, F.M.; Mahouachi, M.; Atti, N. Meat physicochemical properties, fatty acid profile, lipid oxidation and sensory characteristics from three North African lamb breeds, as influenced by concentrate or pasture finishing diets. J. Food Compos. Anal. 2016, 48, 102–110. [Google Scholar] [CrossRef]
- Dettori, M.L.; Carcangiu, V.; Cengarle, L.; Tilloca, G.R.; Vacca, G.M. Fatty acid profile of lamb semitendinous muscle and perirenal adipose tissue from two different genotypes. J. Anim. Feed Sci. 2004, 13, 681–684. [Google Scholar] [CrossRef]
- Elmore, J.S.; Mottram, D.S. Formation of 2-alkyl-(2H)-thiapyrans and 2-alkylthiophenes in cooked beef and lamb. J. Agric. Food Chem. 2000, 48, 2420–2424. [Google Scholar] [CrossRef]
- Madruga, M.S.; Dantas, I.; De Queiroz, A.L.M.; Brasil, L.; Ishihara, Y.M. Volatiles and Water- and Fat-Soluble Precursors of Saanen Goat and Cross Suffolk Lamb Flavour. Molecules 2013, 18, 2150–2165. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, A.R.; Kohram, H.; Shahneh, A.Z.; Nikkhah, A.; Campbell, A. Comparison of the meat quality and fatty acid composition of traditional fat-tailed (Chall) and tailed (Zel) Iranian sheep breeds. Meat Sci. 2012, 92, 417–422. [Google Scholar] [CrossRef]
- Lee, J.H.; Kannan, G.; Eega, K.R.; Kouakou, B.; Getz, W.R. Nutritional and quality characteristics of meat from goats and lambs finished under identical dietary regime. Small Rumin. Res. 2008, 74, 255–259. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the AOAC International, 16th ed.; AOAC International: Arlington, MA, USA, 1995. [Google Scholar]
- Machiels, D.; Istasse, L. Evaluation of two commercial solid-phase microextraction fibres for the analysis of target aroma compounds in cooked beef meat. Talanta 2003, 61, 529–537. [Google Scholar] [CrossRef]
- Guo, S.; Jom, K.N.; Ge, Y. Influence of Roasting Condition on Flavor Profile of Sunflower Seeds. A flavoromics approach. Sci. Rep. 2019, 91, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jandasek, J.; Milerski, M.; Lichovnikova, M. Effect of sire breed on physico-chemical and sensory characteristics of lamb meat. Meat Sci. 2014, 96, 88–93. [Google Scholar] [CrossRef]
- Wachira, A.M.; Sinclair, L.A.; Wilkinson, R.G.; Enser, M.; Wood, J.D.; Fisher, A.V. Effects of dietary fat source and breed on the carcass composition, n-3 polyunsaturated fatty acid and conjugated linoleic acid content of sheep meat and adipose tissue. Br. J. Nutr. 2002, 88, 697–709. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, L.A.; Cooper, S.L.; Chikunya, S.; Wilkinson, R.G.; Hallett, K.G.; Enser, M.; Wood, J.D. Biohydrogenation of n-3 polyunsaturated fatty acids in the rumen and their effects on microbial metabolism and plasma fatty acid concentrations in sheep. Anim. Sci. 2005, 81, 239–248. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; He, Z.; Wang, T.; Qin, G. Study on the flavor contribution of phospholipids and triglycerides to pork. Food Sci. Biotechnol. 2010, 19, 1267–1276. [Google Scholar] [CrossRef]
- Elmore, J.S.; Campo, M.M.; Enser, M.; Mottram, D.S. Effect of lipid composition on meat-like model systems containing cysteine, ribose, and polyunsaturated fatty acids. J. Agric. Food Chem. 2002, 50, 1126–1132. [Google Scholar] [CrossRef]
- Nute, G.R.; Richardson, R.I.; Wood, J.D.; Hughes, S.I.; Wilkinson, R.G.; Cooper, S.L.; Sinclair, L.A. Effect of dietary oil source on the flavour and the colour and lipid stability of lamb meat. Meat Sci. 2007, 77, 547–555. [Google Scholar] [CrossRef]
- Elmore, J.S.; Mottram, D.S.; Enser, M.; Wood, J.D. Effect of the polyunsaturated fatty acid composition of beef muscle on the profile of aroma volatiles. J. Agric. Food Chem. 1999, 47, 1619–1625. [Google Scholar] [CrossRef]
- Diaz, M.T.; Alvarez, I.; La Fuente, J.D.; Sanudo, C.; Campo, M.M.; Oliver, M.A.; Furnols, M.F.I.; Montossi, F.; Julian, R.S.; Nute, G.R. Fatty acid composition of meat from typical lamb production systems of Spain, United Kingdom, Germany and Uruguay. Meat Sci. 2005, 71, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Rohini, A.; Agrawal, N.; Kumar, H.; Kumar, V. Emerging role of branched chain amino acids in metabolic disorders: A mechanistic review. PharmaNutrition 2018, 6, 47–54. [Google Scholar] [CrossRef]
- Shimomura, Y.; Kitaura, Y.J. Physiological and pathological roles of branched-chain amino acids in the regulation of protein and energy metabolism and neurological functions. Pharm. Res. 2018, 133, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.K.; Lindsay, R.C. Volatile Alkylphenols and Thiophenol in Species-related Characterizing Flavors of Red Meats. J. Food Sci. 1991, 56, 1197–1202. [Google Scholar] [CrossRef]
- Moon, S.; Cliff, M.A.; Lichan, E.C.Y. Odour-active components of simulated beef flavour analysed by solid phase microextraction and gas chromatography–mass spectrometry and –olfactometry. Food Res. Int. 2006, 39, 294–308. [Google Scholar] [CrossRef]
- Cerny, C.; Briffod, M. Effect of pH on the Maillard Reaction of [13C5]Xylose, Cysteine, and Thiamin. J. Agric. Food Chem. 2007, 55, 1552–1556. [Google Scholar] [CrossRef] [PubMed]
- Kerscher, R.; Grosch, W. Quantification of 2-methyl-3-furanthiol, 2-furfurylthiol, 3-mercapto-2-pentanone, and 2-mercapto-3-pentanone in heated meat. J. Agric. Food Chem. 1998, 46, 1954–1958. [Google Scholar] [CrossRef]
- Elmore, J.S.; Cooper, S.L.; Enser, M.; Mottram, D.S.; Sinclair, L.A.; Wilkinson, R.G.; Wood, J.D. Dietary manipulation of fatty acid composition in lamb meat and its effect on the volatile aroma compounds of grilled lamb. Meat Sci. 2005, 69, 233–242. [Google Scholar] [CrossRef]
- Resconi, V.C.; Campo, M.M.; Montossi, F.; Ferreira, V.; Sanudo, C.; Escudero, A. Relationship between odour-active compounds and flavour perception in meat from lambs fed different diets. Meat Sci. 2010, 85, 700–706. [Google Scholar] [CrossRef]
- Selli, S.; Cayhan, G.G. Analysis of volatile compounds of wild gilthead sea bream (Sparus aurata) by simultaneous distillation–extraction (SDE) and GC–MS. Microchem. J. 2009, 93, 232–235. [Google Scholar] [CrossRef]
- Calkins, C.R.; Hodgen, J.M. A fresh look at meat flavor. Meat Sci. 2007, 77, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Wurzenberger, M.; Grosch, W. Enzymic oxidation of linolenic acid to 1,Z-5-octadien-3-ol, Z-2,Z-5-octadien-1-ol and 10-oxo-E-8-decenoic acid by a protein fraction from mushrooms (Psalliota bispora). Lipids 1986, 21, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Van, B.H.; Amna, T.; Hwang, I. Significant influence of particular unsaturated fatty acids and pH on the volatile compounds in meat-like model systems. Meat Sci. 2013, 94, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, T.; Xie, J.; Xiao, Q.; Cheng, J.; Chen, F.; Wang, S.; Sun, B. Formation mechanism of aroma compounds in a glutathione-glucose reaction with fat or oxidized fat. Food Chem. 2019, 270, 436–444. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | Content (%) | Nutrient Level | Content |
|---|---|---|---|
| Pellet | ME 2 (MJ/kg) | 9.86 | |
| Corn | 37.33 | Crude protein | 15.78 |
| Wheat bran | 6.67 | Crude fiber | 14.54 |
| Rapeseed cake | 5.33 | Neutral detergent fiber | 45.39 |
| Soybean meal | 5.33 | Acid detergent fiber | 31.06 |
| Rice bran | 4.00 | Ether extract | 2.34 |
| Corn gluten meal | 4.00 | Total Calcium | 0.50 |
| Nahco3 | 1.33 | Total Phosphorus | 0.29 |
| Salt | 1.33 | ||
| Premix 1 | 1.33 | ||
| Alfalfa meal | 33.33 | ||
| Total | 100.00 |
| Trait | Breed | SEM | p Value | ||
|---|---|---|---|---|---|
| Tan (n = 10) | Dorper (n = 10) | Hu (n = 10) | |||
| Moisture (%) | 67.24 b | 71.71 a | 67.92 b | 0.492 | <0.001 |
| Crude Protein (%) | 18.94 b | 21.18 a | 19.20 b | 0.324 | <0.001 |
| Intramuscular fat (%) | 10.96 a | 3.83 b | 10.02 a | 0.623 | 0.005 |
| Ash (%) | 3.06 b | 4.13 a | 3.21 b | 0.118 | <0.001 |
| SFA (%) | Tan (n = 10) | Dorper (n = 10) | Hu (n = 10) | SEM | p Value |
|---|---|---|---|---|---|
| C10:0 | 0.10 | 0.12 | 0.12 | 0.004 | 0.158 |
| C12:0 | 0.21 a | 0.11 b | 0.13 b | 0.013 | 0.001 |
| C14:0 | 3.31 a | 2.08 b | 2.56 b | 0.138 | <0.001 |
| C15:0 | 0.49 a | 0.38 b | 0.44 a | 0.014 | 0.001 |
| C16:0 | 24.55 | 23.93 | 24.25 | 0.227 | 0.554 |
| C17:0 | 1.49 | 1.29 | 1.41 | 0.059 | 0.386 |
| C18:0 | 19.88 b | 19.58 b | 22.10 a | 0.353 | 0.003 |
| C20:0 | 0.13 b | 0.12 b | 0.15 a | 0.003 | <0.001 |
| C21:0 | 0.19 b | 0.31 a | 0.15 b | 0.017 | <0.001 |
| C22:0 | 0.04 b | 0.05 a | 0.03 b | 0.002 | 0.002 |
| C23:0 | 0.04 b | 0.06 a | 0.03 b | 0.008 | <0.001 |
| C24:0 | 0.03 b | 0.04 a | 0.02 b | 0.002 | <0.001 |
| ΣSFA | 50.47 a | 48.07 b | 51.38 a | 0.357 | <0.001 |
| MUFA (%) | Tan (n = 10) | Dorper (n = 10) | Hu (n = 10) | SEM | p Value |
|---|---|---|---|---|---|
| C14:1 | 0.10 a | 0.07 b | 0.08 b | 0.005 | 0.025 |
| C16:1 | 1.55 | 1.44 | 1.44 | 0.037 | 0.371 |
| C18:1n-9c | 40.72 | 40.37 | 40.60 | 0.36 | 0.645 |
| C20:1 | 0.13 b | 0.14 b | 0.15 a | 0.002 | 0.009 |
| C22:1n-9 | 0.04 | 0.03 | 0.04 | 0.001 | 0.159 |
| C24:1 | 0.02 b | 0.04 a | 0.02 b | 0.002 | <0.001 |
| ΣMUFA | 43.04 | 42.09 | 42.32 | 0.378 | 0.581 |
| PUFA (%) | Tan (n = 10) | Dorper (n = 10) | Hu (n = 10) | SEM | p Value |
|---|---|---|---|---|---|
| C18:2n-6c | 5.09 b | 7.17 a | 4.94 b | 0.313 | 0.002 |
| C18:3n-3 | 0.32 b | 0.44 a | 0.43 a | 0.014 | <0.001 |
| C20:2 | 0.02 b | 0.04 a | 0.02 b | 0.002 | <0.001 |
| C20:3n-6 | 0.08 b | 0.17 a | 0.08 b | 0.011 | <0.001 |
| C20:4n-6 | 0.92 b | 1.86 a | 0.76 b | 0.129 | <0.001 |
| C20:3n-3 | 0.01 b | 0.03 a | 0.01 b | 0.002 | <0.001 |
| C20:5n-3 (EPA) | 0.04 b | 0.08 a | 0.03 b | 0.005 | <0.001 |
| C22:6n-3 (DHA) | 0.02 b | 0.05 a | 0.03 a | 0.003 | <0.001 |
| ΣPUFA | 6.49 b | 9.84 a | 6.30 b | 0.468 | 0.001 |
| P/S | 0.13 b | 0.20 a | 0.12 b | 0.010 | <0.001 |
| n-6 PUFA | 6.08 b | 9.20 a | 5.78 b | 0.446 | 0.001 |
| n-3 PUFA | 0.39 c | 0.60 a | 0.50 b | 0.022 | <0.001 |
| n-6/n-3 PUFA | 15.28 a | 15.22 a | 11.55 b | 0.402 | <0.001 |
| Amino Acid (%) | Tan (n = 10) | Dorper (n = 10) | Hu (n = 10) | SEM | p Value |
|---|---|---|---|---|---|
| Aspartic acid | 5.51 b | 7.00 a | 5.53 b | 0.159 | <0.001 |
| Threonine | 2.77 b | 3.50 a | 2.78 b | 0.080 | <0.001 |
| Serine | 2.32 b | 2.95 a | 2.57 b | 0.069 | <0.001 |
| Glutamic acid | 9.00 b | 11.46 a | 9.26 b | 0.252 | <0.001 |
| Proline | 2.32 c | 3.11 a | 2.69 b | 0.084 | <0.001 |
| Glycine | 2.92 b | 3.39 a | 3.23 ab | 0.072 | 0.019 |
| Alanine | 3.17 b | 3.95 a | 3.38 b | 0.100 | 0.002 |
| Cysteine | 0.84 b | 1.01 a | 0.83 b | 0.024 | 0.001 |
| Valine | 2.77 b | 3.87 a | 2.98 b | 0.105 | <0.001 |
| Methionine | 2.00 b | 2.28 a | 1.88 b | 0.060 | 0.015 |
| Isoleucine | 2.67 b | 3.57 a | 2.70 b | 0.92 | <0.001 |
| Leucine | 4.82 b | 6.19 a | 4.88 b | 0.146 | <0.001 |
| Tyrosine | 2.21 b | 2.83 a | 2.10 b | 0.094 | 0.001 |
| Phenylalanine | 2.34 b | 3.04 a | 2.37 b | 0.072 | <0.001 |
| Histidine | 1.84 b | 2.54 a | 1.87 b | 0.076 | <0.001 |
| Lysine | 4.99 b | 6.86 a | 5.34 b | 0.177 | <0.001 |
| Arginine | 3.72 b | 4.81 a | 3.97 b | 0.109 | <0.001 |
| Tryptophan | 0.66 b | 0.85 a | 0.64 b | 0.022 | <0.001 |
| Volatile Compounds | Retention Index | Relative Content/% | SEM | p Value | Odorant Descriptors | |||
|---|---|---|---|---|---|---|---|---|
| Tan (n = 10) | Dorper (n = 10) | Hu (n = 10) | ||||||
| Aldehydes | Hexanal | 1081 | 18.29 a | 18.22 a | 10.46 b | 1.38 | 0.021 | Herbal, grassy |
| Heptanal | 1183 | 14.24 b | 37.00 a | 13.28 b | 3.24 | 0.002 | Oily, stock | |
| (E)-2-hexenal | 1266 | 1.309 | Apple, fruit | |||||
| Octanal | 1290 | 13.01 a | 16.66 a | 4.76 b | 1.28 | <0.001 | Citrus, floral | |
| Nonanal | 1394 | 14.97 | 12.30 | 9.21 | 1.15 | 0.093 | Fatty, sweet | |
| (E)-2-octenal | 1408 | 2.88 b | 6.89 a | 0.95 c | 0.54 | <0.001 | Nutty, meaty | |
| Benzaldehyde | 1495 | 9.85 b | 5.24 c | 25.07 a | 2.39 | <0.001 | Almond, caramel | |
| (E)-2-nonenal | 1538 | 1.263 | Fatty, rancid | |||||
| (E)-2-decenaldehyde | 1621 | 1.39 | 1.08 | 3.73 | 1.38 | 0.688 | Chicken fat, orange | |
| Phenylacetaldehyde | 1639 | 0.82 b | 1.30 a | 0.56 b | 0.11 | 0.008 | Honey, sweet | |
| 4-ethylbenzaldehyde | 1707 | 0.311 | bitter almond | |||||
| (E)-2-undecenal | 1754 | 0.30 b | 1.56 a | 0.20 | <0.001 | Wax, fatty | ||
| (E,E)-2,4-decadienal | 1811 | 1.52 | Chicken fat, poultry | |||||
| Alcohols | Pentanol | 1255 | 1.49 b | 4.28 c | 2.53 b | 0.33 | <0.001 | Oily |
| Hexanol | 1360 | 1.28 | 1.44 | 1.40 | 0.10 | 0.825 | Pine, fruit | |
| 1-octene-3-ol | 1453 | 5.66 b | 9.90 a | 5.28 b | 0.76 | 0.023 | Mushroom, lavender | |
| Heptanol | 1461 | 1.61 | 1.66 | 2.25 | 0.23 | 0.462 | Grassy, fresh | |
| 2-ethylhexanol | 1491 | 3.08 | 1.08 | 2.21 | 0.38 | 0.129 | Rosy, sweet | |
| Linalool | 1537 | 0.38 | 0.06 | 0.26 | 0.06 | 0.105 | Floral, tea | |
| Octanol | 1564 | 2.07 | 2.04 | 2.44 | 0.20 | 0.676 | Burnt | |
| (E)-2-octenol | 1620 | 0.55 | 1.28 | 2.86 | 0.47 | 0.120 | Fatty | |
| Benzyl alcohol | 1806 | 0.78 b | 0.62 b | 2.95 a | 0.34 | 0.001 | Sweet, flora | |
| Heterocyclic compounds | Furfuryl alcohol | 1199 | 0.56 | 0.65 | 0.76 | 0.06 | 0.452 | Bitter, burnt |
| 2-pentylfuran | 1224 | 12.11 | 11.47 | 1.87 | 0.871 | Roast, buttery | ||
| Thiophene-3-carboxaldehyde | 1800 | 0.17 c | 0.61 b | 0.92 a | 0.08 | <0.001 | - | |
| 2-acetylpyrrole | 1974 | 0.56 | 0.57 | 2.45 | 0.42 | 0.125 | Nutty, toast | |
| Ketones | 3-hydroxy-2-butanone | 1287 | 3.44 b | 3.70 b | 17.85 a | 2.36 | 0.005 | Buttery, cream |
| 2-undecanone | 1543 | 0.28 | 0.27 | 0.40 | 0.04 | 0.442 | Orange, grassy | |
| Acids | Hexanoic acid | 1803 | 0.95 a | 0.58 b | 0.07 | 0.006 | Sweat, mutton | |
| Octanoic acid | 2083 | 0.14 a | 0.03 b | 0.12 a | 0.01 | <0.001 | Cheese, mutton | |
| Alkenes | Styrene | 1251 | 12.85 | 9.34 | 1.10 | 0.113 | Sweet, flora | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhang, H.; Liu, M.; Zhao, X.; Luo, H. Effect of Breed on the Volatile Compound Precursors and Odor Profile Attributes of Lamb Meat. Foods 2020, 9, 1178. https://doi.org/10.3390/foods9091178
Zhang C, Zhang H, Liu M, Zhao X, Luo H. Effect of Breed on the Volatile Compound Precursors and Odor Profile Attributes of Lamb Meat. Foods. 2020; 9(9):1178. https://doi.org/10.3390/foods9091178
Chicago/Turabian StyleZhang, Can, Hao Zhang, Ming Liu, Xin’gang Zhao, and Hailing Luo. 2020. "Effect of Breed on the Volatile Compound Precursors and Odor Profile Attributes of Lamb Meat" Foods 9, no. 9: 1178. https://doi.org/10.3390/foods9091178
APA StyleZhang, C., Zhang, H., Liu, M., Zhao, X., & Luo, H. (2020). Effect of Breed on the Volatile Compound Precursors and Odor Profile Attributes of Lamb Meat. Foods, 9(9), 1178. https://doi.org/10.3390/foods9091178





