Effect of Sunlight Exposure on Anthocyanin and Non-Anthocyanin Phenolic Levels in Pomegranate Juices by High Resolution Mass Spectrometry Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Harvesting Procedures, Preparation of Pomegranate Juices (PJs) and Instrumental Color Measurement
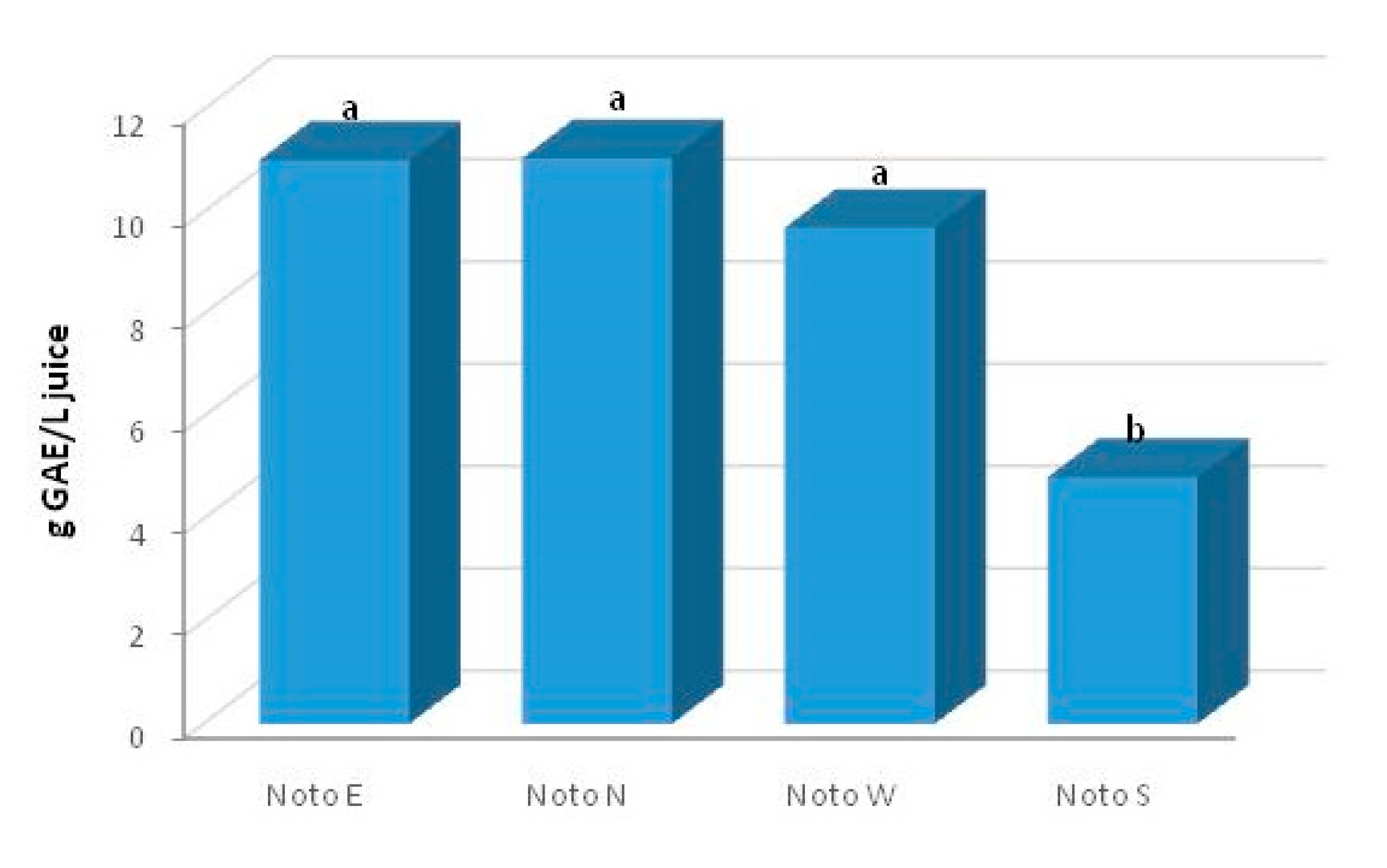
2.2. Total Phenolic Content (TPC)
2.3. Determination of Phenolic Compounds by UHPLC–Orbitrap-MS
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stover, E.; Mercure, E.W. The pomegranate: A new look at the fruit of paradise. HortScience 2007, 42, 1088–1092. [Google Scholar] [CrossRef]
- Qin, G.; Liu, C.; Li, J.; Qi, Y.; Gao, Z.; Zhang, X.; Yi, X.; Pan, H.; Ming, R.; Xu, Y. Diversity of metabolite accumulation patterns in inner and outer seed coats of pomegranate: Exploring their relationship with genetic mechanisms of seed coat development. Hortic. Res. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Cossi, F. Melograno, potenzialità e limiti di un antico frutto italiano. Riv. Fruttic. Ortofloric. 2017, 81, 52–63. [Google Scholar]
- Viuda-Martos, M.; Fernández-Lóaez, J.; Pérez-álvarez, J.A. Pomegranate and its Many Functional Components as Related to Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef]
- Bartual, J.; Fernandez-Zamudio, M.A.; De Miguel, M.D. Situation of the production, research and economics of the pomegranate industry in Spain. Acta Hortic. 2015, 1089, 345–349. [Google Scholar] [CrossRef]
- Rymon, D. Mapping features of the global pomegranate market. Acta Hortic. 2011, 599–601. [Google Scholar] [CrossRef]
- Işık Özgüven, A.; Gültekin, U.; Gözlekçi, S.; Yilmaz, I.; Yilmaz, C.; Küçük, E.; Imrak, B.; Korkmaz, C. A review of the economics and the marketing of the pomegranate industry in Turkey. Acta Hortic. 2015, 221–228. [Google Scholar] [CrossRef]
- Katz, S.R.; Newman, R.A.; Lansky, E.P. Punica granatum: Heuristic Treatment for Diabetes Mellitus. J. Med. Food 2007, 10, 213–217. [Google Scholar] [CrossRef]
- Esmaillzadeh, A.; Tahbaz, F.; Gaieni, I.; Alavi-Majd, H.; Azadbakht, L. Cholesterol-Lowering Effect of Concentrated Pomegranate Juice Consumption in Type II Diabetic Patients with Hyperlipidemia. Int. J. Vitam. Nutr. Res. 2006, 76, 147–151. [Google Scholar] [CrossRef]
- Aviram, M.; Dornfeld, L.; Rosenblat, M.; Volkova, N.; Kaplan, M.; Coleman, R.; Hayek, T.; Presser, D.; Fuhrman, B. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: Studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am. J. Clin. Nutr. 2000, 71, 1062–1076. [Google Scholar] [CrossRef]
- Davidson, M.H.; Maki, K.C.; Dicklin, M.R.; Feinstein, S.B.; Witchger, M.S.; Bell, M.; McGuire, D.K.; Provost, J.C.; Liker, H.; Aviram, M. Effects of Consumption of Pomegranate Juice on Carotid Intima-Media Thickness in Men and Women at Moderate Risk for Coronary Heart Disease. Am. J. Cardiol. 2009, 104, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M.; Gaitini, D.; Nitecki, S.; Hoffman, A.; Dornfeld, L.; Volkova, N.; Presser, D.; Attias, J.; Liker, H.; et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004, 23, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Zhang, X.N.; Wang, W.; Xing, D.M.; Xie, W.D.; Su, H.; Du, L.J. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int. J. Obes. 2007, 31, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Boussetta, T.; Raad, H.; Lettéron, P.; Gougerot-Pocidalo, M.A.; Marie, J.C.; Driss, F.; El-Benna, J. Punicic acid a conjugated linolenic acid inhibits TNFα-induced neutrophil hyperactivation and protects from experimental colon inflammation in rats. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Romier-Crouzet, B.; Van De Walle, J.; During, A.; Joly, A.; Rousseau, C.; Henry, O.; Larondelle, Y.; Schneider, Y.J. Inhibition of inflammatory mediators by polyphenolic plant extracts in human intestinal Caco-2 cells. Food Chem. Toxicol. 2009, 47, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.K.; Gupta, S.K.; Jacob, M.R.; Khan, S.I.; Ferreira, D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007, 73, 461–467. [Google Scholar] [CrossRef]
- Gerardi, C.; Albano, C.; Calabriso, N.; Carluccio, M.A.; Durante, M.; Mita, G.; Renna, M.; Serio, F.; Blando, F. Techno-functional properties of tomato puree fortified with anthocyanin pigments. Food Chem. 2018, 240, 1184–1192. [Google Scholar] [CrossRef]
- Kabakcı, S.A.; Türkyılmaz, M.; Özkan, M. Changes in the quality of kefir fortified with anthocyanin-rich juices during storage. Food Chem. 2020, 326, 126977. [Google Scholar] [CrossRef]
- El-Seideek, L.; Zaied, S.F.; Hassan, M.I.; Elgammal, M.H. Antimicrobial, biochemical, organoleptic and stability properties of cookies fortified by pomegranate juice during storage. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 288–299. [Google Scholar]
- Lee, D.W.; Gould, K.S. Anthocyanins in Leaves and Other Vegetative Organs: An introduction; Elsevier B.V.: Amsterdam, The Nethersland, 2002; pp. 1–16. ISBN 9780080953052. [Google Scholar]
- Kühnau, J. The flavonoids. A class of semi-essential food components: Their role in human nutrition. World Rev. Nutr. Diet. 1976, 24, 117–191. [Google Scholar]
- Jaldappagari, S.; Motohashi, N.; Gangeenahalli, M.P.; Naismith, J.H. Bioactive Mechanism of Interaction Between Anthocyanins and Macromolecules Like DNA and Proteins. In Bioactive Heterocycles VI.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 49–65. [Google Scholar]
- Di Stefano, V.; Pitonzo, R.; Novara, M.E.; Bongiorno, D.; Indelicato, S.; Gentile, C.; Avellone, G.; Bognanni, R.; Scandurra, S.; Melilli, M.G. Antioxidant activity and phenolic composition in pomegranate (Punica granatum L.) genotypes from south Italy by UHPLC-Orbitrap-MS approach. J. Sci. Food Agric. 2019, 99, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Pereira, J.A.; Lopéz-Cortés, I.; Salazar, D.M.; González-Álvarez, J.; Ramalhosa, E. Physicochemical composition and antioxidant activity of several pomegranate (Punica granatum L.) cultivars grown in Spain. Eur. Food Res. Technol. 2017, 243, 1799–1814. [Google Scholar] [CrossRef]
- Fischer, U.A.; Dettmann, J.S.; Carle, R.; Kammerer, D.R. Impact of processing and storage on the phenolic profiles and contents of pomegranate (Punica granatum L.) juices. Eur. Food Res. Technol. 2011, 233, 797–816. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Verardo, V.; Toselli, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Caboni, M.F. Determination of the Major Phenolic Compounds in Pomegranate Juices by HPLC–DAD–ESI-MS. J. Agric. Food Chem. 2013, 61, 5328–5337. [Google Scholar] [CrossRef] [PubMed]
- Ballaré, C.L. Light Regulation of Plant Defense. Annu. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, L.; Karppinen, K.; Escobar, A.L.; Häggman, H.; Jaakola, L. Light-controlled flavonoid biosynthesis in fruits. Front. Plant Sci. 2014, 5, 534. [Google Scholar] [CrossRef]
- Li, J.-H.; Guan, L.; Fan, P.-G.; Li, S.-H.; Wu, B.-H. Effect of Sunlight Exclusion at Different Phenological Stages on Anthocyanin Accumulation in Red Grape Clusters. Am. J. Enol. Vitic. 2013, 64, 349–356. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Zlatev, Z.S.; Lidon, F.J.C.; Kaimakanova, M. Plant physiological responses to UV-B radiation. Emirates J. Food Agric. 2012, 24, 481–501. [Google Scholar] [CrossRef]
- Azuma, A.; Yakushiji, H.; Koshita, Y.; Kobayashi, S. Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta 2012, 236, 1067–1080. [Google Scholar] [CrossRef]
- Matus, J.T.; Loyola, R.; Vega, A.; Peña-Neira, A.; Bordeu, E.; Arce-Johnson, P.; Alcalde, J.A. Post-veraison sunlight exposure induces MYB-mediated transcriptional regulation of anthocyanin and flavonol synthesis in berry skins of Vitis vinifera. J. Exp. Bot. 2009, 60, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Li, X.X.; Chu, Y.N.; Zhang, M.X.; Wen, Y.Q.; Duan, C.Q.; Pan, Q.H. Three types of ultraviolet irradiation differentially promote expression of shikimate pathway genes and production of anthocyanins in grape berries. Plant Physiol. Biochem. 2012, 57, 74–83. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Sánchez-Díaz, M.; Delrot, S.; Aguirreolea, J.; Pascual, I.; Gomès, E. Ultraviolet-B radiation and water deficit interact to alter flavonol and anthocyanin profiles in grapevine berries through transcriptomic regulation. Plant Cell Physiol. 2014, 55, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry to total phenolics with phosphomolybdic acid reagents. Am. J. Enol. Vinic. 1965, 16, 144–158. [Google Scholar]
- Melilli, M.G.; Di Stefano, V.; Sciacca, F.; Pagliaro, A.; Bognanni, R.; Scandurra, S.; Virzì, N.; Gentile, C.; Palumbo, M. Improvement of Fatty Acid Profile in Durum Wheat Breads Supplemented with Portulaca oleracea L. Quality Traits of Purslane-Fortified Bread. Foods 2020, 9, 764. [Google Scholar] [CrossRef]
- Barden, C.L.; Bramlage, W.J. Accumulation of antioxidants in apple peel as related to preharvest factors and superficial scald susceptibility of the fruit. J. Am. Soc. Hortic. Sci. 1994, 119, 264–269. [Google Scholar] [CrossRef]
- Todaro, A.; Cavallaro, R.; Lamalfa, S.; Continella, A.; Gentile, A.; Fischer, U.A.; Carle, R.; Spagna, G. Anthocyanin profile and antioxidant activity of freshly squeezed pomegranate (Punica Granatum L.) Juices of sicilian and Spanish provenances. Ital. J. Food Sci. 2016, 28, 464–479. [Google Scholar] [CrossRef]
- Awad, M.A.; De Jager, A.; Van Westing, L.M. Flavonoid and chlorogenic acid levels in apple fruit: Characterisation of variation. Sci. Hortic. 2000, 83, 249–263. [Google Scholar] [CrossRef]
- Tezcan, F.; Gültekin-Özgüven, M.; Diken, T.; Özçelik, B.; Erim, F.B. Antioxidant activity and total phenolic, organic acid and sugar content in commercial pomegranate juices. Food Chem. 2009, 115, 873–877. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Erim, F.B. Total phenolic contents, antioxidant activities, and bioactive ingredients of juices from pomegranate cultivars worldwide. Food Chem. 2017, 221, 496–507. [Google Scholar] [CrossRef]
- Munera, S.; Hernández, F.; Aleixos, N.; Cubero, S.; Blasco, J. Maturity monitoring of intact fruit and arils of pomegranate cv. ‘Mollar de Elche’ using machine vision and chemometrics. Postharvest Biol. Technol. 2019, 156, 110936. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Zapata, P.J.; Valero, D. Preharvest or a combination of preharvest and postharvest treatments with methyl jasmonate reduced chilling injury, by maintaining higher unsaturated fatty acids, and increased aril colour and phenolics content in pomegranate. Postharvest Biol. Technol. 2020, 167, 111226. [Google Scholar] [CrossRef]
- Barden, C.L.; Bramlage, W.J. Separating the effects of low temperature, ripening, and light on loss of scald susceptibility in apples before harvest. J. Am. Soc. Hortic. Sci. 1994, 119, 54–58. [Google Scholar] [CrossRef]
- Li, P.; Ma, F.; Cheng, L. Primary and secondary metabolism in the sun-exposed peel and the shaded peel of apple fruit. Physiol. Plant. 2013, 148, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Yuan, Y.; Liu, C.; Zhan, S.; Wang, M. Relationships among simple phenol, flavonoid and anthocyanin in apple fruit peel at harvest and scald susceptibility. Postharvest Biol. Technol. 1996, 8, 83–93. [Google Scholar] [CrossRef]

| Fruit Weight (g) | Arils Weight (g Fruit−1) | Juice Yield (%) | Soluble Sugar (°Brix) | L * | a * | b * | |
|---|---|---|---|---|---|---|---|
| Noto E | 276 ± 13.25 b | 170 ± 2.04 b | 56.9 ± 1.19 b | 13.2 ± 0.16 b | 40.7 ± 2.12 | 11.5 ± 0.37 | 21.88 ± 0.70 |
| Noto N | 319 ± 13.40 a | 189 ± 4.54 ab | 61.5 ± 2.58 a | 13.7 ± 0.33 b | 40.6 ± 0.97 | 11.4 ± 0.48 | 21.75 ± 0.83 |
| Noto W | 282 ± 9.02 b | 162 ± 8.26 b | 63.2 ± 1.52 a | 14.7 ± 0.35 a | 41.2 ± 1.77 | 11.7 ± 0.47 | 21.85 ± 0.59 |
| Noto S | 342 ± 8.21 a | 218 ± 6.10 a | 66.7 ± 3.47 a | 14.8 ± 0.77 a | 41.3 ±1.16 | 11.9 ± 0.44 | 21.7 ± 1.24 |
| Means | 297 ± 10.97 | 179 ± 5.24 | 59.2 ± 2.19 | 13.4 ± 0.40 | 40.9 ± 1.50 | 11.6 ± 0.44 | 21.8 ± 0.84 |
| Phenolic Compounds | Noto E | Noto N | Noto W | Noto S |
|---|---|---|---|---|
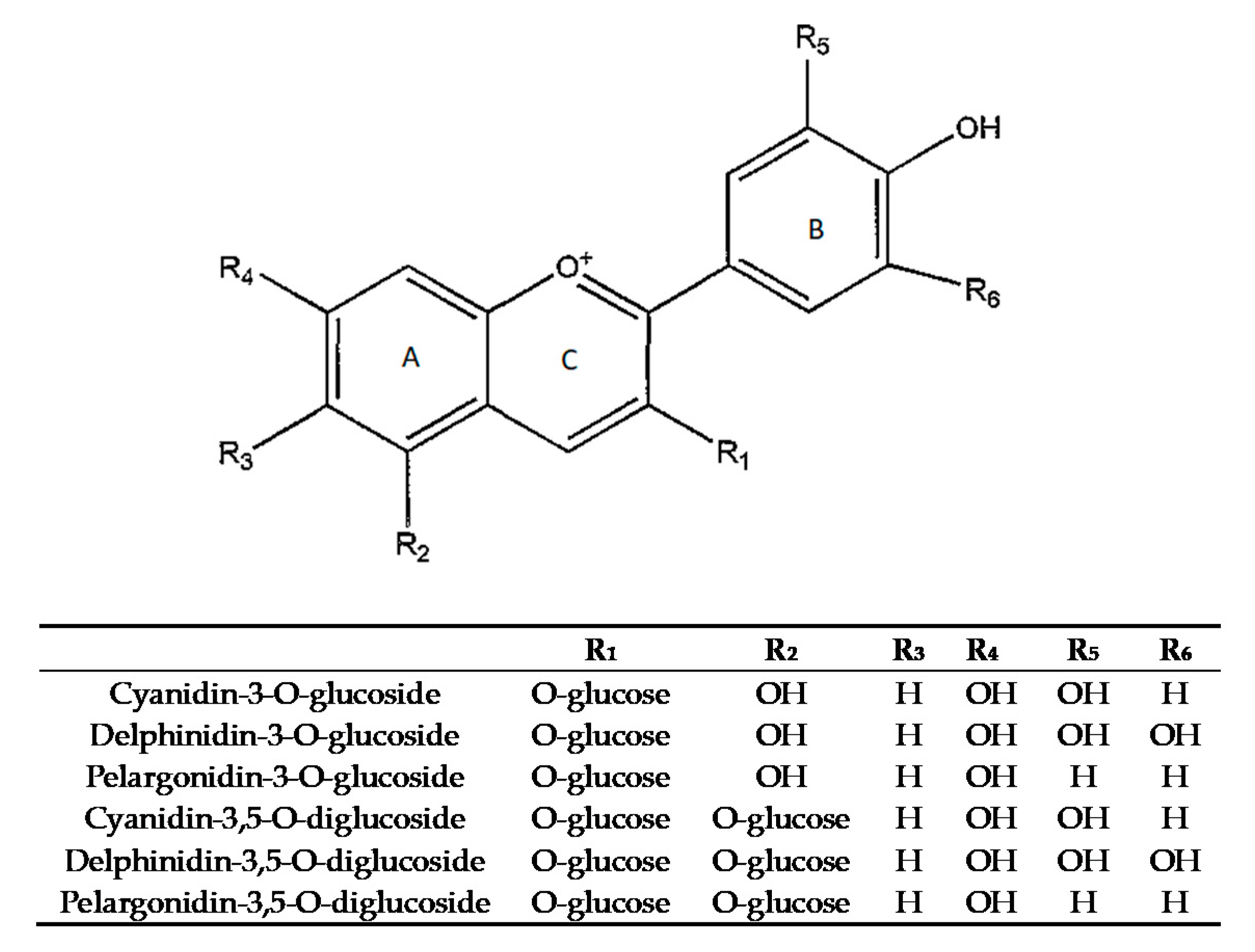
| Cyanidin-3-O-glucoside * | 69.85 ± 1.06 cd | 145.81 ± 1.77 b | 87.47 ± 0.39 d | 169.74 ± 0.25 a |
| Delphinidin-3-O-glucoside | 21.08 ± 0.19 cd | 41.93 ± 0.19 b | 19.66 ± 0.23 d | 45.83 ± 0.06 a |
| Pelargonidin-3-O-glucoside | 8.61 ± 0.08 d | 17.41 ± 0.10 b | 9.91 ± 0.03 c | 21.66 ± 0.03 a |
| Pelargonidin-3,5-O-diglucoside | 36.87 ± 0.22 c | 50.21 ± 0.17 b | 24.52 ± 0.02 d | 56.02 ± 0.11 a |
| Cyanidin-3,5-O-diglucoside * | 374.00 ± 1.97 c | 639.78 ± 1.02 b | 305.63 ± 0.14 d | 765.69 ± 0.51 a |
| Delphinidin-3,5-O-diglucoside | 118.66 ± 1.42 c | 212.11 ± 0.21 b | 69.43 ± 0.27 d | 225.98 ± 0.14 a |
| Total anthocyanins | 510.41 | 1107.25 | 516.62 | 1284.92 |
| Compounds | [M]+ m/z | MS/MS m/z | Molecular Formula | Retention Time (min) |
|---|---|---|---|---|
| Pelargonidin-3-O-glucoside | 433.1129 | 271.0593 | C21H21O10 | 14.08 |
| Cyanidin-3-O-glucoside * | 449.1078 | 287.0542 | C21H21O11 | 13.56 |
| Delphinidin-3-O-glucoside | 465.1028 | 303.0492 | C21H21O12 | 13.17 |
| Pelargonidin-3,5-O-diglucoside | 595.1658 | 433.1120271.0594 | C27H31O15 | 13.44 |
| Cyanidin-3,5-O-diglucoside * | 611.1606 | 449.1018287.0542 | C27H31O16 | 12.79 |
| Delphinidin-3,5-O-diglucoside | 627.1556 | 465.1018303.0492 | C27H31O17 | 12.20 |
| Compounds | [M-H]− m/z | MS/MS m/z | Molecular Formula | Retention Time (min) |
|---|---|---|---|---|
| Gallic acid * | 169.01344 | 125.02334 | C7H6O5 | 11.12 |
| Kaempferol-3-O-glucoside | 447.09414 | 285.00387 | C21H20O11 | 13.56 |
| Quercetin 3-O-hexoside | 463.08932 | 300.9990 | C21H20O12 | 13.16 |
| Rutin * | 609.14805 | 447.0571284.9679 | C27H30O16 | 12.87 |
| Vanillic acid hexoside | 329.08895 | 167.03417101.02327 | C14H18O9 | 13.09 |
| Ferulic acid hexoside | 355.10476 | 175.03937160.0130 | C16H20O9 | 14.25 |
| Ellagic acid pentoside | 433.04277 | 300.9990 | C19H14O12 | 19.85 |
| Ellagic acid deoxyhexoside | 447.05824 | 300.9990 | C20H16O12 | 20.67 |
| Corilagin | 633.07469 | 470.98416 | C27H22O18 | 14.20 |
| Lagerstannin C | 649.07055 | 486.97901 | C27H22O19 | 8.52 |
| Phenolic Compounds | Noto E | Noto N | Noto W | Noto S |
|---|---|---|---|---|
| Gallic acid * | 1.12 ± 0.00 b | 0.9 ± 0.04 b | 1.21 ± 0.03 b | 0.94 ± 0.05 b |
| Kaempferol-3-O-glucoside | 74.94 ± 0.09 c | 103.59 ± 0.19 b | 62.99 ± 0.11 d | 130.80 ± 0.16 a |
| Quercetin 3-O-hexoside | 26.60 ± 0.06 c | 33.60 ± 0.03 b | 16.96 ± 0.02 ef | 37.55 ± 0.04 a |
| Rutin * | 0.74 ± 0.02 b | 1.02 ± 0.00 a | 0.77 ± 0.00 b | 1.13 ± 0.01 a |
| Vanillic acid hexoside | 67.75 ± 0.12 b | 70.34 ± 0.01 a | 47.52 ± 0.07 e | 58.00 ± 0.01 c |
| Ferulic acid hexoside | 60.35 ± 0.01 a | 59.97 ± 0.04 a | 33.93 ± 0.10 c | 53.77 ± 0.26 b |
| Ellagic acid pentoside | 10.48 ± 0.26 b | 10.60 ± 0.04 b | 13.18 ± 0.08 a | 12.36 ± 0.08 a |
| Ellagic acid deossihexoside | 26.64 ± 0.09 c | 23.77 ± 0.03 d | 29.94 ± 0.06 a | 28.32 ± 0.04 b |
| Corilagin | 8.70 ± 0.17 b | 6.86 ± 0.02 c | 12.66 ± 0.03 a | 7.90 ± 0.02 bc |
| Lagerstannin C | 10.03 ± 0.05 d | 12.66 ± 0.04 c | 22.01 ± 0.06 a | 13.01 ± 0.06 c |
| Total phenolic acids | 1.12 | 0.90 | 1.21 | 0.94 |
| Total phenolic acids hexoside | 128.10 | 130.31 | 81.45 | 111.77 |
| Total flavonoids | 102.28 | 138.21 | 80.72 | 169.48 |
| Total hydrolysable tannins | 55.85 | 53.89 | 77.79 | 61.59 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Stefano, V.; Scandurra, S.; Pagliaro, A.; Di Martino, V.; Melilli, M.G. Effect of Sunlight Exposure on Anthocyanin and Non-Anthocyanin Phenolic Levels in Pomegranate Juices by High Resolution Mass Spectrometry Approach. Foods 2020, 9, 1161. https://doi.org/10.3390/foods9091161
Di Stefano V, Scandurra S, Pagliaro A, Di Martino V, Melilli MG. Effect of Sunlight Exposure on Anthocyanin and Non-Anthocyanin Phenolic Levels in Pomegranate Juices by High Resolution Mass Spectrometry Approach. Foods. 2020; 9(9):1161. https://doi.org/10.3390/foods9091161
Chicago/Turabian StyleDi Stefano, Vita, Salvatore Scandurra, Antonella Pagliaro, Vincenzo Di Martino, and Maria Grazia Melilli. 2020. "Effect of Sunlight Exposure on Anthocyanin and Non-Anthocyanin Phenolic Levels in Pomegranate Juices by High Resolution Mass Spectrometry Approach" Foods 9, no. 9: 1161. https://doi.org/10.3390/foods9091161
APA StyleDi Stefano, V., Scandurra, S., Pagliaro, A., Di Martino, V., & Melilli, M. G. (2020). Effect of Sunlight Exposure on Anthocyanin and Non-Anthocyanin Phenolic Levels in Pomegranate Juices by High Resolution Mass Spectrometry Approach. Foods, 9(9), 1161. https://doi.org/10.3390/foods9091161