Extended Harvest Date Alter Flavonoid Composition and Chromatic Characteristics of Plavac Mali (Vitis vinifera L.) Grape Berries
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Environmental Conditions and Vineyard Design
2.3. Harvesting, Grape Sampling and Preparation for Analysis
2.4. Analysis of Physicochemical Components of Fresh Juice
2.5. Organic Acids Analysis
2.6. Extraction of Flavonoid Compounds from Grape Berries
2.7. Analysis of Flavonoid Compounds by HPLC
2.8. Colorimetric Analyses (CIELab)
2.9. Statistical Analysis
3. Results
3.1. Climate Conditions
3.2. Physicochemical Grape Berry Composition
3.3. Evaluation of Grape Berry Flavonoid Composition
3.4. Characterization of Green Berries
3.5. Evaluation of External Color: CIELab Skin Parameters
4. Discussion
4.1. Conventional Indicators of the Grape Ripeness
4.2. Flavonoid Indicators of Grape Ripeness
4.2.1. Flavonoid Compositional Changes in Grape Skin
4.2.2. Flavonoid Compositional Changes in Grape Seeds
4.3. Green Berries
4.4. External Color CIELab Skin Parameters as Indicators of Ripeness
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bindon, K.; Varela, C.; Kennedy, J.; Holt, H.; Herderich, M. Relationships between harvest time and wine composition in Vitis vinifera L. cv. Cabernet Sauvignon 1. Grape and wine chemistry. Food Chem. 2013, 138, 1696–1705. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Gambetta, G.A.; Wada, H.; Krasnow, M.N.; Cramer, G.R.; Peterlunger, E.; Shackel, K.A.; Matthews, M.A. Characterization of major ripening events during softening in grape: Turgor, sugar accumulation, abscisic acid metabolism, colour development, and their relationship with growth. J. Exp. Bot. 2016, 67, 709–722. [Google Scholar] [CrossRef]
- Bindon, K.A.; Madani, S.H.; Pendleton, P.; Smith, P.A.; Kennedy, J.A. Factors affecting skin tannin extractability in ripening grapes. J. Agric. Food Chem. 2014, 62, 1130–1141. [Google Scholar] [CrossRef]
- de Rosas, I.; Ponce, M.T.; Malovini, E.; Deis, L.; Cavagnaro, B.; Cavagnaro, P. Loss of anthocyanins and modification of the anthocyanin profiles in grape berries of Malbec and Bonarda grown under high temperature conditions. Plant Sci. 2017, 258, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Bañuelos, G.; Buica, A.; Schückel, J.; Zietsman, A.J.J.; Willats, W.G.T.; Moore, J.P.; Du Toit, W.J. Investigating the relationship between grape cell wall polysaccharide composition and the extractability of phenolic compounds into Shiraz wines. Part I: Vintage and ripeness effects. Food Chem. 2019, 278, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Rienth, M.; Grimplet, J.; Chatbanyong, R.; Torregrosa, L.; Romieu, C.; Agorges, A. Transcriptional response to temperature of ripening microvine (DRCF) depends on daytime. Acta Hortic. 2017, 1157, 321–328. [Google Scholar] [CrossRef]
- Melino, V.J.; Soole, K.L.; Ford, C.M. Ascorbate metabolism and the developmental demand for tartaric and oxalic acids in ripening grape berries. BMC Plant Biol. 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Guyot, S.; Vercauteren, J.; Cheynier, V. Colourless and yellow dimers resulting from (+)-catechin oxidative coupling catalysed by grape polyphenoloxidase. Phytochemistry 1996, 42, 1279–1288. [Google Scholar] [CrossRef]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef]
- Gouthu, S.; O’Neil, S.T.; Di, Y.; Ansarolia, M.; Megraw, M.; Deluc, L.G. A comparative study of ripening among berries of the grape cluster reveals an altered transcriptional programme and enhanced ripening rate in delayed berries. J. Exp. Bot. 2014, 65, 5889–5902. [Google Scholar] [CrossRef]
- Schelezki, O.J.; Smith, P.A.; Hranilovic, A.; Bindon, K.A.; Jeffery, D.W. Comparison of consecutive harvests versus blending treatments to produce lower alcohol wines from Cabernet Sauvignon grapes: Impact on polysaccharide and tannin content and composition. Food Chem. 2018, 244, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Lasanta, C.; Caro, I.; Gómez, J.; Pérez, L. The influence of ripeness grade on the composition of musts and wines from Vitis vinifera cv. Tempranillo grown in a warm climate. Food Res. Int. 2014, 64, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Kontoudakis, N.; Esteruelas, M.; Fort, F.; Canals, J.M.; De Freitas, V.; Zamora, F. Influence of the heterogeneity of grape phenolic maturity on wine composition and quality. Food Chem. 2011, 124, 767–774. [Google Scholar] [CrossRef]
- Rousserie, P.; Lacampagne, S.; Vanbrabant, S.; Rabot, A.; Geny-Denis, L. Influence of berry ripeness on seed tannins extraction in wine. Food Chem. 2020, 315, 126307. [Google Scholar] [CrossRef]
- Allegro, G.; Pastore, C.; Valentini, G.; Muzzi, E.; Filippetti, I. Influence of berry ripeness on accumulation, composition and extractability of skin and seed flavonoids in cv. Sangiovese (Vitis vinifera L.). J. Sci. Food Agric. 2016, 96, 4553–4559. [Google Scholar] [CrossRef]
- Black, J.A.K.; di Profio, F.; Le Dauphin, V.; Moreno, L.H.; Reynolds, A.G. Impact of crop level and harvest date on anthocyanins and phenolics of red wines from Ontario. Can. J. Plant Sci. 2016, 96, 1045–1059. [Google Scholar] [CrossRef]
- Moreno Luna, H.L.; Reynolds, G.A.; Di Profio, F. Crop level and harvest date impact composition of four Ontario wine grape cultivars. I. Yield, fruit, and wine composition. Am. J. Enol. Vitic. 2017, 68, 431–446. [Google Scholar] [CrossRef]
- Laget, F.; Tondut, J.L.; Deloire, A.; Kelly, M.T. Climate trends in a specific Mediterranean viticultural area between 1950 and 2006. OENO One 2008. [Google Scholar] [CrossRef]
- Zyprian, E.; Eibach, R.; Trapp, O.; Schwander, F.; Töpfer, R. Grapevine breeding under climate change: Applicability of a molecular marker linked to veraison. Vitis-J. Grapevine Res. 2018, 57, 119–123. [Google Scholar] [CrossRef]
- Salazar-Parra, C.; Aranjuelo, I.; Pascual, I.; Aguirreolea, J.; Sánchez-Díaz, M.; Irigoyen, J.J.; Araus, J.L.; Morales, F. Is vegetative area, photosynthesis, or grape C uploading involved in the climate change-related grape sugar/anthocyanin decoupling in Tempranillo? Photosynth. Res. 2018, 138, 115–128. [Google Scholar] [CrossRef]
- Greer, D.H.; Weedon, M.M. The impact of high temperatures on Vitis vinifera cv. Semillon grapevine performance and berry ripening. Front. Plant Sci. 2013. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lüscher, J.; Kizildeniz, T.; Vucetic, V.; Dai, Z.; Luedeling, E.; van Leeuwen, C.; Gomès, E.; Pascual, I.; Irigoyen, J.J.; Morales, F.; et al. Sensitivity of grapevine phenology to water availability, temperature and CO2 concentration. Front. Environ. Sci. 2016. [Google Scholar] [CrossRef]
- Crespo, J.; Rigou, P.; Romero, V.; García, M.; Arroyo, T.; Cabellos, J.M. Effect of seasonal climate fluctuations on the evolution of glycoconjugates during the ripening period of grapevine cv. Muscat à petits grains blancs berries. J. Sci. Food Agric. 2018, 98, 1803–1812. [Google Scholar]
- Martínez de Toda, F.; Sancha, J. Balda, P. Reducing the sugar and pH of the grape (Vitis vinifera L. cvs. ‘Grenache’ and ‘Tempranillo’) through a single shoot trimming. S. Afr. J. Enol. Vitic. 2013, 34, 246–251. [Google Scholar] [CrossRef]
- Arias-Pérez, I.; Ferrero-Del-Teso, S.; Sáenz-Navajas, M.P.; Fernández-Zurbano, P.; Lacau, B.; Astraín, J.; Barón, C.; Ferreira, V.; Escudero, A. Some clues about the changes in wine aroma composition associated to the maturation of “neutral” grapes. Food Chem. 2020, 320, 126610. [Google Scholar] [CrossRef]
- Cadot, Y.; Caillé, S.; Samson, A.; Barbeau, G.; Cheynier, V. Sensory representation of typicality of Cabernet franc wines related to phenolic composition: Impact of ripening stage and maceration time. Anal. Chim. Acta 2012, 732, 91–99. [Google Scholar] [CrossRef]
- Budić-Leto, I.; Zdunić, G.; Gajdoš-Kljusurić, J.; Mucalo, A.; Vrhovšek, U. Differentiation between Croatian dessert wine Prošek and dry wines based on phenolic composition. J. Food Compos. Anal. 2017, 62. [Google Scholar] [CrossRef]
- Casassa, L.F.; Beaver, C.W.; Mireles, M.; Larsen, R.C.; Hopfer, H.; Heymann, H.; Harbertson, J.F. Influence of fruit maturity, maceration length, and ethanol amount on chemical and sensory properties of Merlot wines. Am. J. Enol. Vitic. 2013, 64, 437–449. [Google Scholar] [CrossRef]
- Ćurko, N.; Kovačević Ganić, K.; Gracin, L.; Dapić, M.; Jourdes, M.; Teissedre, P.L. Characterization of seed and skin polyphenolic extracts of two red grape cultivars grown in Croatia and their sensory perception in a wine model medium. Food Chem. 2014, 145, 15–22. [Google Scholar] [CrossRef]
- Preiner, D.; Tomaz, I.; Markovic, Z.; Stupic, D.; Andabaka, Z.; Sikuten, I.; Cenbauer, D.; Maletic, E.; Kontic, J.K. Differences in chemical composition of “Plavac Mali” grape berries. Vitis-J. Grapevine Res. 2017, 56, 95–102. [Google Scholar] [CrossRef]
- Zdunić, G.; Maletić, E.; Vokurka, A.; Karoglan Kontić, J.; Pezo, I.; Pejić, I. Phenotypical, sanitary and ampelometric variability within the population of cv. Plavac Mali (Vitis vinifera L.). Agric. Conspec. Sci. 2007, 72, 117–128. [Google Scholar]
- Bucalossi, G.; Fia, G.; Dinnella, C.; De Toffoli, A.; Canuti, V.; Zanoni, B.; Servili, M.; Pagliarini, E.; Gallina Toschi, T.; Monteleone, E. Functional and sensory properties of phenolic compounds from unripe grapes in vegetable food prototypes. Food Chem. 2020, 315, 126291. [Google Scholar] [CrossRef] [PubMed]
- Coombe, B.G. Growth Stages of the Grapevine: Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995. [Google Scholar] [CrossRef]
- Office International de la Vigne et du Vin. Compendium of International Methods of Wine and Must Analysis—Vol I; Organisation Internationale de la Vigne et du Vin (OIV): Paris, France, 2012. [Google Scholar]
- Dukes, B.C.; Butzke, C.E. Rapid determination of primary amino acids in grape juice using an o-phthaldialdehyde/N-acetyl-L-cysteine spectrophotometric assay. Am. J. Enol. Vitic. 1998, 49, 125–134. [Google Scholar]
- Zoecklein, B.W.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. Fruit Quality and Soluble Solids. In Production Wine Analysis; Springer Science and Business Media: New York, NY, USA, 2012; p. 476. [Google Scholar]
- Tomaz, I.; Maslov, L. Simultaneous determination of phenolic compounds in different matrices using phenyl-hexyl stationary phase. Food Anal. Methods 2016, 9, 401–410. [Google Scholar] [CrossRef]
- Berente, B.; De La Calle García, D.; Reichenbächer, M.; Danzer, K. Method development for the determination of anthocyanins in red wines by high-performance liquid chromatography and classification of German red wines by means of multivariate statistical methods. J. Chromatogr. A 2000. [Google Scholar] [CrossRef]
- Carreño, J.; Martínez, A.; Almela, L.; Fernández-López, J.A. Proposal of an index for the objective evaluation of the colour of red table grapes. Food Res. Int. 1995, 28, 373–377. [Google Scholar] [CrossRef]
- Rinaldi, A.; Villano, C.; Lanzillo, C.; Tamburrino, A.; Jourdes, M.; Teissedre, P.L.; Moio, L.; Frusciante, L.; Carputo, D.; Aversano, R. Metabolic and RNA profiling elucidates proanthocyanidins accumulation in Aglianico grape. Food Chem. 2017, 233, 52–59. [Google Scholar] [CrossRef]
- Riebel, M.; Fronk, P.; Tenzer, S.; Distler, U.; Decker, H. Proteomic profiling of German Dornfelder grape berries using data-independent acquisition. Plant Physiol. Biochem. 2017, 118, 64–70. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Coetzee, Z.A.; Walker, R.R.; Deloire, A.; Tyerman, S.D. Potassium in the grape (Vitis vinifera L.) berry: Transport and function. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Barril, C. Impact of short temperature exposure of Vitis vinifera L. cv. Shiraz grapevine bunches on berry development, primary metabolism and tannin accumulation. Environ. Exp. Bot. 2019, 168, 103866. [Google Scholar] [CrossRef]
- Sweetman, C.; Sadras, V.O.; Hancock, R.D.; Soole, K.L.; Ford, C.M. Metabolic effects of elevated temperature on organic acid degradation in ripening Vitis vinifera fruit. J. Exp. Bot. 2014, 65, 5975–5988. [Google Scholar] [CrossRef] [PubMed]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié-A-Mbéguié, D.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Ampofo-Asiama, J.; Baiye, V.M.M.; Hertog, M.L.A.T.M.; Waelkens, E.; Geeraerd, A.H.; Nicolai, B.M. The metabolic response of cultured tomato cells to low oxygen stress. Plant Biol. 2014, 16, 594–606. [Google Scholar] [CrossRef]
- Xiao, Z.; Liao, S.; Rogiers, S.Y.; Sadras, V.O.; Tyerman, S.D. Effect of water stress and elevated temperature on hypoxia and cell death in the mesocarp of Shiraz berries. Aust. J. Grape Wine Res. 2018, 24, 487–497. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Moreno-Simunovic, Y. Location effects on ripening and grape phenolic composition of eight ‘Carignan’ vineyards from Maule valley (Chile). Chil. J. Agric. Res. 2018, 78, 139–149. [Google Scholar] [CrossRef]
- Cramer, G.R.; Cochetel, N.; Ghan, R.; Destrac-Irvine, A.; Delrot, S. A sense of place: Transcriptomics identifies environmental signatures in Cabernet Sauvignon berry skins in the late stages of ripening. BMC Plant Biol. 2020, 20, 1–26. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Tregoat, O.; Choné, X.; Bois, B.; Pernet, D.; Gaudillère, J.P. Vine water status is a key factor in grape ripening and vintage quality for red Bordeaux wine. How can it be assessed for vineyard management purposes? OENO One 2009, 43, 121–134. [Google Scholar] [CrossRef]
- Mucalo, A.; Zdunić, G.; Will, F.; Budić-Leto, I.; Pejić, I.; Ma-Letić, E. Changes in anthocyanins and berry color of “Plavac Mali” grape during ripening. Mitteilungen Klosterneubg. 2015, 65, 130–142. [Google Scholar]
- Tarara, M.J.; Lee, J.; Spayd, E.S.; Scagel, F.C. Berry temperature and solar radiation alter acylation, proportion, and concentration of anthocyanin in Merlot grapes. Am. J. Enol. Vitic. 2008, 59, 235–247. [Google Scholar]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Del-Castillo-Alonso, M.Á.; Diago, M.P.; Tomás-Las-Heras, R.; Monforte, L.; Soriano, G.; Martínez-Abaigar, J.; Núñez-Olivera, E. Effects of ambient solar UV radiation on grapevine leaf physiology and berry phenolic composition along one entire season under Mediterranean field conditions. Plant Physiol. Biochem. 2016, 109, 374–386. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; Peña-Neira, A.; López-Solís, R.; Cáceres-Mella, A.; Toledo-Araya, H.; López-Rivera, A. Phenolic composition of skins from four Carmenet grape varieties (Vitis vinifera L.) during ripening. LWT 2013, 54, 404–413. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Hayasaka, Y.; Vidal, S.; Waters, E.J.; Jones, G.P. Composition of grape skin proanthocyanidins at different stages of berry development. J. Agric. Food Chem. 2001, 49, 5348–5355. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lüscher, J.; Plank, C.M.; Brillante, L.; Cooper, M.L.; Smith, R.J.; Al-Rwahnih, M.; Yu, R.; Oberholster, A.; Girardello, R.; Kurtural, S.K. Grapevine Red Blotch Virus may reduce carbon translocation leading to impaired grape berry ripening. J. Agric. Food Chem. 2019, 67, 2437–2448. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H.; Uefuji, H.; Sakihama, Y. Bleaching of the red anthocyanin induced by superoxide radical. Arch. Biochem. Biophys. 1996, 332, 183–186. [Google Scholar] [CrossRef]
- Baranac, J.M.; Petranovic, N.A.; Dimitric-Markovic, J.M. Spectrophotometric study of anthocyan copigmentation reactions. 2. Malvin and the nonglycosidized flavone quercetin. J. Agric. Food Chem. 1997, 45, 1694–1697. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Matthews, M.A.; Waterhouse, A.L. Effect of maturity and vine water status on grape skin and wine flavonoids. Am. J. Enol. Vitic. 2002, 53, 268–274. [Google Scholar]
- Li, X.X.; He, F.; Wang, J.; Li, Z.; Pan, Q.H. Simple rain-shelter cultivation prolongs accumulation period of anthocyanins in wine grape berries. Molecules 2014, 19, 14843–14861. [Google Scholar] [CrossRef]
- Lorrain, B.; Ky, I.; Pechamat, L.; Teissedre, P.L. Evolution of analysis of polyhenols from grapes, wines, and extracts. Molecules 2013, 18, 1076–1100. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Influence of climatic conditions on the phenolic composition of Vitis vinifera L. cv. Graciano. Anal. Chim. Acta 2012, 732, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Ikeda, H.; Poudel, P.R.; Goto-Yamamoto, N. Light quality affects flavonoid biosynthesis in young berries of Cabernet Sauvignon grape. Phytochemistry 2012, 78, 54–64. [Google Scholar] [CrossRef]
- Cáceres-Mella, A.; Talaverano, M.I.; Villalobos-González, L.; Ribalta-Pizarro, C.; Pastenes, C. Controlled water deficit during ripening affects proanthocyanidin synthesis, concentration and composition in Cabernet Sauvignon grape skins. Plant Physiol. Biochem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Genebra, T.; Santos, R.R.; Francisco, R.; Pinto-Marijuan, M.; Brossa, R.; Serra, A.T.; Duarte, C.M.M.; Chaves, M.M.; Zarrouk, O. Proanthocyanidin accumulation and biosynthesis are modulated by the irrigation regime in Tempranillo seeds. Int. J. Mol. Sci. 2014, 15, 11862–11877. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Matthews, M.A.; Waterhouse, A.L. Changes in grape seed polyphenols during fruit ripening. Phytochemistry 2000, 55, 77–85. [Google Scholar] [CrossRef]
- Jordão, A.M.; Ricardo-da-Silva, J.M.; Laureano, O. Evolution of Catechins and Oligomeric Procyanidins during grape maturation of Castelão Francês and Touriga Francesa. Am. J. Enol. Vitic. 2001, 52, 230–234. [Google Scholar]
- Liu, Y.X.; Pan, Q.H.; Yan, G.L.; He, J.J.; Duan, C.Q. Changes of Flavan-3-ols with different degrees of polymerization in seeds of “Shiraz”, “Cabernet Sauvignon” and ‘Marselan’ grapes after veraison. Molecules 2010, 15, 7763. [Google Scholar] [CrossRef]
- Bogs, J.; Downey, M.O.; Harvey, J.S.; Ashton, A.R.; Tanner, G.J.; Robinson, S.P. Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol. 2005, 139, 652–663. [Google Scholar] [CrossRef]
- Edo-Roca, M.; Nadal, M.; Lampreave, M. How terroir affects bunch uniformity, ripening and berry composition in Vitis vinifera cvs. Carignan and Grenache. OENO One 2013, 47, 1–20. [Google Scholar] [CrossRef]
- Martínez-Gil, A.M.; Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Pérez-Álvarez, E.P.; Moreno-Simunovic, Y. Characterization of phenolic composition in Carignan noir grapes (Vitis vinifera L.) from six wine-growing sites in Maule Valley, Chile. J. Sci. Food Agric. 2018. [Google Scholar] [CrossRef]
- Roberto, S.R.; de Assis, A.M.; Yamamoto, L.Y.; Miotto, L.C.V.; Sato, A.J.; Koyama, R.; Genta, W. Application timing and concentration of abscisic acid improve color of “Benitaka” table grape. Sci. Hortic. 2012, 142, 44–48. [Google Scholar] [CrossRef]
- Ferrara, G.; Mazzeo, A.; Matarrese, A.M.S.; Pacucci, C.; Pacifico, A.; Gambacorta, G.; Faccia, M.; Trani, A.; Gallo, V.; Cafagna, I.; et al. Application of Abscisic Acid (S-ABA) to “Crimson Seedless” grape berries in a Mediterranean climate: Effects on color, chemical characteristics, metabolic profile, and S-ABA concentration. J. Plant Growth Regul. 2013, 32, 491–505. [Google Scholar] [CrossRef]
- Shahab, M.; Roberto, S.R.; Ahmed, S.; Colombo, R.C.; Silvestre, J.P.; Koyama, R.; de Souza, R.T. Relationship between anthocyanins and skin color of table grapes treated with abscisic acid at different stages of berry ripening. Sci. Hortic. 2020, 259, 108859. [Google Scholar] [CrossRef]
- Fernandez-Lopez, J.A.; Almela, L.; Munoz, J.A.; Hidalgo, V.; Carreno, J. Dependence between colour and individual anthocyanin content in ripening grapes. Food Res. Int. 1998, 31, 667–672. [Google Scholar] [CrossRef]
- Vergara, A.E.; Díaz, K.; Carvajal, R.; Espinoza, L.; Alcalde, J.A.; Pérez-Donoso, A.G. Exogenous applications of brassinosteroids improve color of red table grape (Vitis vinifera L. Cv. “Redglobe”) berries. Front. Plant Sci. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Spayd, S.E.; Tarara, J.M.; Mee, D.L.; Ferguson, J.C. Separation of light and temp on Merlot. Am. J. Enol. Vitic. 2002, 3, 171–182. [Google Scholar]
| Split | Zadar | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | H1 | H2 | H3 | H4 | H1 | H2 | H3 | H4 | Location | HD × L |
| Weight 1 | 221.78 ± 8.40 b | 165.48 ± 7.04 d | 244.41 ± 6.17 a | 185.29 ± 3.53 c | 271.09 ± 7.49 b | 295.58 ± 1.94 a | 297.65 ± 14.82 a | 302.16 ± 2.28 a | *** | *** |
| TSS 2 (°Brix) | 18.39 ± 0.40 c | 20.68 ± 0.59 b | 20.53 ± 0.13 b | 22.42 ± 0.06 a | 16.83 ± 0.38 c | 18.69 ± 0.43 b | 20.04 ± 0.17 a | 20.38 ± 0.13 a | *** | ** |
| TA 3 (gL−1) | 6.76 ± 0.31 a | 4.84 ± 0.02 b | 4.03 ± 0.09 c | 3.98 ± 0.14 c | 7.28 ± 0.17 a | 5.45 ± 0.07 b | 4.73 ± 0.18 c | 4.82 ± 0.17 c | *** | ns |
| pH | 3.38 ± 0.02 d | 3.57 ± 0.01 c | 3.63 ± 0.02 b | 3.75 ± 0.01 a | 3.27 ± 0.02 c | 3.42 ± 0.02 b | 3.49 ± 0.01 a | 3.50 ± 0.02 a | *** | *** |
| Potassium (g L−1) | 1316.36 ± 50.5 b | 1784.38 ± 46.3 a | 1274.31 ± 28.9 b | 1731.28 ± 96.2 a | 739.15 ± 13.6 b | 1108.49 ± 14.2a | 1191.04 ± 43.1 a | 1174.66 ± 79.9 a | *** | *** |
| Citric a 4 (g L−1) | 0.16 ± 0.01 b | 0.15 ± 0.01 b | 0.16 ± 0 b | 0.35 ± 0.07 a | 0.14 ± 0.01 b | 0.15 ± 0.01 b | 0.14 ± 0.02 b | 0.34 ± 0.01 a | ns | ns |
| Tartaric a 5 (g L−1) | 3.49 ± 0.25 b | 3.57 ± 0.09 b | 4.32 ± 0.03 a | 3.88 ± 0.33 b | 5.73 ± 0.06 a | 5.14 ± 0.08 b | 4.88 ± 0.08 c | 4.37 ± 0.14 d | *** | *** |
| Malic a 6 (g L−1) | 2.17 ± 0.33 a | 1.12 ± 0.04 b | 0.68 ± 0.02 c | 0.25 ± 0.03 d | 3.43 ± 0.06 a | 2.20 ± 0.05 b | 1.58 ± 0.08 c | 0 d | *** | *** |
| Glucose (g L−1) | 105.99 ± 7.59 a | 118.74 ± 19.81 a | 99.36 ± 8.19 a | 108.20 ± 7.48 a | 82.51 ±7.40 b | 98.51 ± 7.07 a | 102.49 ± 5.25 a | 111.73 ± 8.35 a | * | * |
| Fructose (g L−1) | 89.53 ± 21.93 a | 58.77 ± 17.50 b | 92.28 ± 11.24 a | 105.83 ± 11.44 a | 68.77 ± 8.74 ab | 33.89 ± 12.92 c | 52.53 ± 29.13 bc | 94.05 ± 3.53 a | ** | ns |
| G:F 7 | 1.21 ± 0.19 b | 2.14 ± 0.63 a | 1.08 ± 0.05 b | 1.03 ± 0.14 b | 1.22 ± 0.24 b | 3.37 ± 1.81 a | 2.34 ± 1.06 ab | 1.19 ± 0.08 b | ns | ns |
| Panopa 8 (g L−1) | 52.61 ± 5.29 b | 48.54 ± 6.66 b | 75.65 ± 12.55 a | 53.23 ± 8.04 b | 120.58 ± 21.21 a | 127.96 ± 4.08 a | 111.44 ± 14.32 a | 118.37 ± 11.71 a | *** | * |
| Split | Zadar | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HD 1 | H1 | H2 | H3 | H4 | H1 | H2 | H3 | H4 | L 2 | I 3 | |
| Anthocyanins | D3g 4 | 382.9 ± 15.6 d | 692.9 ± 12.6 c | 812.0 ±15.8 b | 855.3 ± 21.2 a | 729.2 ± 7.8 d | 806.4 ± 6.1 c | 1087.7 ± 6.0 b | 1126.1 ± 14.6 a | *** | *** |
| C3g 5 | 27.8 ± 1.2 b | 41.3 ± 3.9 a | 37.6 ± 2.5 a | 40.1 ± 3.8 a | 24.4 ± 2.4 d | 34.1 ± 2.4 c | 54.8 ± 4.5 b | 68.5 ± 7.7 a | *** | *** | |
| Pet3g 6 | 450.3 ± 6.3 c | 673.4 ± 16.4 b | 666.9 ±10.7 b | 853.4 ± 10.0 a | 740.1 ± 12.2 c | 800.1 ± 4.9 b | 1111.0 ± 53.3 a | 1081.3 ± 19.8 a | *** | *** | |
| Peo3g 7 | 110.7 ± 3.4 c | 147.3 ± 3.8 a | 132.3 ± 3.4 b | 127.0 ± 3.7 b | 103.3 ± 6.2 d | 133.9 ± 2.7 c | 200.3 ± 11.0 b | 223.5 ± 3.2 a | *** | *** | |
| Mal3g 8 | 2720.5 ± 90.5 d | 3759.5 ± 103.4 c | 4406.6 ± 60.8 a | 4245.9 ± 77.3 b | 4256.2 ± 104.6 d | 4655.1 ± 87.0 c | 6389.5 ± 254.8 a | 6106.0 ± 50.6 b | *** | *** | |
| Flavonols | M3Og 9 | 53.4 ± 2.3 d | 72.3 ± 3.6 c | 89.4 ± 3.8 b | 100.2 ± 3.5 a | 50.1 ± 5.5 c | 61.6 ± 0.9 b | 90.4 ± 3.0 a | 84.6 ± 2.7 a | *** | ** |
| Rut 10 | 50.0 ± 5.2 a | 38.7 ± 0.8 b | 31.5 ± 3.3 c | 23.6 ± 1.4 d | 14.4 ± 1.0 b | 14.7 ± 0.6 b | 16.6 ± 0.3 a | 14.8 ± 0.6 b | *** | *** | |
| Hyper 11 | 23.5 ± 3.4 a | 20.5 ± 1.3 a | 21.3 ± 3.1 a | 22.2 ± 3.5 a | 12.1 ± 1.0 d | 20.7 ± 0.3 c | 28.3 ± 2.5 b | 46.2 ± 0.8 a | *** | *** | |
| Q3Og 12 | 320.1 ± 7.9 a | 319.1 ± 1.9 a | 249.7 ± 4.8 b | 245.0 ± 11.7 b | 170.4 ± 7.9 d | 195.5 ± 5.3 c | 239.7 ± 0.6 b | 326.9 ± 3.6 a | *** | *** | |
| K3Og 13 | 21.7 ± 1.8 a | 18.4 ± 0.8 b | 22.1 ± 0.2 a | 16.1 ± 2.2 b | 8.8 ± 1.8 d | 20.6 ± 1.5 c | 28.6 ± 2.6 b | 51.7 ± 1.0 a | *** | *** | |
| Iso3g 14 | 24.8 ± 1.9 c | 25.9 ± 1.8 c | 30.8 ± 3.2 b | 36.0 ± 1.8 a | 15.3 ± 1.3 d | 23.5 ± 2.0 c | 37.2 ± 2.3 b | 48.7 ± 4.2 a | ns | *** | |
| Flavan-3-ols monomeric forms | GC 15 | 3.5 ± 0.4 d | 5.0 ± 0.2 c | 6.7 ± 0.6 b | 8.8 ± 0.4 a | 3.2 ± 0.4 a | 2.3 ± 0.1 b | 3.2 ± 0.4 a | 1.2 ± 0.1 c | *** | *** |
| EGC 16 | 92.6 ± 3.7 a | 88.9 ± 2.1 a b | 84.2 ± 4.2 b | 60.0 ± 3.8 c | 107.8 ± 3.1 a | 93.4 ± 2.4 b | 103.7 ± 5.8 a | 83.0 ± 0.9 c | *** | ** | |
| C 17 | 43.2 ± 1.8 a | 44.4 ± 4.0 a | 31.8 ± 2.8 b | 28.1 ± 2.3 b | 34.0 ± 1.6 a | 28.6 ± 2.0 b | 32.3 ± 1.5 a | 26.0 ± 0.9 b | *** | *** | |
| EC 18 | 4.2 ± 0.3 c | 7.0 ± 0.7 b | 8.6 ± 0.5 a | 7.5 ± 1.7 ab | 4.3 ± 1.2 b | 4.2 ± 0.5 b | 15.4 ± 2.5 a | 17.6 ± 1.8 a | *** | *** | |
| Flavan-3-ols dimeric forms | B1 | 85.3 ± 2.3 a | 69.4 ± 5.3 b | 72.4 ± 0.8 b | 46.3 ± 3.3 c | 154.7 ±3.1 a | 136.7 ± 5.5 b | 147.1 ± 6.5 a | 118.5 ± 1.4 c | *** | ns |
| B2 | 12.7 ± 0.5 b | 15.5 ± 2.1 a | 12.7 ± 0.8 b | 13.1 ± 1.7 ab | 10.8 ± 0.4 c | 11.3 ± 0.3 c | 11.9 ± 0.3 b | 12.9 ± 0.4 a | *** | ** | |
| B3 | 15.0 ± 0.8 a | 15.4 ± 1.6 a | 11.6 ± 1.0 b | 10.4 ± 0.7 b | 14.5 ± 0.8 a | 11.6 ± 0.5 b | 14.3 ± 1.6 a | 10.2 ± 0.4 b | ns | *** | |
| B4 | 14.3 ± 0.6 b | 15.9 ± 0.9 a | 14.5 ± 0.9 ab | 14.3 ± 0.5 b | 17.4 ± 0.2 a | 16.3 ± 0.2 b | 17.6 ± 0.7 a | 15.4 ± 0.2 c | *** | ** | |
| Split | Zadar | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HD 1 | H1 | H2 | H3 | H4 | H1 | H2 | H3 | H4 | L 2 | I 3 | |
| Flavan-3-ols monomeric forms | ECG 4 | 93 ± 21 a | 108 ± 2 a | 54 ± 17 b | 46 ± 17 b | 184 ± 4 a | 152 ± 35 a | 160 ± 25 a | 108 ± 12 b | *** | ns |
| GC 5 | 160 ± 7 a | 166 ± 9 a | 152 ± 8 a | 137 ± 14 b | 166 ± 9 a | 168 ± 12 a | 167 ± 23 a | 164 ± 5 a | * | ns | |
| EGC 6 | 205 ± 14 a | 215 ± 15 a | 210 ± 8 a | 178 ± 11 b | 282 ± 25 a | 261 ± 32 a | 259 ± 45 a | 232 ± 20 a | *** | ns | |
| C 7 | 7453 ± 393 a | 6811 ± 264 a | 4799 ± 277 b | 4507 ± 799 b | 19,142 ± 822 a | 17,901 ± 848 ab | 15,223 ± 2246 c | 15,410 ± 993 bc | *** | ns | |
| EC 8 | 6670 ± 157 a | 6821 ± 105 a | 5775 ± 40 b | 5292 ± 890 b | 14,871 ± 548 a | 13,664 ± 854 a | 12,888 ± 1685 a | 12,793 ± 1188 a | *** | ns | |
| Flavan-3-ols dimeric forms | B1 | 3625 ± 219 ab | 3929 ± 294 a | 3520 ± 178 ab | 3270 ± 566 b | 5599 ± 485 a | 5486 ± 545 a | 5644 ± 1291 a | 5919 ± 397 a | *** | ns |
| B2 | 2864 ± 196 a | 3201 ± 171 a | 3030 ± 77 a | 2763 ± 455 a | 4460 ± 442 a | 4230 ± 397 a | 4452 ± 934 a | 4518 ± 326 a | *** | ns | |
| B4 | 969 ± 61 a | 970 ± 48 a | 843 ± 32 ab | 753 ± 121 b | 1427 ± 96 a | 1331 ± 122 a | 1278 ± 269 a | 1310 ± 86 a | *** | ns | |
| Quality Variable | Split | Zadar | Significance | |
|---|---|---|---|---|
| Morphological parameters | Total mass (150) berries (g) | 104.11 ± 18.2 b | 152.78 ± 12.5 a | * (0.0187) |
| Mass 1 berry (g) | 0.69 ± 0.12 b | 1.02 ± 0.08 a | * (0.0187) | |
| Total number of seeds in 150 berries | 195.33 ± 6.51 a | 172 ± 10.15 b | * (0.0285) | |
| Total seed mass (g) | 4.16 ± 0.58 a | 2.87 ± 0.29 b | * (0.0270) | |
| Average mass of 100 seeds (g) | 2.33 ± 0.45 | 1.66 ± 0.28 | ns | |
| Primary metabolites | TSS 1(°Brix) | 10.71 ± 0.26 a | 8.94 ± 0.15 b | *** (0.0004) |
| pH | 2.87 ± 0.02 | 2.87 ± 0.02 | ns | |
| Potassium (g L−1) | 747.96 ± 152.4 | 591.2 ± 23.2 | ns | |
| TA 2 | 18.06 ± 0.65 | 18.51 ± 0.34 | ns | |
| Citric acid (g L−1) | 0.54 ± 0.06 a | 0.39 ± 0.01 b | * (0.0133) | |
| Tartaric acid (g L−1) | 10.42 ± 0.33 | 10.63 ± 0.24 | ns | |
| Malic acid (g L−1) | 7.88 ± 0.34 b | 10.67 ± 0.21 a | *** (0.0003) | |
| Succinic acid (g L−1) | 1.68 ± 0.04 | nd | nd | |
| Glucose (g L−1) | 49.25 ± 31.74 | 47.39 ± 19.81 | ns | |
| Fructose (g L−1) | 41.2 ± 24.52 | 38.81 ± 11.55 | ns | |
| Panopa (g L−1) | 62.27 ± 24.92 b | 161.02 ± 54.08 a | * (0.0453) | |
| Flavonols | Rutin | 76.82 ± 4.81 a | 27.88 ± 0.61 b | **** (0.0001) |
| Hyper | 25.18 ± 0.91 | nd | nd | |
| Q3Og 3 | 591.31 ± 16.64 a | 422.05 ± 19.69 b | *** (0.0003) | |
| K3Og 4 | 18.25 ± 2.73 | nd | nd | |
| Flavan-3-ol monomers (skin) | GC 5 | 12.21 ± 0.38 | 13.81 ± 1.13 | ns |
| EGC 6 | 252.64 ± 7.24 | 236.69 ± 9.82 | ns | |
| C 7 | 126.11 ± 5.31 a | 82.22 ± 3.23 b | *** (0.0003) | |
| EC 8 | 7.25 ± 0.8 | nd | nd | |
| Dimers | B1 | 58.02 ± 2.56 | 52.35 ± 2.88 | ns |
| B2 | 12.61 ± 0.51 | 12.07 ± 1.96 | ns | |
| B4 | 11.10 ± 1.28 | 10.99 ± 1.39 | ns | |
| Flavan-3-ol monomers (seed) | ECG 9 | 27.89 ± 9.82 b | 110.36 ± 1.44 a | **** (0.0001) |
| GC | 125.04 ± 17.10 | 96.08 ± 6.34 | ns | |
| EGC | 103.11 ± 20.55 b | 185.90 ± 7.14 a | ** (0.0027) | |
| C | 7161.02 ± 528.24 b | 10,197.26 ± 997.97 a | ** (0.0096) | |
| EC | 8720.9 ± 336.7 a | 6885.45 ± 318.68 b | ** (0.0024) | |
| Dimers | B1 | 2686.73 ± 398.33 | 2028.01 ± 105.89 | ns |
| B2 | 3169.40 ± 673.1 a | 1780.64 ± 57.84 b | * (0.0236) | |
| B4 | 804.19 ± 81.63 | 715.99 ± 23.54 | ns |
| Split | Zadar | L | I | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HD | H1 | H2 | H3 | H4 | H1 | H2 | H3 | H4 | ||
| L* 1 | 25.15 ± 1.51 a | 25.17 ± 1.06 a | 25.00 ± 0.99 a | 24.37 ± 1.30 b | 25.07 ± 0.97 a | 24.70 ± 1.05 b | 24.29 ± 0.92 c | 23.61 ± 0.92 d | *** | *** |
| a* 2 | 4.37 ± 2.68 a | 2.87 ± 1.82 b | 1.99 ± 1.12 c | 1.90 ± 1.06 c | 2.35 ± 1.04 a | 1.87 ± 1.04 b | 1.47 ± 0.55 c | 1.60 ± 0.64 c | *** | *** |
| b* 3 | 0.73 ± 0.92 a | 0.35 ± 0.59 b | 0.21 ± 0.47 c | 0.35 ± 0.47 b | 0.61 ± 0.41 a | 0.46 ± 0.35 b | 0.45 ± 0.31 b | 0.42 ± 0.35 b | ** | *** |
| C 4 | 4.46 ± 2.78 a | 2.94 ± 1.83 b | 2.06 ± 1.11 c | 1.99 ± 1.05 c | 2.45 ± 1.08 a | 1.95 ± 1.06 b | 1.57 ± 0.55 c | 1.69 ± 0.63 c | *** | *** |
| h° 5 | 8.65 ± 7.11 a | 6.35 ± 12.67 b | 6.29 ± 15.32 b | 10.56 ± 16.53 a | 14.23 ± 7.71 b | 14.03 ± 9.78 b | 17.00 ± 11.71 a | 14.84 ± 13.78 b | *** | *** |
| CIRG 6 | 5.88 ± 0.71 c | 6.22 ± 0.64 b | 6.43 ± 0.60 a | 6.45 ± 0.68 a | 6.04 ± 0.45 c | 6.25 ± 0.53 b | 6.32 ± 0.53 b | 6.54 ± 0.56 a | ns | ** |
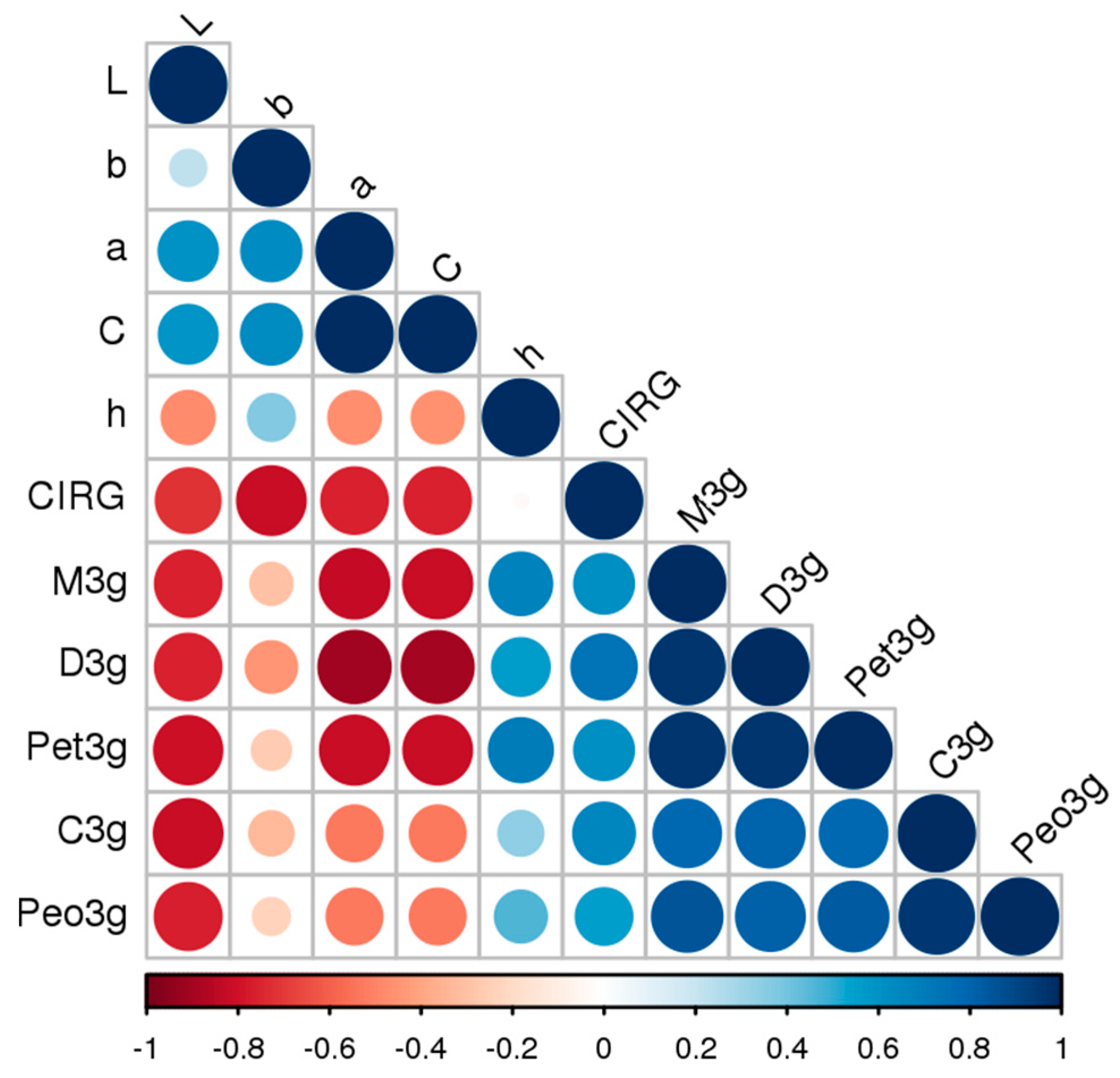
| CIELab | Del3g 1 | Cya3g 2 | Pet3g 3 | Peo3g 4 | Mal3g 5 |
|---|---|---|---|---|---|
| L* 6 | −0.76 *** | −0.81 *** | −0.80 *** | −0.77 *** | −0.76 *** |
| a* 7 | −0.90 *** | −0.53 ** | −0.82 *** | −0.53 ** | −0.83 *** |
| b* 8 | −0.46 * | −0.33 n.s. | −0.27 n.s. | −0.23 n.s. | −0.30 n.s. |
| C 9 | −0.89 *** | −0.53 ** | −0.81 *** | −0.53 ** | −0.82 *** |
| H 10 | 0.57 ** | 0.34 n.s. | 0.70 *** | 0.46 * | 0.67 *** |
| CIRG 11 | 0.73 *** | 0.65 *** | 0.61 ** | 0.55 ** | 0.61 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mucalo, A.; Maletić, E.; Zdunić, G. Extended Harvest Date Alter Flavonoid Composition and Chromatic Characteristics of Plavac Mali (Vitis vinifera L.) Grape Berries. Foods 2020, 9, 1155. https://doi.org/10.3390/foods9091155
Mucalo A, Maletić E, Zdunić G. Extended Harvest Date Alter Flavonoid Composition and Chromatic Characteristics of Plavac Mali (Vitis vinifera L.) Grape Berries. Foods. 2020; 9(9):1155. https://doi.org/10.3390/foods9091155
Chicago/Turabian StyleMucalo, Ana, Edi Maletić, and Goran Zdunić. 2020. "Extended Harvest Date Alter Flavonoid Composition and Chromatic Characteristics of Plavac Mali (Vitis vinifera L.) Grape Berries" Foods 9, no. 9: 1155. https://doi.org/10.3390/foods9091155
APA StyleMucalo, A., Maletić, E., & Zdunić, G. (2020). Extended Harvest Date Alter Flavonoid Composition and Chromatic Characteristics of Plavac Mali (Vitis vinifera L.) Grape Berries. Foods, 9(9), 1155. https://doi.org/10.3390/foods9091155








