Transcriptome Analysis and Metabolic Profiling of Green and Red Mizuna (Brassica rapa L. var. japonica)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. RNA Extraction and Illumina Sequencing
2.3. Assembly and Functional Annotation
2.4. HPLC Analysis of Desulfo-Glucosinolates
2.5. HPLC Analysis of the Phenolic Compounds
2.6. HPLC and LC-ESI-MS/MS Analysis of Anthocyanins
2.7. GC-TOFMS Analysis of Polar Metabolites
2.8. Statistical Analysis of Metabolites
3. Results and Discussion
3.1. Functional Annotation and Classification of the Green and Red Mizuna Transcriptomes
3.2. Identification of Secondary Metabolite Biosynthetic Genes from the Green and Red Mizuna Transcriptomes
3.3. Quantification of Glucosinolates in Green and Red Mizuna
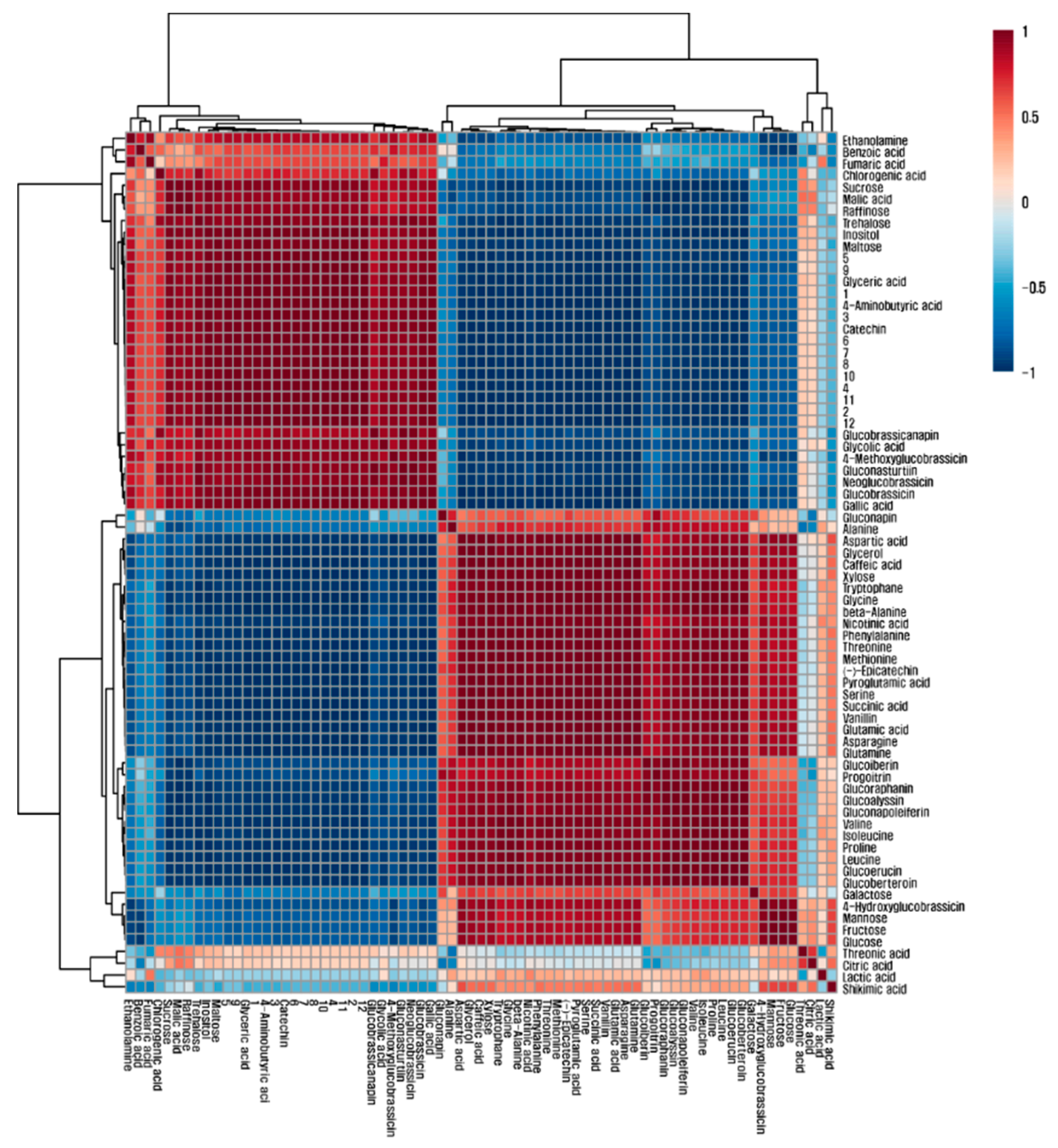
3.4. Quantification of Phenolic and Organic Compounds in Green and Red Mizuna
3.5. Quantification of Phenolic and Organic Compounds in Green and Red Mizuna
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bjorkman, M.; Klingen, I.; Birch, A.N.E.; Bones, A.M.; Bruce, T.J.A.; Johansen, T.J.; Meadow, R.; Molmann, J.; Seljasen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in plant protection and human health—Influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef] [PubMed]
- Cardone, M.; Mazzoncini, M.; Menini, S.; Rocco, V.; Senatore, A.; Seggiani, M.; Vitolo, S. Brassica carinata as an alternative oil crop for the production of biodiesel in Italy: Agronomic evaluation, fuel production by transesterification and characterization. Biomass Bioenergy 2003, 25, 623–636. [Google Scholar] [CrossRef]
- Dominguez-Perles, R.; Mena, P.; Garcia-Viguera, C.; Moreno, D.A. Brassica Foods as a Dietary Source of Vitamin C: A Review. Crit. Rev. Food Sci. 2014, 54, 1076–1091. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Health-Affecting Compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009, 8, 31–43. [Google Scholar] [CrossRef]
- Prakash, D.; Gupta, C. The phytochemicals of nutraceutical importance. J. Complement Integr. Med. 2012, 9, 13. [Google Scholar] [CrossRef]
- Sun, J.H.; Zhang, M.L.; Chen, P. GLS-Finder: A Platform for Fast Profiling of Glucosinolates in Brassica Vegetables. J. Agric. Food Chem. 2016, 64, 4407–4415. [Google Scholar] [CrossRef]
- Herr, I.; Buchler, M.W. Dietary constituents of broccoli and other cruciferous vegetables: Implications for prevention and therapy of cancer. Cancer Treat. Rev. 2010, 36, 377–383. [Google Scholar] [CrossRef]
- Kim, Y.B.; Li, X.; Kim, S.J.; Kim, H.H.; Lee, J.; Kim, H.; Park, S.U. MYB Transcription Factors Regulate Glucosinolate Biosynthesis in Different Organs of Chinese Cabbage (Brassica rapa ssp pekinensis). Molecules 2013, 18, 8682–8695. [Google Scholar] [CrossRef]
- Douglas, C.J. Phenylpropanoid metabolism and lignin biosynthesis: From weeds to trees. Trends Plant Sci. 1996, 1, 171–178. [Google Scholar] [CrossRef]
- Higdon, J.V.; Delage, B.; Williams, D.E.; Dashwood, R.H. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharm. Res. 2007, 55, 224–236. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Hoch, W.A.; Zeldin, E.L.; McCown, B.H. Physiological significance of anthocyanins during autumnal leaf senescence. Tree Physiol. 2001, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Nystrand, O.; Granstrom, A. Post-dispersal predation on Pinus sylvestris seeds by Fringilla spp: Ground substrate affects selection for seed color. Oecologia 1997, 110, 353–359. [Google Scholar] [CrossRef]
- Wrolstad, R.E. Interaction of natural colors with other ingredients—Anthocyanin pigments—Bioactivity and coloring properties. J. Food Sci. 2004, 69, C419–C421. [Google Scholar] [CrossRef]
- Kalisz, A.; Sekara, A.; Kostrzewa, J. Effect of growing date and cultivar on the morphological parameters and yield of Brassica rapa var. japonica. Acta Sci. Pol. Hortorum Cultus 2012, 11, 131–143. [Google Scholar]
- Khanam, U.K.S.; Oba, S.; Yanase, E.; Murakami, Y. Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J. Funct. Foods 2012, 4, 979–987. [Google Scholar] [CrossRef]
- Kim, O.K.; Murakami, A.; Nakamura, Y.; Ohigashi, H. Screening of edible Japanese plants for nitric oxide generation inhibitory activities in RAW 264.7 cells. Cancer Lett. 1998, 125, 199–207. [Google Scholar] [CrossRef]
- Li, D.J.; Deng, Z.; Qin, B.; Liu, X.H.; Men, Z.H. De novo assembly and characterization of bark transcriptome using Illumina sequencing and development of EST-SSR markers in rubber tree (Hevea brasiliensis Muell. Arg.). BMC Genom. 2012, 13, 192. [Google Scholar] [CrossRef]
- Tong, C.B.; Wang, X.W.; Yu, J.Y.; Wu, J.; Li, W.S.; Huang, J.Y.; Dong, C.H.; Hua, W.; Liu, S.Y. Comprehensive analysis of RNA-seq data reveals the complexity of the transcriptome in Brassica rapa. BMC Genom. 2013, 14, 689. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Lim, C.J.; Kim, S.; Choe, J.K.; Jo, S.H.; Baek, N.; Kwon, S.Y. High-Throughput Sequencing and De Novo Assembly of Brassica oleracea var. Capitata, L. for Transcriptome Analysis. PLoS ONE 2014, 9, e92087. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sinha, S.; Raxwal, V.K.; Joshi, B.; Jagannath, A.; Katiyar-Agarwal, S.; Goel, S.; Kumar, A.; Agarwal, M. De novo transcriptome profiling of cold-stressed siliques during pod filling stages in Indian mustard (Brassica juncea L.). Front. Plant Sci. 2015, 6, 932. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.J.; Yu, X.X.; Ma, F.M.; Li, J. RNA-Seq Analysis of Transcriptome and Glucosinolate Metabolism in Seeds and Sprouts of Broccoli (Brassica oleracea var. italic). PLoS ONE 2014, 9, e88804. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Morgan, A.M.A.; Park, B.B.; Lee, S.Y.; Lee, S.; Kim, J.K.; Park, S.U. Metabolic Analysis of Four Cultivars of Liriope platyphylla. Metabolites 2019, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Kim, J.K.; Kim, H.; Kim, Y.J.; Park, Y.J.; Kim, S.J.; Kim, C.; Park, S.U. Transcriptome analysis and metabolic profiling of green and red kale (Brassica oleracea var. acephala) seedlings. Food Chem. 2018, 241, 7–13. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Park, S.Y.; Kim, J.K.; Park, S.U. Comparative Phytochemical Analyses and Metabolic Profiling of Different Phenotypes of Chinese Cabbage (Brassica Rapa ssp. Pekinensis). Foods 2019, 8, 587. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, N.S.; Park, J.S.; Lee, S.Y.; Lee, J.W.; Park, S.U. Effects of Light-Emitting Diodes on the Accumulation of Glucosinolates and Phenolic Compounds in Sprouting Canola (Brassica napus L.). Foods 2019, 8, 76. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Kim, N.S.; Eun, P.Y.; Kim, S.J.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.Y.; Kim, J.K.; Park, S.U. Metabolic profiling of pale green and purple kohlrabi (Brassica oleracea var. gongylodes). Appl. Biol. Chem. 2017, 60, 249–257. [Google Scholar] [CrossRef]
- Viant, M.R.; Kurland, I.J.; Jones, M.R.; Dunn, W.B. How close are we to complete annotation of metabolomes? Curr. Opin. Chem. Biol. 2017, 36, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yeo, H.J.; Park, Y.E.; Baek, S.A.; Kim, J.K.; Park, S.U. Transcriptome Analysis and Metabolic Profiling of Lycoris Radiata. Biology 2019, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Chen, H.C.; Cao, B.; Lei, J.J.; Chen, G.J. Molecular Characterization of MYB28 Involved in Aliphatic Glucosinolate Biosynthesis in Chinese Kale (Brassica oleracea var. alboglabra Bailey). Front. Plant Sci. 2017, 8, 1083. [Google Scholar] [CrossRef] [PubMed]
- Frerigmann, H.; Gigolashvili, T. MYB34, MYB51, and MYB122 Distinctly Regulate Indolic Glucosinolate Biosynthesis in Arabidopsis thaliana. Mol. Plant 2014, 7, 814–828. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yeo, H.J.; Kim, N.S.; Park, Y.E.; Park, S.Y.; Kim, J.K.; Park, S.U. Metabolomic Profiling of the White, Violet, and Red Flowers of Rhododendron schlippenbachii Maxim. Molecules 2018, 23, 827. [Google Scholar] [CrossRef]
- Maloney, V.J.; Park, J.Y.; Unda, F.; Mansfield, S.D. Sucrose phosphate synthase and sucrose phosphate phosphatase interact in planta and promote plant growth and biomass accumulation. J. Exp. Bot. 2015, 66, 4383–4394. [Google Scholar] [CrossRef]
- Babb, V.M.; Haigler, C.H. Sucrose phosphate synthase activity rises in correlation with high-rate cellulose synthesis in three heterotrophic systems. Plant Physiol. 2001, 127, 1234–1242. [Google Scholar] [CrossRef]
- Lastdrager, J.; Hanson, J.; Smeekens, S. Sugar signals and the control of plant growth and development. J. Exp. Bot. 2014, 65, 799–807. [Google Scholar] [CrossRef]
- Shin, D.H.; Choi, M.G.; Lee, H.K.; Cho, M.; Choi, S.B.; Choi, G.; Park, Y.I. Calcium dependent sucrose uptake links sugar signaling to anthocyanin biosynthesis in Arabidopsis. Biochem. Bioph. Res. Commun. 2013, 430, 634–639. [Google Scholar] [CrossRef]
- Zakhleniuk, O.V.; Raines, C.A.; Lloyd, J.C. pho3: A phosphorus-deficient mutant of Arabidopsis thaliana (L.) Heynh. Planta 2001, 212, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.C.; Park, C.H.; Yang, J.L.; Yeo, H.J.; Kim, T.J.; Kim, J.K.; Park, S.U. Molecular characterization of anthocyanin and betulinic acid biosynthesis in red and white mulberry fruits using high-throughput sequencing. Food Chem. 2019, 279, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, X.C.; Xu, Y.Q.; Li, L.; Aghdam, M.S.; Luo, Z.S. Effect of exogenous sucrose on anthocyanin synthesis in postharvest strawberry fruit. Food Chem. 2019, 289, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Righetti, L.; Tassoni, A. Increasing sucrose concentrations promote phenylpropanoid biosynthesis in grapevine cell cultures. J. Plant Physiol. 2011, 168, 189–195. [Google Scholar] [CrossRef] [PubMed]

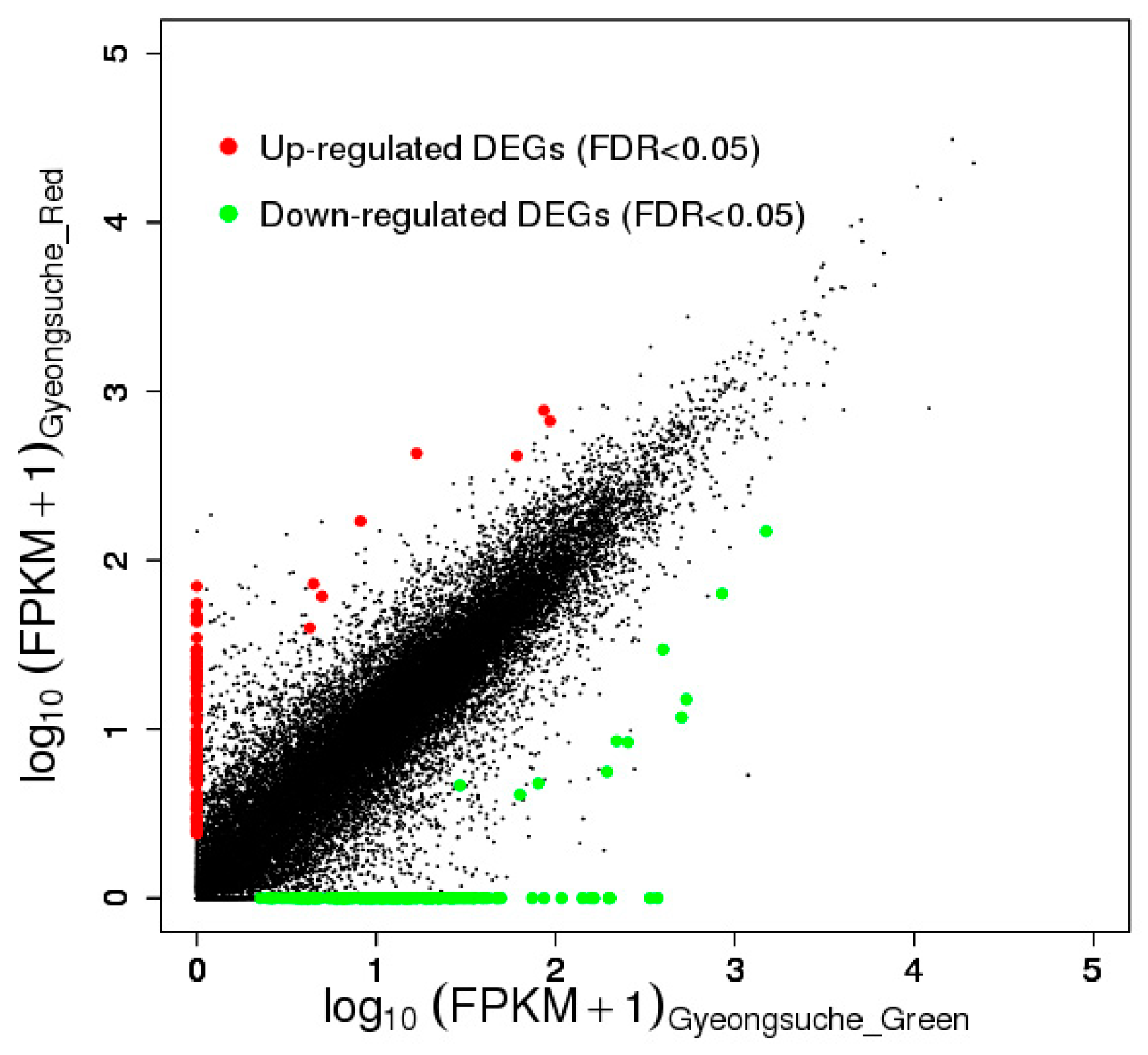
| Category | Term | Count | p-Value | Fold Enrichment |
|---|---|---|---|---|
| Biological process | GO:0008219~cell death | 10 | 0.00092 | 4 |
| GO:0006915~apoptosis | 8 | 0.0016 | 4.7 | |
| GO:0009407~toxin catabolic process | 4 | 0.0104 | 8.8 | |
| GO:0009404~toxin metabolic process | 4 | 0.0104 | 8.8 | |
| GO:0005985~sucrose metabolic process | 3 | 0.0119 | 17.8 | |
| GO:0006952~defense response | 19 | 0.0125 | 1.9 | |
| GO:0045087~innate immune response | 8 | 0.0192 | 2.9 | |
| GO:0009266~response to temperature stimulus | 9 | 0.024 | 2.6 | |
| GO:0019748~secondary metabolic process | 10 | 0.0242 | 2.4 | |
| GO:0009617~response to bacterium | 7 | 0.0339 | 2.9 | |
| GO:0006855~multidrug transport | 4 | 0.0379 | 5.4 | |
| GO:0055114~oxidation reduction | 19 | 0.0434 | 1.6 | |
| GO:0015893~drug transport | 4 | 0.0445 | 5 | |
| GO:0042493~response to drug | 4 | 0.0459 | 5 | |
| GO:0000302~response to reactive oxygen species | 5 | 0.0497 | 3.6 | |
| GO:0020037~heme binding | 11 | 0.00382 | 3.0 | |
| GO:0046906~tetrapyrrole binding | 11 | 0.00653 | 2.8 | |
| GO:0019825~oxygen binding | 8 | 0.00818 | 3.5 | |
| Molecular function | GO:0005506~iron ion binding | 15 | 0.01239 | 2.1 |
| GO:0004364~glutathione transferase activity | 4 | 0.01243 | 8.2 | |
| GO:0009055~electron carrier activity | 14 | 0.01295 | 2.1 | |
| GO:0015238~drug transporter activity | 4 | 0.04875 | 4.9 |
| Class | Name | Green Mizuna | Red Mizuna |
|---|---|---|---|
| Glucoiberin | 0.14 ± 0.03 1 | 0.07 ± 0.00 | |
| Progoitrin | 0.31 ± 0.05 *,2 | 0.16 ± 0.04 | |
| Glucoraphanin | 0.14 ± 0.04 | N.D. 3 | |
| Glucoalyssin | 0.32 ± 0.08 | N.D. | |
| Aliphatic glucosinolate | Gluconapoleiferin | 0.22 ± 0.05 | N.D. |
| Gluconapin | 6.45 ± 1.09 | 4.67 ± 1.63 | |
| Glucobrassicanapin | 0.40 ± 0.07 | 2.37 ± 0.84 | |
| Glucoerucin | 3.45 ± 0.59 | N.D. | |
| Glucoberteroin | 2.71 ± 0.47 | N.D. | |
| Phenethyl glucosinolate | Gluconasturtiin | N.D. | 0.16 ± 0.04 |
| Indolic glucosinolate | 4-Hydroxyglucobrassicin | 0.26 ± 0.11 * | 0.05 ± 0.02 |
| Glucobrassicin | 0.84 ± 0.09 | 1.43 ± 0.03 *** | |
| 4-Methoxyglucobrassicin | 0.48 ± 0.05 | 0.70 ± 0.03 ** | |
| Neoglucobrassicin | 0.08 ± 0.01 | 0.28 ± 0.05 ** | |
| Total | 15.81 ± 2.51 * | 9.88 ± 2.66 |
| Class | Name | Green Mizuna | Red Mizuna |
|---|---|---|---|
| Phenolic acid | Gallic acid | 1.48 ± 0.18 | 4.71 ± 0.25 *** 1 |
| Chlorogenic acid | 176.51 ± 2.88 | 184.86 ± 5.56 | |
| Caffeic acid | 120.09 ± 6.03 *** | 64.82 ± 1.47 | |
| Flavonoid | Catechin | 154.16 ± 0.90 | 234.07 ± 6.15 ** |
| (−)-Epicatechin | 1351.16 ± 21.66 *** | 847.38 ± 42.12 | |
| Organic compound | Vanillin | 64.71 ± 3.82 *** | 5.09 ± 1.28 |
| Benzoic acid | 195.20 ± 22.33 | 220.89 ± 14.38 | |
| Total | 2063.30 ± 23.08 *** | 1561.82 ± 66.89 |
| Class | Name | Green Mizuna | Red Mizuna |
|---|---|---|---|
| Flavonoids | Cyanidin 3-diglucoside-5-glucoside | N.D. 1 | 0.02 ± 0.00 2 |
| Cyanidin 3-(sinapoyl)diglucoside-5-glucoside | N.D. | 0.16 ± 0.01 | |
| Cyanidin 3-(caffeoyl)(p-coumaroyl)diglucoside-5-glucoside | N.D. | 0.03 ± 0.00 | |
| Cyanidin 3-(glycopyranosyl-sinapoyl)diglucoside-5-glucoside | N.D. | 0.13 ± 0.01 | |
| Cyanidin 3-(p-coumaroyl)(sinapoyl)triglucoside-5-glucoside | N.D. | 0.02 ± 0.00 | |
| Cyanidin 3-(sinapoyl)glucoside-5-glucoside | N.D. | 0.20 ± 0.02 | |
| Cyanidin 3-(p-coumaroyl)diglucoside-5-glucoside | N.D. | 0.11 ± 0.01 | |
| Cyanidin 3-(sinapoyl)diglucoside-5-glucoside | N.D. | 0.25 ± 0.02 | |
| Cyanidin 3-(p-coumaroyl)(sinapoyl)diglucoside-5-glucoside | N.D. | 0.04 ± 0.01 | |
| Cyanidin 3-(feruloyl)(sinapoyl)diglucoside-5-glucoside | N.D. | 0.35 ± 0.02 | |
| Cyanidin 3-(sinapoyl)(sinapoyl)diglucoside-5-glucoside | N.D. | 0.76 ± 0.04 | |
| Cyanidin 3-(feruloyl)(sinapoyl)diglucoside-5-glucoside | N.D. | 0.15 ± 0.01 | |
| Total | 2.49 ± 0.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.H.; Bong, S.J.; Lim, C.J.; Kim, J.K.; Park, S.U. Transcriptome Analysis and Metabolic Profiling of Green and Red Mizuna (Brassica rapa L. var. japonica). Foods 2020, 9, 1079. https://doi.org/10.3390/foods9081079
Park CH, Bong SJ, Lim CJ, Kim JK, Park SU. Transcriptome Analysis and Metabolic Profiling of Green and Red Mizuna (Brassica rapa L. var. japonica). Foods. 2020; 9(8):1079. https://doi.org/10.3390/foods9081079
Chicago/Turabian StylePark, Chang Ha, Sun Ju Bong, Chan Ju Lim, Jae Kwang Kim, and Sang Un Park. 2020. "Transcriptome Analysis and Metabolic Profiling of Green and Red Mizuna (Brassica rapa L. var. japonica)" Foods 9, no. 8: 1079. https://doi.org/10.3390/foods9081079
APA StylePark, C. H., Bong, S. J., Lim, C. J., Kim, J. K., & Park, S. U. (2020). Transcriptome Analysis and Metabolic Profiling of Green and Red Mizuna (Brassica rapa L. var. japonica). Foods, 9(8), 1079. https://doi.org/10.3390/foods9081079





