A Novel Approach to Structure Plant-Based Yogurts Using High Pressure Processing
Abstract
1. Introduction
2. Methods
2.1. Materials and Sample Preparation
2.2. High Pressure Processing (HPP)
2.3. Rheological Analyses
3. Results and Discussion
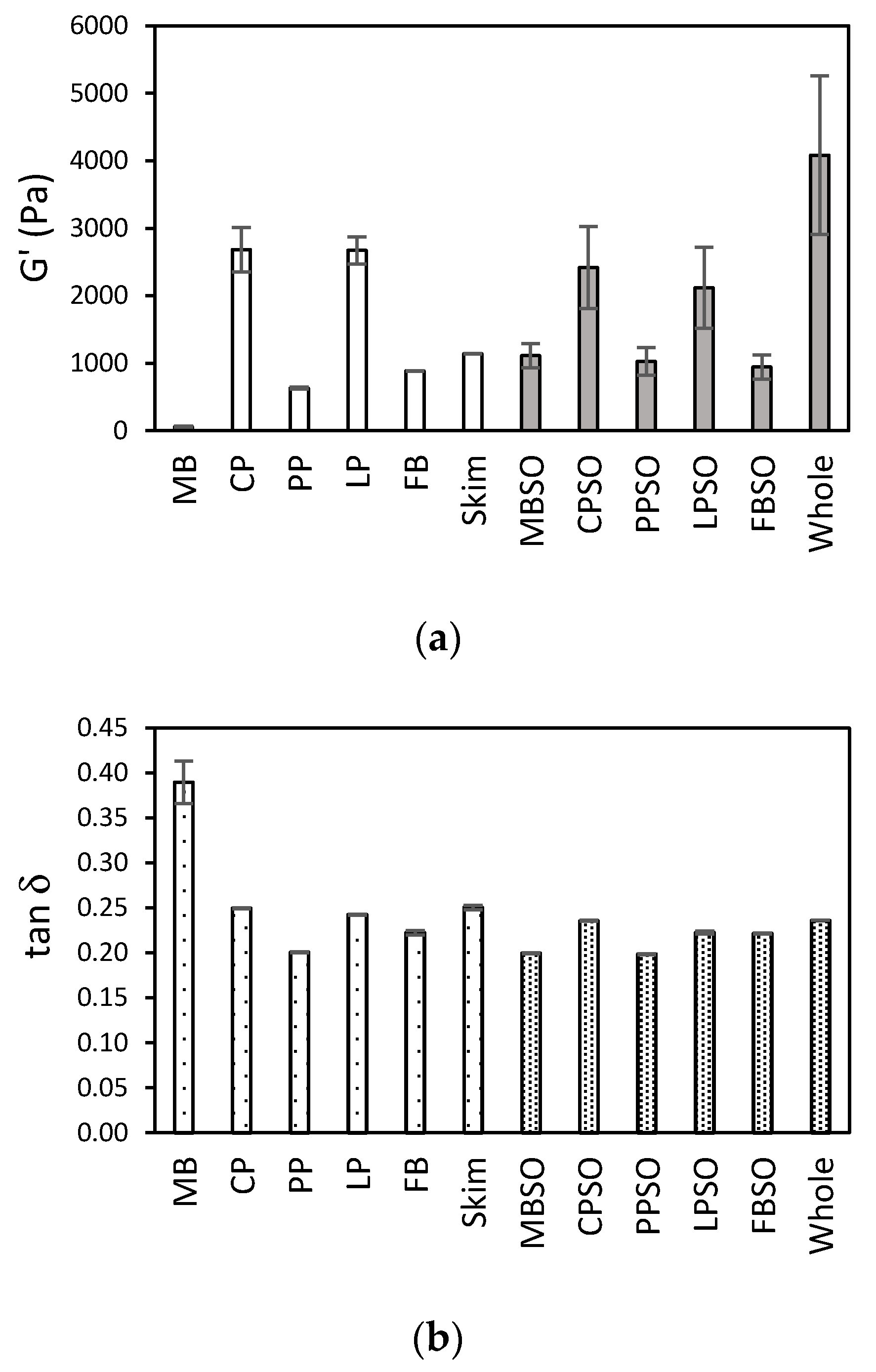
3.1. Viscoelastic Properties of the HPP-Treated Plant Protein Samples
3.2. Viscosity of the HPP-Treated Plant Protein Samples
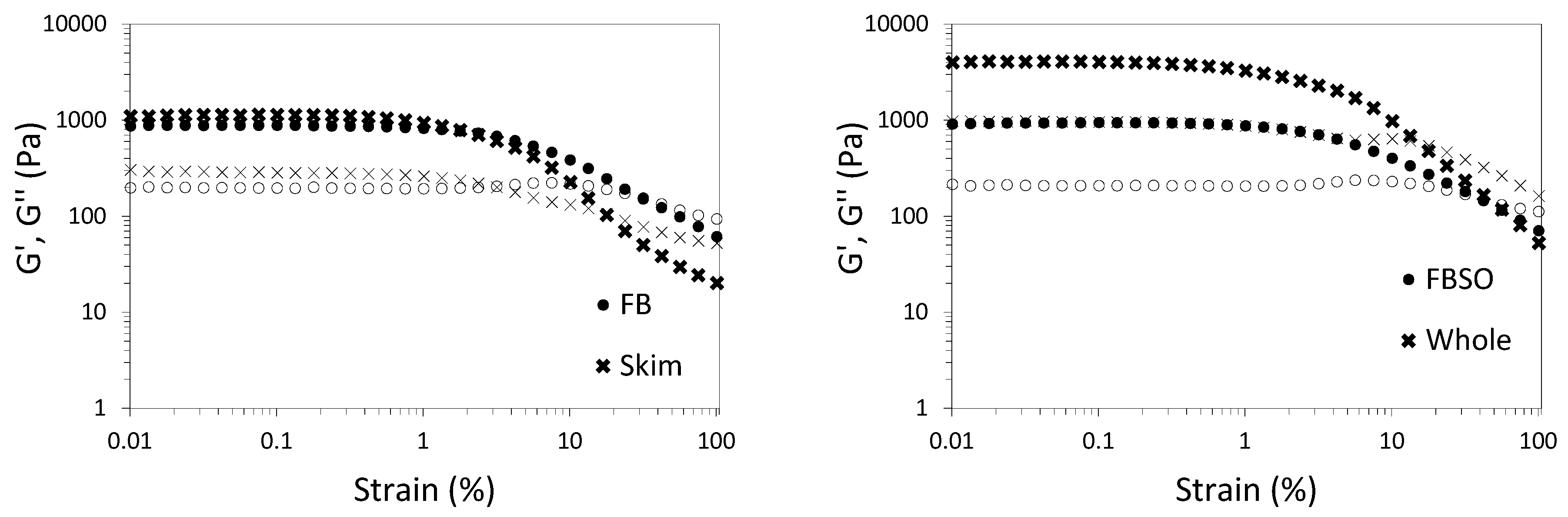
3.3. Feasibility of Using HPP to Develop Plant-Based Yogurts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Day, L. Proteins from land plants—Potential resources for human nutrition and food security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- McClements, D.J. Development of Next-Generation Nutritionally Fortified Plant-Based Milk Substitutes: Structural Design Principles. Foods 2020, 9, 421. [Google Scholar] [CrossRef]
- Kizer, L.; Renninger, N.; Schelle, M. Dairy Product Analogs and Processes for Making Same. U.S. Patent 16/095,117, 16 May 2019. [Google Scholar]
- Margolis, G.; Myers, S.; Newbold, D. Manufacturing of Plant-Based Yogurt. U.S. Patent 16/293,282, 12 September 2019. [Google Scholar]
- Grasso, N.; Alonso-Miravalles, L.; O’Mahony, J.A. Composition, Physicochemical and Sensorial Properties of Commercial Plant-Based Yogurts. Foods 2020, 9, 252. [Google Scholar] [CrossRef]
- Jeske, S.; Zannini, E.; Arendt, E.K. Past, present and future: The strength of plant-based dairy substitutes based on gluten-free raw materials. Food Res. Int. 2018, 110, 42–51. [Google Scholar] [CrossRef]
- Balasubramaniam, V.M.; Barbosa-Cánovas, G.V.; Lelieveld, H.L.M. High Pressure Processing of Food: Principles, Technology and Applications; Springer: New York, NY, USA, 2016; pp. 4–16. [Google Scholar]
- Queirós, R.P.; Saraiva, J.A.; da Silva, J.A.L. Tailoring structure and technological properties of plant proteins using high hydrostatic pressure. Crit. Rev. Food Sci. Nutr. 2018, 58, 1538–1556. [Google Scholar] [CrossRef]
- Dickinson, E.; James, J.D. Rheology and Flocculation of High-Pressure-Treated β-Lactoglobulin-Stabilized Emulsions: Comparison with Thermal Treatment. J. Agric. Food Chem. 1998, 46, 2565–2571. [Google Scholar] [CrossRef]
- Puppo, M.C.; Beaumal, V.; Speroni, F.; de Lamballerie, M.; Añón, M.C.; Anton, M. β-Conglycinin and glycinin soybean protein emulsions treated by combined temperature–high-pressure treatment. Food Hydrocoll. 2011, 25, 389–397. [Google Scholar] [CrossRef]
- Jarpa-Parra, M. Lentil protein: A review of functional properties and food application. An overview of lentil protein functionality. Int. J. Food Sci. Technol. 2018, 53, 892–903. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Petersen, I.L.; Joehnke, M.S.; Sørensen, J.C.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; O’Mahony, J.A.; Arendt, E.K.; et al. Comparison of Faba Bean Protein Ingredients Produced Using Dry Fractionation and Isoelectric Precipitation: Techno-Functional, Nutritional and Environmental Performance. Foods 2020, 9, 322. [Google Scholar] [CrossRef]
- Sim, S.Y.J.; Karwe, M.V.; Moraru, C.I. High pressure structuring of pea protein concentrates. J. Food Process. Eng. 2019, 42, e13261. [Google Scholar] [CrossRef]
- Brishti, F.H.; Zarei, M.; Muhammad, S.K.S.; Ismail-Fitry, M.R.; Shukri, R.; Saari, N. Evaluation of the functional properties of mung bean protein isolate for development of textured vegetable protein. Int. Food Res. J. 2017, 24, 1595–1605. [Google Scholar]
- Sim, S.Y.J.; Moraru, C.I. High-pressure processing of pea protein–starch mixed systems: Effect of starch on structure formation. J. Food Process. Eng. 2020, 43, e13352. [Google Scholar] [CrossRef]
- Buerman, E.C.; Worobo, R.W.; Padilla-Zakour, O.I. High pressure processing of spoilage fungi as affected by water activity in a diluted apple juice concentrate. Food Control 2020, 107, 106779. [Google Scholar] [CrossRef]
- Foudazi, R.; Qavi, S.; Masalova, I.; Malkin, A.Y. Physical chemistry of highly concentrated emulsions. Adv. Colloid Interface Sci. 2015, 220, 78–91. [Google Scholar] [CrossRef]
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S.H. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- Schmitt, C.; Silva, J.V.C.; Amagliani, L.; Chassenieux, C.; Nicolai, T. Heat-induced and acid-induced gelation of dairy/plant protein dispersions and emulsions. Curr. Opin. Food Sci. 2019, 27, 43–48. [Google Scholar] [CrossRef]
- Dickinson, E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for Special Dietary Needs: Non-dairy Plant-based Milk Substitutes and Fermented Dairy-type Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef]
- Fazilah, N.F.; Ariff, A.B.; Khayat, M.E.; Rios-Solis, L.; Halim, M. Influence of probiotics, prebiotics, synbiotics and bioactive phytochemicals on the formulation of functional yogurt. J. Funct. Foods 2018, 48, 387–399. [Google Scholar] [CrossRef]
- Almnura, A.-M.; Arabia, S. Fundamental elements to produce sesame yoghurt from sesame milk. Am. J. Appl. Sci. 2011, 8, 1086–1092. [Google Scholar]
- Kieserling, K.; Vu, T.M.; Drusch, S.; Schalow, S. Impact of pectin-rich orange fibre on gel characteristics and sensory properties in lactic acid fermented yoghurt. Food Hydrocoll. 2019, 94, 152–163. [Google Scholar] [CrossRef]
- Soares, M.; Christen, P.; Pandey, A.; Soccol, C.R. Fruity flavour production by Ceratocystis fimbriata grown on coffee husk in solid-state fermentation. Process. Biochem. 2000, 35, 857–861. [Google Scholar] [CrossRef]
- Mantzouridou, F.T.; Paraskevopoulou, A.; Lalou, S. Yeast flavour production by solid state fermentation of orange peel waste. Biochem. Eng. J. 2015, 101, 1–8. [Google Scholar] [CrossRef]
- Bosse, A.K.; Fraatz, M.A.; Zorn, H. Formation of complex natural flavours by biotransformation of apple pomace with basidiomycetes. Food Chem. 2013, 141, 2952–2959. [Google Scholar] [CrossRef]
- Cheng, H. Volatile Flavor Compounds in Yogurt: A Review. Crit. Rev. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef]
- Tsevdou, M.; Ouli-Rousi, M.; Soukoulis, C.; Taoukis, P. Impact of High-Pressure Process on Probiotics: Viability Kinetics and Evaluation of the Quality Characteristics of Probiotic Yoghurt. Foods 2020, 9, 360. [Google Scholar] [CrossRef]
- Carroll, T.; Chen, P.; Harnett, M.; Harnett, J. Pressure Treating Food to Reduce Spoilage. U.S. Patent 10/530,536, 21 December 2010. [Google Scholar]
- Farmer, S.; Keller, D.; Lefkowitz, A.R. Probiotic Sports Nutrition Compositions. U.S. Patent 15/466,037, 6 July 2017. [Google Scholar]




| Formulation | % Protein (w/w) | % Fat (w/w) | % Carbohydrate (w/w) | % Sugars (w/w) | % Dietary Fiber (w/w) | % Starch (w/w) | % Ash (w/w) | % Moisture (w/w) |
|---|---|---|---|---|---|---|---|---|
| MB | 12.0 | <0.1 | <0.9 | n.a. | n.a. | n.a. | 0.7 | 86.4 |
| CP | 12.0 | 0.2 | 6.1 | n.a. | 2.6 | n.a. | 0.7 | 81.0 |
| PP | 12.0 | 1.0 | 7.9 | 0.5 | 3.8 | 0.4 | 1.3 | 77.8 |
| LP | 12.0 | 1.0 | 7.3 | 0.4 | 3.1 | 0.9 | 1.2 | 78.5 |
| FB | 12.0 | 0.8 | 5.2 | 0.3 | 2.7 | 0.4 | 1.2 | 80.8 |
| Skim | 9.7 | 0.2 | 4.2 | 3.3 | n.a. | n.a. | n.a. | <85.9 |
| MBSO | 12.0 | 5.0 | <0.9 | n.a. | n.a. | n.a. | 0.7 | 81.4 |
| CPSO | 12.0 | 5.2 | 6.1 | n.a. | 2.6 | n.a. | 0.7 | 76.0 |
| PPSO | 12.0 | 5.0 | 7.9 | 0.5 | 3.8 | 0.4 | 1.3 | 73.8 |
| LPSO | 12.0 | 5.0 | 7.3 | 0.4 | 3.1 | 0.9 | 1.2 | 74.5 |
| FBSO | 12.0 | 5.0 | 5.2 | 0.3 | 2.7 | 0.4 | 1.2 | 76.6 |
| Whole | 9.0 | 5.0 | 3.0 | 3.0 | n.a. | n.a. | n.a. | <83.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, S.Y.J.; Hua, X.Y.; Henry, C.J. A Novel Approach to Structure Plant-Based Yogurts Using High Pressure Processing. Foods 2020, 9, 1126. https://doi.org/10.3390/foods9081126
Sim SYJ, Hua XY, Henry CJ. A Novel Approach to Structure Plant-Based Yogurts Using High Pressure Processing. Foods. 2020; 9(8):1126. https://doi.org/10.3390/foods9081126
Chicago/Turabian StyleSim, Shaun Y. J., Xin Yi Hua, and Christiani Jeyakumar Henry. 2020. "A Novel Approach to Structure Plant-Based Yogurts Using High Pressure Processing" Foods 9, no. 8: 1126. https://doi.org/10.3390/foods9081126
APA StyleSim, S. Y. J., Hua, X. Y., & Henry, C. J. (2020). A Novel Approach to Structure Plant-Based Yogurts Using High Pressure Processing. Foods, 9(8), 1126. https://doi.org/10.3390/foods9081126





