Microencapsulation of Saccharomyces cerevisiae into Alginate Beads: A Focus on Functional Properties of Released Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Microencapsulation into Alginate Beads
2.3. Yeast Viability During Storage
2.4. Release of Yeasts from Beads
2.5. Hydrophobicity
2.6. Auto-Aggregation
2.7. Simulated Gastrointestinal Conditions
- Nine different sterile tubes, containing 45 mL of SS and 5 g of beads, were prepared and incubated at 37 °C for 5 min. Then, viable count was evaluated on beads and in SS from 3 tubes.
2.8. Biofilm Formation
- 40 mL YPG broth+ beads (5 g)
- 40 mL saline solution + beads (5 g)
- 40 mL YPG broth + free cells (5 log cfu/mL)
- 40 mL saline solution + free cells (5 log cfu/mL)
2.9. Statistic
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- de Vos, P.; Faas, M.M.; Spasojevic, M.; Sikkema, J. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. Int. Dairy J. 2010, 20, 292–302. [Google Scholar] [CrossRef]
- Champagne, C.P.; Fustier, P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr. Opin. Biotechnol. 2007, 18, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.; Shami, T.C.; Bhasker Rao, K.U. Microencapsulation technology and applications. Def. Sci. J. 2009, 59, 82–95. [Google Scholar]
- Nazzaro, F.; Orlando, P.; Fratianni, F.; Coppola, R. Microencapsulation in food science and biotechnology. Curr. Opin. Biotechnol. 2012, 23, 182–186. [Google Scholar] [CrossRef]
- Yao, M.; Wu, J.; Li, B.; Xiao, H.; McClements, D.J.; Lanjuan, L. Microencapsulation of Lactobacillus salivarious Li01 for enhanced storage viability and targeted delivery to gut microbiota. Food Hydrocoll. 2017, 72, 228–236. [Google Scholar] [CrossRef]
- D’Orazio, G.; Di Gennaro, P.; Boccarusso, M.; Presti, I.; Bizzaro, G.; Giardina, S.; Michelotti, A.; Labra, M.; La Ferla, B. Microencapsulation of new probiotic formulations for gastrointestinal delivery: In vitro study to assess viability and biological properties. Appl. Microbiol. Biotechnol. 2015, 99, 9779–9789. [Google Scholar] [CrossRef]
- Kailasapathy, K. Microencapsulation of probiotic bacteria: Technology and potential applications. Curr. Issues Int. Microbiol. 2002, 3, 39–48. [Google Scholar]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Evaluation of encapsulation techniques of probiotics for yoghurt. Int. Dairy J. 2003, 13, 3–13. [Google Scholar] [CrossRef]
- Gbassi, G.K.; Vandamme, T.; Ennahar, S.; Marchioni, E. Microencapsulation of Lactobacillus plantarum in an alginate matrix coated with whey proteins. Int. J. Food Microbiol. 2009, 129, 103–105. [Google Scholar] [CrossRef]
- Kavitake, D.; Kandasamya, S.; Bruntha Devi, P.; Shetty, P.H. Recent developments on encapsulation of lactic acid bacteria as potential starter culture in fermented foods–A review. Food Biosci. 2018, 21, 34–44. [Google Scholar] [CrossRef]
- Martín, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Microencapsulation of bacteria: A review of different technologies and their impact on the probiotic effects. Innov. Food Sci. Emerg. Technol. 2015, 27, 15–25. [Google Scholar] [CrossRef]
- Muzzafar, A.; Sharma, V. Microencapsulation of probiotics for incorporation in cream biscuits. J. Food Meas. Charact. 2018, 12, 2193–2201. [Google Scholar] [CrossRef]
- Ye, Q.; Georges, N.; Selomulya, C. Microencapsulation of active ingredients in functional foods: From research stage to commercial food products. Trends Food Sci. Technol. 2018, 78, 167–179. [Google Scholar] [CrossRef]
- Qi, W.; Liang, X.; Yun, T.; Guo, W. Growth and survival of microencapsulated probiotics prepared by emulsion and internal gelation. J. Food Sci. Technol. 2019, 56, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Bevilacqua, A.; Speranza, B.; Sinigaglia, M.; Corbo, M.R. Alginate beads and apple pieces as carriers for Saccharomyces cerevisiae var. boulardii, as representative of yeast functional starter cultures. Int. J. Food Sci. Technol. 2014, 49, 2092–2100. [Google Scholar] [CrossRef]
- Zamora-Vega, R.; Montañez-Soto, J.L.; Martínez-Flores, H.E.; Flores-Magallon, R.; Muñoz-Ruiz, C.V.; Venegas-González, J.; Ariza Ortega, T.D.J. Effect of incorporating prebiotics in coating materials for the microencapsulation of Saccharomyces boulardii. Int. J. Food Sci. Nutr. 2012, 63, 930–935. [Google Scholar] [CrossRef]
- Rodriguez, E.T.; Flores, H.E.M.; Lopez, J.O.R.; Vega, R.Z.; Garciglia, R.S.; Sanchez, R.E.P. Survival rate of Saccharomyces boulardii adapted to a functional freeze-dried yogurt: Experimental study related to processing, storage and digestion by Wistar rats. Funct. Foods Health Dis. 2017, 7, 98–114. [Google Scholar]
- Bevilacqua, A.; Speranza, B.; Santillo, A.; Albenzio, M.; Gallo, M.; Sinigaglia, M.; Corbo, M.R. Alginate-microencapsulation of Lactobacillus casei and Bifidobacterium bifidum: Performances of encapsulated microorganisms and bead-validation in lamb rennet. LWT Food Sci. Technol. 2019, 113, 108349. [Google Scholar] [CrossRef]
- Petruzzi, L.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Ochratoxin A removal by yeasts after exposure to simulated human gastrointestinal conditions. J. Food Sci. 2016, 81, M2756–M2760. [Google Scholar] [CrossRef]
- Perricone, M.; Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Technological characterization and probiotic traits of yeasts isolated from Altamura sourdough to select promising microorganisms as functional starter cultures for cereal-based products. Food Microbiol. 2014, 38, 26–35. [Google Scholar] [CrossRef]
- Corbo, M.R.; Bevilacqua, A.; Gallo, M.; Speranza, B.; Sinigaglia, M. Immobilization and microencapsulation of Lactobacillus plantarum: Performances and in vivo applications. Innov. Food Sci. Emerg. Technol. 2013, 18, 196–201. [Google Scholar] [CrossRef]
- Chávarri, M.; Marañón, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; Villarán, M. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Gallego, J.; Arroyo-López, F.N.; Rantsiou, K.; Jiménez-Díaz, R.; Garrido-Fernández, A.; Cocolin, L. Screening of lactic acid bacteria isolated from fermented table olives with probiotic potential. Food Res. Int. 2013, 50, 135–142. [Google Scholar] [CrossRef]
- Russo, P.; Fernández de Palencia, P.; Romano, A.; Fernández, M.; Lucas, P.; Spano, G.; López, P. Biogenic amine production by the wine Lactobacillus brevis IOEB 9809 in systems that partially mimic the gastrointestinal tract stress. BMC Microbiol. 2012, 12, 247. [Google Scholar] [CrossRef]
- Kos, B.; Suskovic, J.; Goreta, J.; Matosic, S. Effect of protectors on the viability of Lactobacillus acidophilus M92 in simulated gastrointestinal conditions. Food Technol. Biotechnol. 2000, 2, 121–127. [Google Scholar]
- Pizzolitto, R.P.; Armando, M.R.; Combina, M.; Cavaglieri, L.R.; Dalcero, A.M.; Salvano, M.A. Evaluation of Saccharomyces cerevisiae strains as probiotic agent with aflatoxin B₁ adsorption ability for use in poultry feedstuffs. J. Environ. Sci. Health 2012, 47, 933–941. [Google Scholar] [CrossRef]
- Speranza, B.; Corbo, M.R.; Sinigaglia, M. Effects of nutritional and environmental conditions on Salmonella spp biofilm formation. J. Food Sci. 2011, 76, M12–M16. [Google Scholar] [CrossRef]
- Corbo, M.R.; Bevilacqua, A.; Sinigaglia, M. Shelf life of alginate beads containing lactobacilli and bifidobacteria. Characterization of microspheres containing Lactobacillus delbrueckii subsp. bulgaricus. Int. J. Food Sci. Technol. 2011, 46, 2212–2217. [Google Scholar] [CrossRef]
- Suvarna, S.; Dsouza, J.; Ragavan, M.L.; Das, N. Potential probiotic characterization and effect of encapsulation of probiotic yeast strains on survival in simulated gastrointestinal tract condition. Food Sci. Biotechnol. 2018, 27, 745–753. [Google Scholar] [CrossRef]
- Koyama, K.; Seki, M. Cultivation of yeast and plant cells entrapped in the low-viscous liquid-core of an alginate membrane capsule prepared using polyethylene glycol. J. Biosci. Bioeng. 2004, 97, 111–118. [Google Scholar] [CrossRef]
- Qi, W.T.; Yu, W.T.; Xie, Y.B.; Ma, X.J. Optimization of Saccharomyces cerevisiae culture in alginatechitosan-alginate microcapsule. Biochem. Eng. J. 2005, 25, 151–157. [Google Scholar]
- George, M.A. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and Chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.; Younesi, H.; Sari, A.B.; Nayafpour, G. Cane molasses fermentation for continuous ethanol production in an immobilized cells reactor by Saccharomyces cerevisiae. Renew. Energ. 2011, 36, 503–509. [Google Scholar] [CrossRef]
- Ding, W.K.; Shah, N.P. Acid, bile, and heat tolerance of free and microencapsulated probiotic bacteria. J. Food Sci. 2007, 72, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, K.; Mustapha, A.; Grun, I.U.; Fernando, L. Viability of microencapsulated Bifidobacteria in set yogurt during refrigerated storage. J. Dairy Sci. 2000, 83, 1946–1951. [Google Scholar] [CrossRef]
- Sultana, K.; Godward, G.; Reynolds, N.; Arumugaswamy, R.; Peiris, P.; Kailasapathy, K. Encapsulation of probiotic bacteria with alginate-starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. Int. J. Food Microbiol. 2000, 62, 47–55. [Google Scholar] [CrossRef]
- Truelstrup, H.L.; Wojtas, A.P.M.; Jin, Y.L.; Paulson, A.T. Survival of Ca-alginate microencapsulated Bifidobacterium spp. in milk and simulated gastrointestinal conditions. Food Microbiol. 2002, 19, 34–45. [Google Scholar]
- Dohnal, J.; Štěpánek, F. Inkjet fabrication and characterization of calcium alginate microcapsules. Powder Technol. 2010, 200, 254–259. [Google Scholar] [CrossRef]
- Jyothi, N.V.N.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G.Y. Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef]
- Wandrey, C.; Bartkowiak, A.; Harding, S.E. Materials for Encapsulation. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Zuidam, N.J., Nedovic, V.K., Eds.; Springer: New York, NY, USA, 2010; pp. 31–100. [Google Scholar]
- Graff, S.; Hussain, S.; Chaumeil, J.C.; Charrueau, C. Increased intestinal delivery of viable Saccharomyces boulardii by encapsulation in microspheres. Pharm. Res. 2008, 25, 1290–1296. [Google Scholar] [CrossRef]
- Iurciuc (Tincu), C.E.; Peptu, C.; Savin, A.; Atanase, L.I.; Souidi, K.; Mackenzie, G.; Martin, P.; Riess, G.; Popa, M. Microencapsulation of baker’s yeast in gellan gum beads used in repeated cycles of glucose fermentation. Int. J. Polym. Sci. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Duarte, J.C.; Rodrigues, J.A.R.; Moran, P.J.S.; Valenca, G.P.; Nunhez, J.R. Effect of immobilized cells in calcium alginate beads in alcoholic fermentation. AMB Express 2013, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De Wouters, T.; Jans, C.; Niederberger, T.; Fischer, P.; Rühs, P.A. Adhesion potential of intestinal microbes predicted by physico-chemical characterization methods. PLoS ONE 2015, 10, e0136437. [Google Scholar] [CrossRef] [PubMed]
- Haddaji, N.; Mahdhi, A.K.; Krifi, B.; Ben Ismail, M.; Bakhrouf, A. Change in cell surface properties of Lactobacillus casei under heat shock treatment. FEMS Microbiol. Lett. 2015, 362, fnv047. [Google Scholar] [CrossRef]
- Del Re, B.; Sgorbati, B.; Miglioli, M.; Palenzona, D. Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett. Appl. Microbiol. 2000, 31, 438–442. [Google Scholar] [CrossRef]
- Kotzamanidis, C.; Kourelis, A.; Litopoulou-Tzanetaki, E.; Tzanetakis, N.; Yiangou, M. Evaluation of adhesion capacity, cell surface traits and immunomodulatory activity of presumptive probiotic Lactobacillus strains. Int. J. Food Microbiol. 2010, 140, 154–163. [Google Scholar] [CrossRef]
- World Health Organization, Food and Agriculture Organization of the United Nations (WHO/FAO). Probiotics in Food. Health and nutritional properties and guidelines for evaluation. FAO Food Nutr. Pap. 2006, 85. [Google Scholar]
- Zamora-Vega, R.; Martínez-Flores, E.; Montañez-Soto, J.L.; Rodiles-Lopez, J.O. Viabilidad de Saccharomyces boullardii en queso fresco bajo condiciones de acidez. Nova Sci. 2015, 15, 68–80. [Google Scholar] [CrossRef]
- Chandramouli, V.; Kailasapathy, K.; Peiris, P.; Jones, M. An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. J. Microbiol. Meth. 2004, 56, 27–35. [Google Scholar] [CrossRef]
- Corona-Hernandez, R.I.; Alvarez-Parilla, E.; Lizardi-Mendoza, J.; Islas-Rubio, A.R.; De la Rosa, L.A.; Wall-Medrano, A. Structural stability and viability of microencapsulated probiotic bacteria: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 614–628. [Google Scholar] [CrossRef]
- Pinpimai, K.; Rodkhum, C.; Chansue, N.; Katagiri, T.; Maita, M.; Pirarat, N. The study on the candidate probiotic properties of encapsulated yeast Saccharomyces cerevisiae JCM 7255, in Nile Tilapia (Oreochromis niloticus). Res. Vet. Sci. 2015, 102, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Speranza, B.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Novel microbial immobilization techniques. In Novel Food Fermentation Technologies, Food Engineering Series; Ojha, K., Tiwari, B., Eds.; Springer: Cham, Switzerland, 2016; pp. 35–55. [Google Scholar]
- Arenales-Sierra, I.M.; Lobato-Calleros, C.; Vernon-Carter, E.J.; Hernández-Rodríguez, L.; Alvarez-Ramirez, J. Calcium alginate beads loaded with Mg(OH)2 improve L. casei viability under simulated gastric condition. LWT Food Sci. Technol. 2019, 112, 108220. [Google Scholar] [CrossRef]
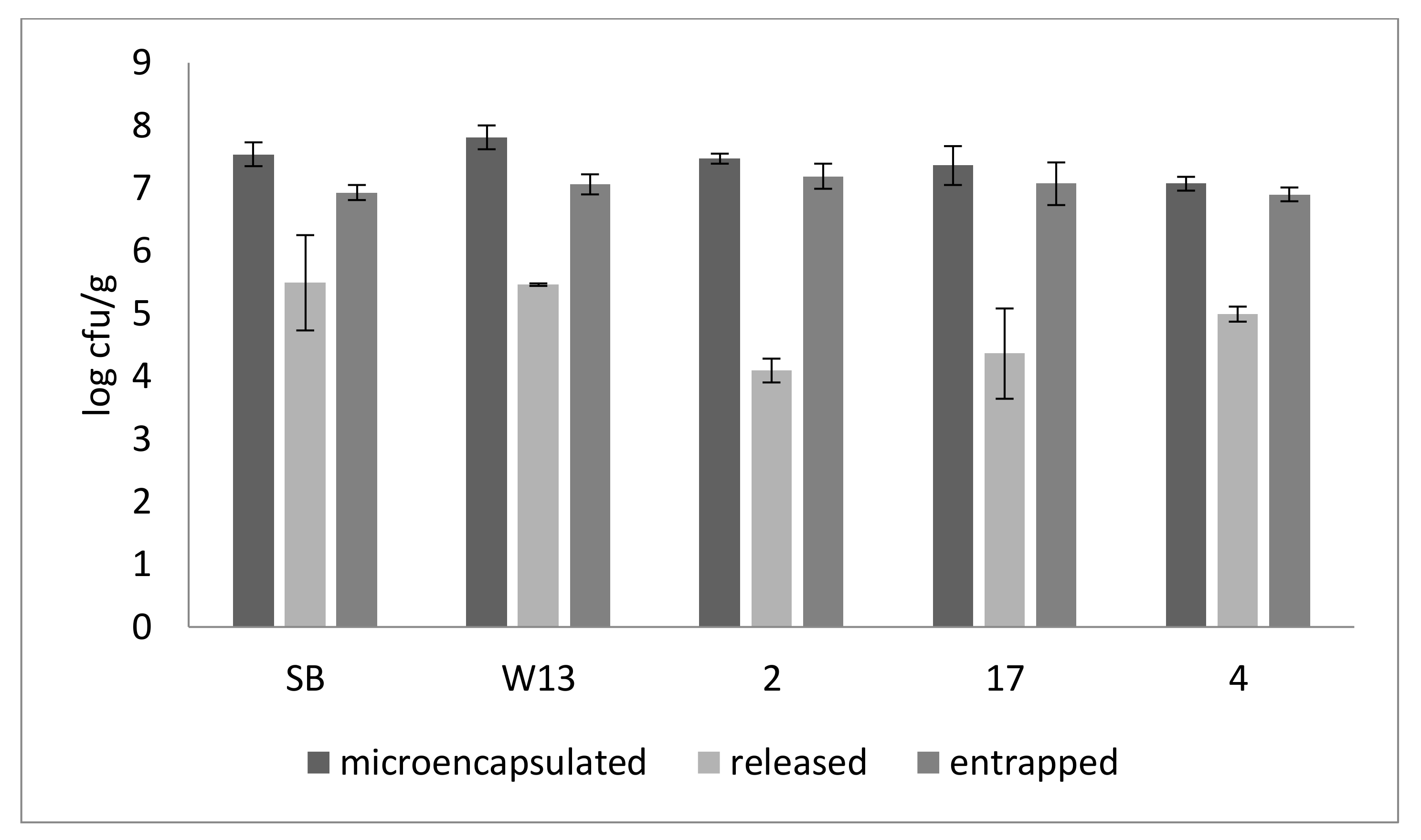

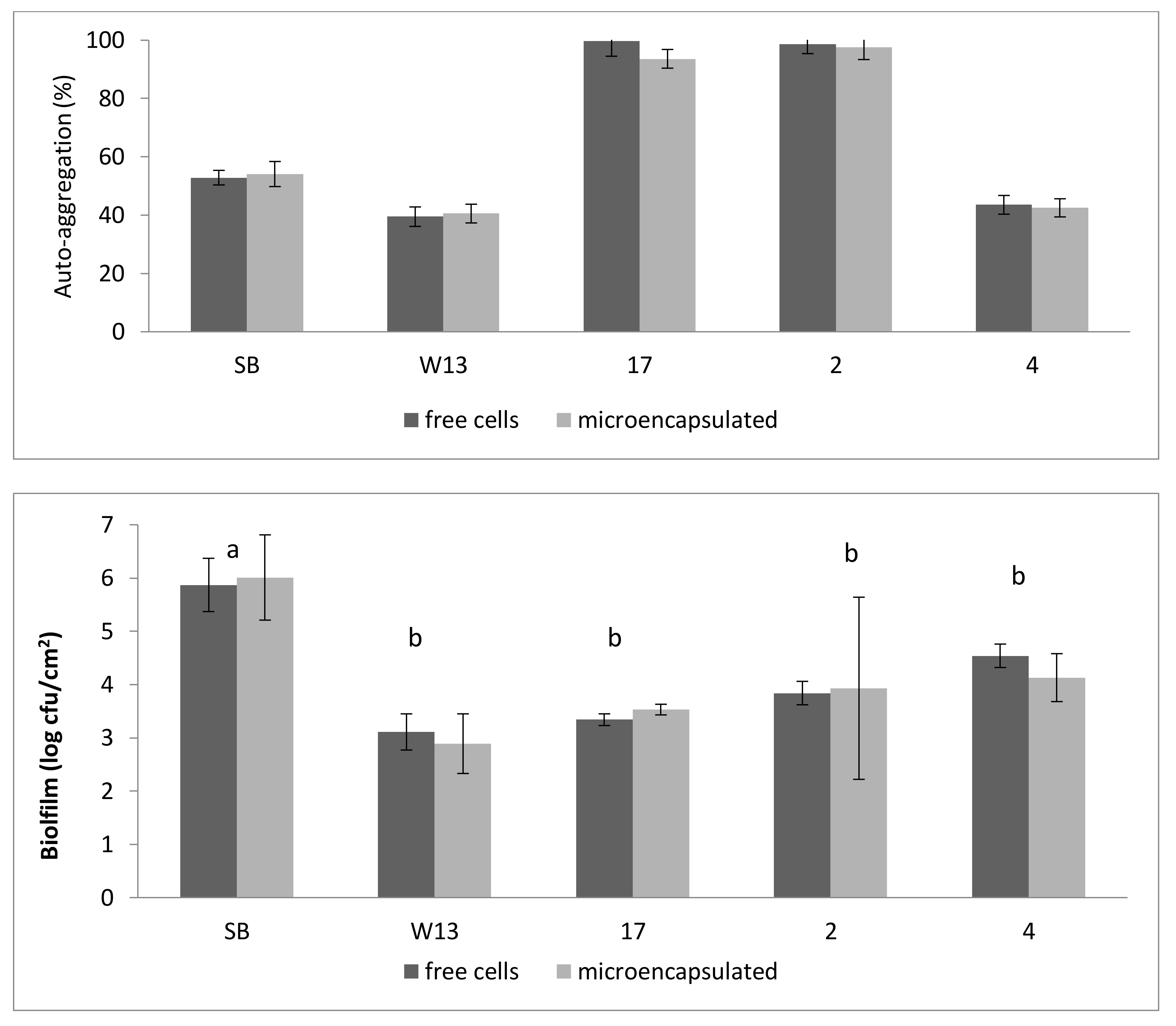
| Strains | EY (%) |
|---|---|
| SB | 93.75 ± 0.36d |
| W13 | 62.78 ± 0.34b |
| 17 | 108.94 ± 0.32e |
| 2 | 54.07 ± 0.14a |
| 4 | 87.80 ± 0.61c |
| Strains | |||||
|---|---|---|---|---|---|
| Days at 4 °C | 2 | 4 | 17 | W13 | SB |
| 0 | 7.54 ± 0.19a | 7.81 ± 0.19a | 7.47 ± 0.08a | 7.36 ± 0.32a | 7.07 ± 0.11a |
| 15 | 7.21 ± 0.19a | 7.53 ± 0.20a | 7.07 ± 0.40a | 7.42 ± 0.08a | 7.13 ± 0.29a |
| 30 | 7.33 ± 0.23a | 7.24 ± 0.17a | 7.70 ± 0.16a | 7.50 ± 0.18a | 7.02 ± 0.32a |
| Days at 25 °C | |||||
| 7 | 4.35 ± 0.23b | 4.11 ± 0.35b | 4.53 ± 0.23b | 3.99 ± 0.11b | 4.01 ± 0.22b |
| Strains | |||||
|---|---|---|---|---|---|
| Time (h) | SB | W13 | 2 | 17 | 4 |
| Dynamic Conditions | |||||
| 0 | - * | - | - | - | - |
| 6 | 4.26 ± 0.68a,b | 4.17 ± 0.08a | 2.97 ± 0.06b | 2.53 ± 0.08c | 3.17 ± 0.13b |
| 24 | 4.49 ± 0.76a | 4.32 ± 0.05a | 3.06 ± 0.09b | 2.62 ± 0.10c | 3.37 ± 0.29b |
| 48 | 3.90 ± 0.18a | 4.46 ± 0.02b | 3.09 ± 0.19c | 3.36 ± 0.72d | 3.99 ± 0.12a |
| Static Conditions | |||||
| 0 | - | - | - | - | - |
| 6 | 3.30 ± 0.09 | 3.06 ± 0.00 | - | - | - |
| 24 | 3.28 ± 0.03 | 3.04 ± 0.02 | 3.18 ± 1.42 | - | - |
| 48 | - | 3.20 ± 0.04 | 3.03 ± 0.23 | - | - |
| SB | ||||
|---|---|---|---|---|
| Time (h) | YF | YB | SF | SB |
| 0 | -P | - | - | - |
| 5 | - | 2.45 ± 0.12d | - | - |
| 8 | 3.24 ± 0.01c | 4.46 ± 0.21a | - | - |
| 14 | 3.17 ± 0.01c | 4.25 ± 0.02b | - | - |
| 21 | 3.06 ± 0.12c | 4.29 ± 0.02b | - | - |
| 17 | ||||
| 0 | - | - | - | - |
| 5 | - | - | - | - |
| 8 | 3.07 ± 0.05c | 2.95 ± 0.02c,d | - | - |
| 14 | 3.13 ± 0.02c | 3.21 ± 0.04c | - | - |
| 21 | 2.86 ± 0.14c,d | 3.28 ± 0.01c | - | |
| Separate Phases | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strains | Salivary Conditions | Gastric Conditions | Intestinal Conditions | |||||||||
| F | B | F | B | F | B | |||||||
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | |
| 2 | 7.65 ± 0.21 | 7.61 ± 0.25 | 7.40 ± 0.11 | 7.22 ± 0.17 | 7.15 ± 0.62 | 7.18 ± 0.61 | 7.40 ± 0.11 | 6.74 ± 0.18 | 7.15 ± 0.62 | 6.98 ± 0.44 | 7.40 ± 0.11 | 5.76 ± 0.22 |
| 4 | 7.52 ± 0.14 | 7.22 ± 0.08 | 7.40 ± 0.23 | 7.05 ± 0.24 | 7.2 ± 0.11 | 6.90 ± 0.53 | 7.44 ± 0.33 | 7.01 ± 0.17 | 7.23 ± 0.15 | 7.07 ± 0.02 | 7.44 ± 0.23 | 6.97 ± 0.25 |
| 17 | 7.35 ± 0.11 | 7.11 ± 0.35 | 7.40 ± 0.20 | 7.68 ± 0.06 | 7.35 ± 0.62 | 7.38 ± 0.21 | 7.20 ± 0.20 | 7.78 ± 0.19 | 7.25 ± 0.62 | 6.97 ± 0.44 | 7.20 ± 0.20 | 7.20 ± 0.27 |
| W13 | 7.65 ± 0.01 | 7.60 ± 0.06 | 7.40 ± 0.11 | 7.40 ± 0.18 | 7.27 ± 0.22 | 7.29 ± 0.05 | 7.40 ± 0.11 | 7.42 ± 0.22 | 7.4 ± 0.03 | 7.30 ± 0.1 | 7.40 ± 0.11 | 7.22 ± 0.08 |
| SB | 7.42 ± 0.10 | 7.4 ± 0.11 | 7.40 ± 0.11 | 7.60 ± 0.07 | 7.06 ± 0.03 | 7.30 ± 0.08 | 7.40 ± 0.11 | 7.74 ± 0.25 | 7.35 ± 0.25 | 7.25 ± 0.15 | 7.40 ± 0.11 | 6.76 ± 0.28 |
| Sequential Protocol | ||||||||||||
| Strains | Before | Salivary Conditions | Gastric Conditions | Intestinal Conditions | ||||||||
| F | B | F | B | F | B | F | B | |||||
| 4 | 7.52 ± 0.15 | 7.44 ± 0.30 | 7.22 ± 0.20 | 7.05 ± 0.24 | 7.29 ± 0.24 | 7.35 ± 0.09 | 7.21 ± 0.13 | 6.72 ± 0.60 | ||||
| 17 | 7.40 ± 0.10 | 7.20 ± 0.20 | 7.30 ± 0.15 | 7.38 ± 0.30 | 7.35 ± 0.13 | 6.82 ± 0.60 | 7.41 ± 0.17 | 7.20 ± 0.27 | ||||
| W13 | 7.66 ± 0.01 | 7.40 ± 0.21 | 7.60 ± 0.05 | 7.40 ± 0.20 | 7.30 ± 0.29 | 7.42 ± 0.11 | 7.45 ± 0.03 | 6.62 ± 0.15 | ||||
| SB | 7.42 ± 0.10 | 7.30 ± 0.19 | 7.66 ± 0.06 | 7.35 ± 0.33 | 7.48 ± 0.25 | 7.29 ± 0.23 | 7.39 ± 0.14 | 7.31 ± 0.28 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bevilacqua, A.; Campaniello, D.; Speranza, B.; Racioppo, A.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Microencapsulation of Saccharomyces cerevisiae into Alginate Beads: A Focus on Functional Properties of Released Cells. Foods 2020, 9, 1051. https://doi.org/10.3390/foods9081051
Bevilacqua A, Campaniello D, Speranza B, Racioppo A, Altieri C, Sinigaglia M, Corbo MR. Microencapsulation of Saccharomyces cerevisiae into Alginate Beads: A Focus on Functional Properties of Released Cells. Foods. 2020; 9(8):1051. https://doi.org/10.3390/foods9081051
Chicago/Turabian StyleBevilacqua, Antonio, Daniela Campaniello, Barbara Speranza, Angela Racioppo, Clelia Altieri, Milena Sinigaglia, and Maria Rosaria Corbo. 2020. "Microencapsulation of Saccharomyces cerevisiae into Alginate Beads: A Focus on Functional Properties of Released Cells" Foods 9, no. 8: 1051. https://doi.org/10.3390/foods9081051
APA StyleBevilacqua, A., Campaniello, D., Speranza, B., Racioppo, A., Altieri, C., Sinigaglia, M., & Corbo, M. R. (2020). Microencapsulation of Saccharomyces cerevisiae into Alginate Beads: A Focus on Functional Properties of Released Cells. Foods, 9(8), 1051. https://doi.org/10.3390/foods9081051







