HPLC-Based Chemometric Analysis for Coffee Adulteration
Abstract
1. Introduction
2. Materials and Methods
2.1. Coffee and Adulterants
2.2. Roasting
2.3. Color Evaluation
2.4. Experimental Design
2.5. Chromatograms Acquisition of Brewed Coffee and Adulterated Coffee
2.6. Statistical Analysis
3. Results and Discussion
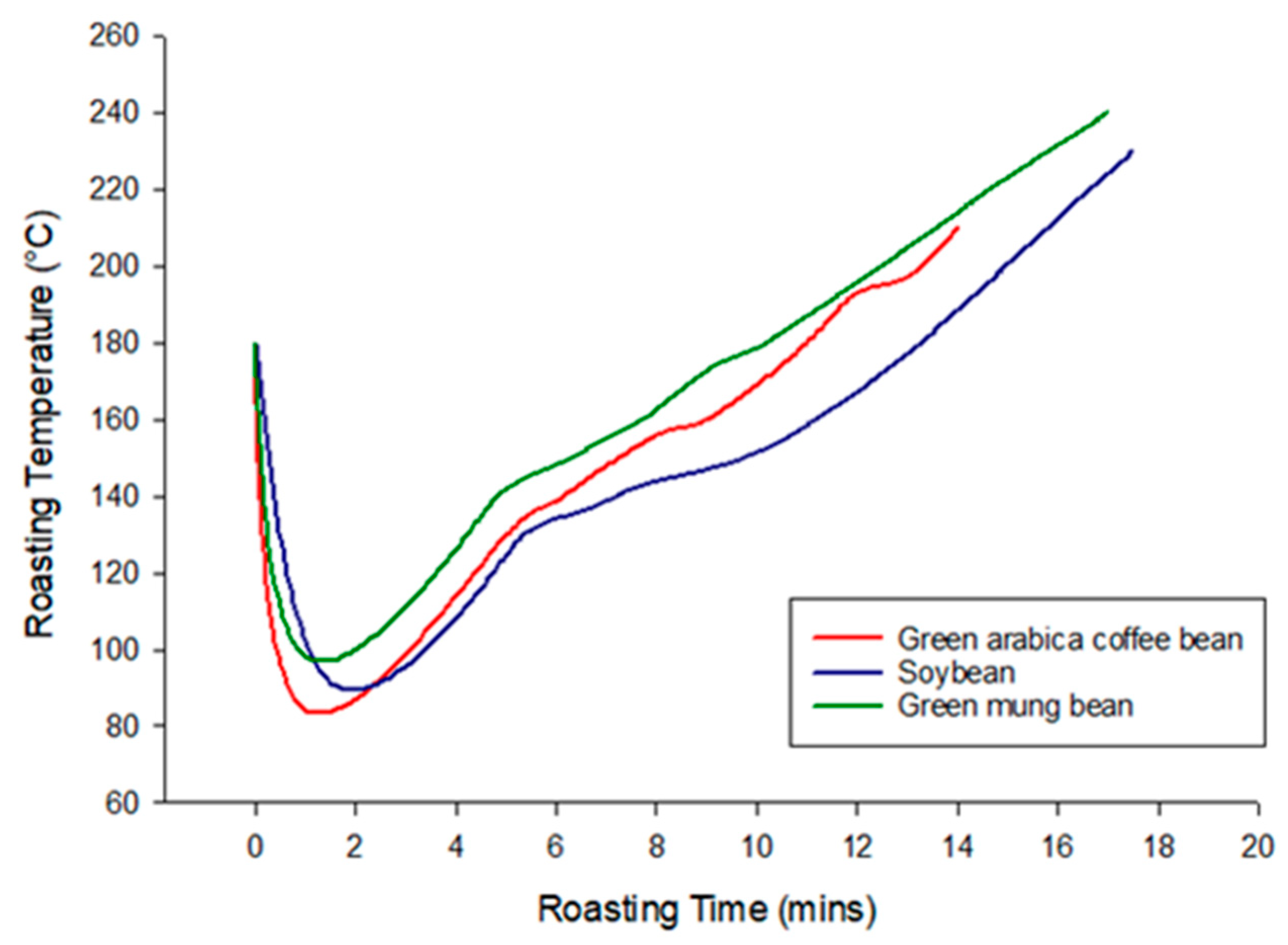
3.1. Coffee Roasting and Color Measurement
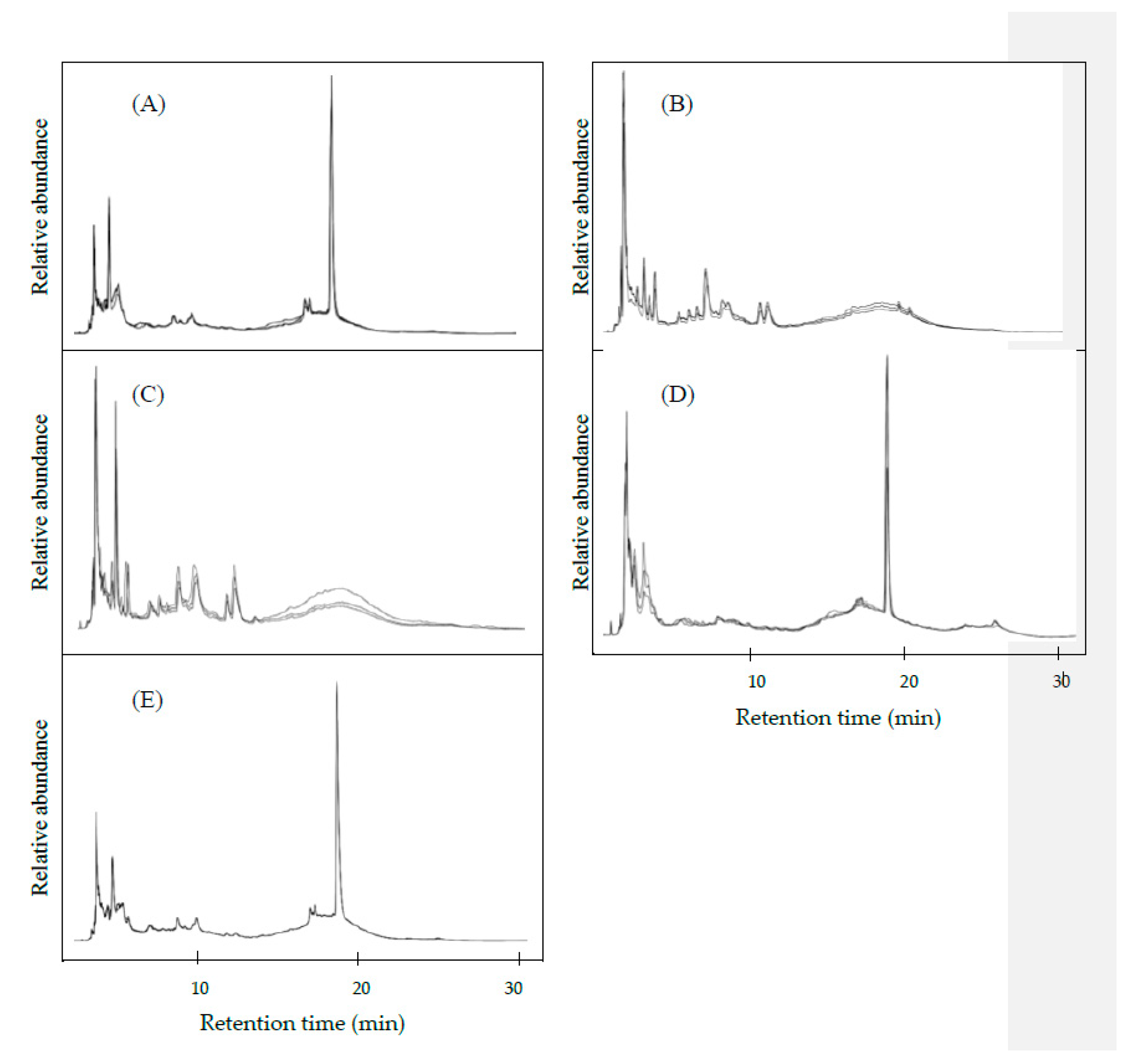
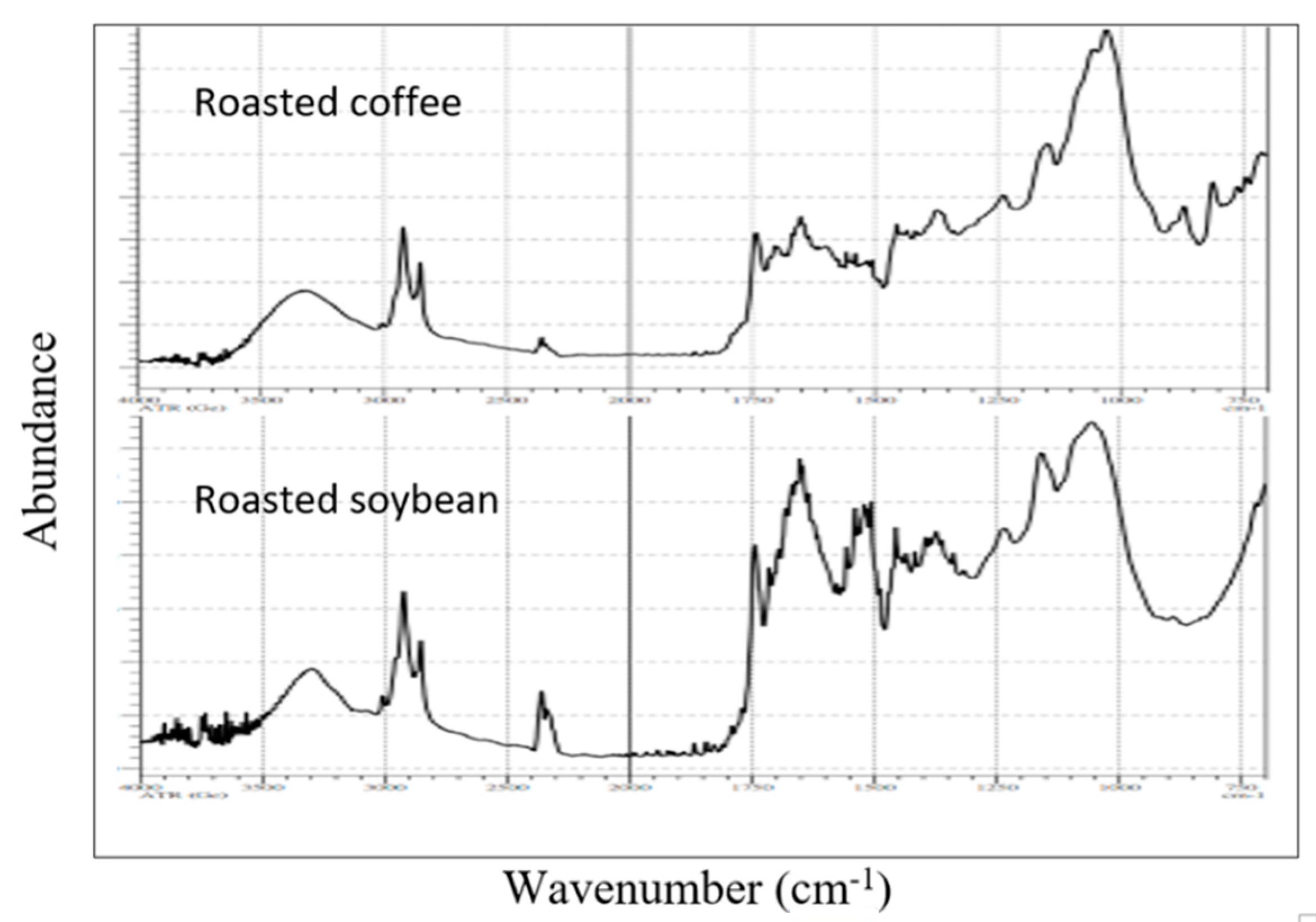
3.2. HPLC Chromatograms
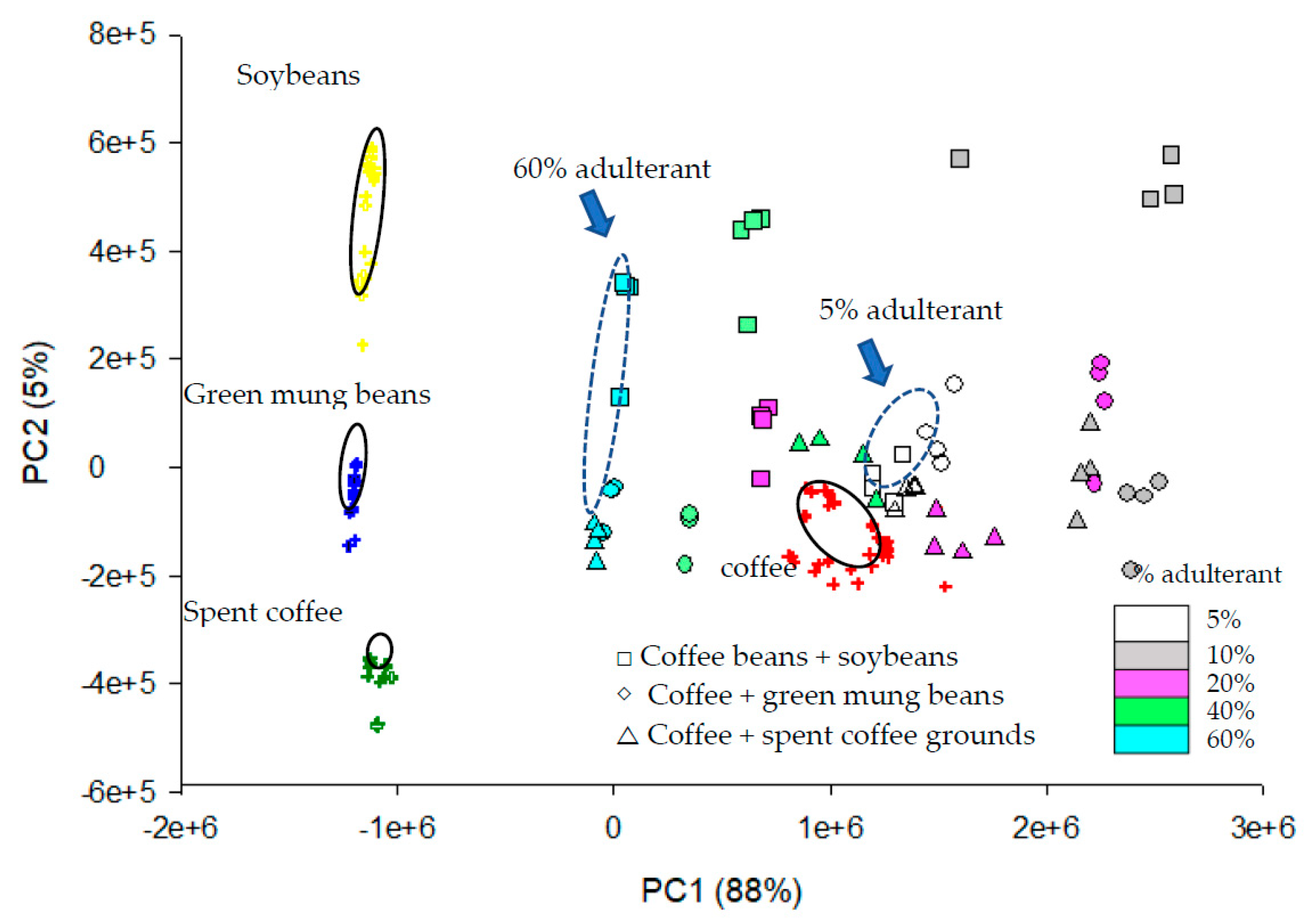
3.3. Principal Component Analysis (PCA)
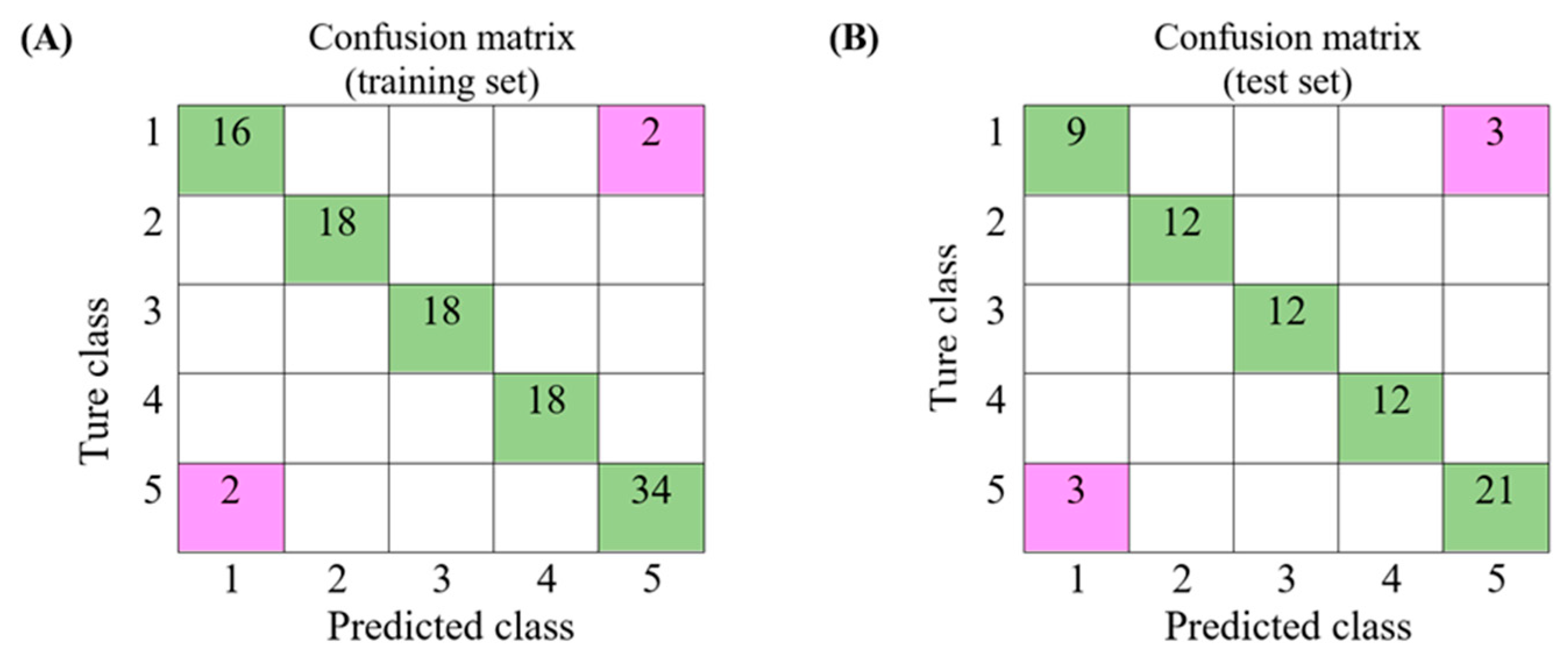
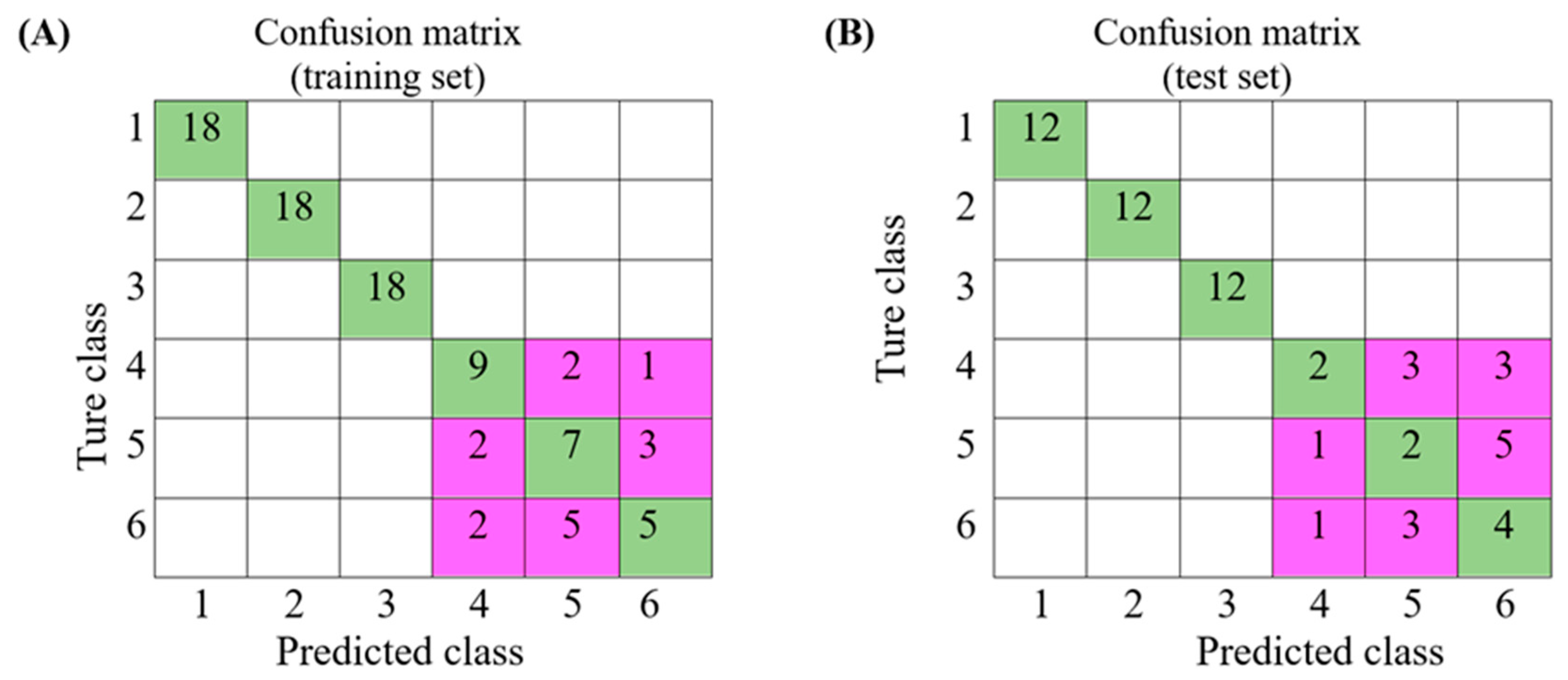
3.4. Discriminatory Power of Models Built
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- International Coffee Organization. Coffee Market Report: November 2019. Available online: http://www.ico.org/documents/cy2019-20/cmr-1119-e.pdf (accessed on 9 February 2020).
- Song, H.Y.; Jang, H.W.; Debnath, T.; Lee, K.G. Food Chemicals Codex, 8th ed.; United States Pharmacopeia: Rockville, MD, USA, 2013. [Google Scholar]
- Oliveira, R.C.; Oliveira, L.S.; Franca, A.S.; Augusti, R. Evaluation of the potential of SPME-GC-MS and chemometrics to detect adulteration of ground roasted coffee with roasted barley. J. Food Compos. Anal. 2009, 22, 257–261. [Google Scholar] [CrossRef]
- Toci, A.T.; Farah, A.; Pezza, H.R.; Pezza, L. Coffee Adulteration: More than Two Decades of Research. Crit. Rev. Anal. Chem. 2015, 46, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Hullah, W.; Trail, A.W.; Mills, D.; Cringle, J.; Drive, P.; Albrecht, S.; Ave, M. Instant Coffee Substitute from Soybeans and Method of Making. U.S. Patent H673, 1989. [Google Scholar]
- FDA Recalls. Market Withdrawals, & Safety Alerts. 2019. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/brian-richardson-dba-tha-pink-issues-voluntary-nationwide-recall-kopi-jantan-tradisional-natural (accessed on 19 March 2020).
- Domingues, D.S.; Pauli, E.D.; de Abreu, J.E.; Massura, F.W.; Cristiano, V.; Santos, M.J.; Nixdorf, S.L. Detection of roasted and ground coffee adulteration by HPLC by amperometric and by post-column derivatization UV–Vis detection. Food Chem. 2014, 146, 353–362. [Google Scholar] [CrossRef]
- Pauli, E.D.; Barbieri, F.; Garcia, P.S.; Madeira, T.B.; Acquaro, V.R.; Scarminio, I.S.; Da Camara, C.A.P.; Nixdorf, S.L. Detection of ground roasted coffee adulteration with roasted soybean and wheat. Food Res. Int. 2014, 61, 112–119. [Google Scholar] [CrossRef]
- Song, H.Y.; Jang, H.W.; Debnath, T.; Lee, K.-G. Analytical method to detect adulteration of ground roasted coffee. Int. J. Food Sci. Technol. 2018, 54, 256–262. [Google Scholar] [CrossRef]
- Barbosa, M.F.; Souto, U.T.D.C.P.; Dantas, H.V.; de Pontes, A.S.; Lyra, W.D.S.; Diniz, P.H.G.D.; Araujo, M.C.U.; Da Silva, E.C. Identification of adulteration in ground roasted coffees using UV–Vis spectroscopy and SPA-LDA. LWT 2015, 63, 1037–1041. [Google Scholar] [CrossRef]
- Cai, T.; Ting, H.; Jin-Lan, Z. Novel identification strategy for ground coffee adulteration based on UPLC–HRMS oligosaccharide profiling. Food Chem. 2016, 190, 1046–1049. [Google Scholar] [CrossRef]
- Reis, N.; Botelho, B.; Franca, A.S.; Oliveira, L.S. Simultaneous Detection of Multiple Adulterants in Ground Roasted Coffee by ATR-FTIR Spectroscopy and Data Fusion. Food Anal. Methods 2017, 10, 2700–2709. [Google Scholar] [CrossRef]
- De Morais, T.C.B.; Rodrigues, D.R.; Souto, U.T.D.C.P.; Lemos, S. A simple voltammetric electronic tongue for the analysis of coffee adulterations. Food Chem. 2019, 273, 31–38. [Google Scholar] [CrossRef]
- Kaufmann, A.; Butcher, P.; Maden, K.; Walker, S.; Widmer, M. Reliability of veterinary drug residue confirmation: High resolution mass spectrometry versus tandem mass spectrometry. Anal. Chim. Acta 2015, 856, 54–67. [Google Scholar] [CrossRef]
- Kaufmann, A. Combining UHPLC and high-resolution MS: A viable approach for the analysis of complex samples? TrAC Trends Anal. Chem. 2014, 63, 113–128. [Google Scholar] [CrossRef]
- Reis, N.; Franca, A.S.; Oliveira, L.S. Performance of diffuse reflectance infrared Fourier transform spectroscopy and chemometrics for detection of multiple adulterants in roasted and ground coffee. LWT 2013, 53, 395–401. [Google Scholar] [CrossRef]
- Okaru, A.O.; Scharinger, A.; de Rezende, T.R.; Teipel, J.C.; Kuballa, T.; Walch, S.G.; Lachenmeier, D.W. Validation of a Quantitative Proton Nuclear Magnetic Resonance Spectroscopic Screening Method for Coffee Quality and Authenticity (NMR Coffee Screener). Foods 2020, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, J.C.; Franca, A.S.; Oliveira, L.S. Physical characterization of non-defective and defective Arabica and Robusta coffees before and after roasting. J. Food Eng. 2009, 92, 474–479. [Google Scholar] [CrossRef]
- Olivieri, A.C. Analytical Figures of Merit: From Univariate to Multiway Calibration. Chem. Rev. 2014, 114, 5358–5378. [Google Scholar] [CrossRef]
- Szymanska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-check: Validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics 2011, 8, 3–16. [Google Scholar] [CrossRef]
- Baratloo, A.; Safari, S.; Elfil, M.; Negida, A. Evidence Based Emergency Medicine Part 3: Positive and Negative Likelihood Ratios of Diagnostic Tests. Emergency 2015, 3, 170–171. [Google Scholar]
- Sloane, P.D.; Slatt, L.M.; Ebell, M.H.; Jacques, L.B.; Smith, M.A. Essentials of Family Medicine, 5th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2008; p. 44. [Google Scholar]
- Patay-Éva, B.; Bencsik, T.; Papp, N. Phytochemical overview and medicinal importance of Coffea species from the past until now. Asian Pac. J. Trop. Med. 2016, 9, 1127–1135. [Google Scholar] [CrossRef]
- Ciabotti, S.; Silva, A.C.B.B.; Juhasz, A.C.P.; Mendonça, C.D.; Tavano, O.L.; Mandarino, J.M.G.; Gonçalves, C.A.A. Chemical composition, protein profile, and isoflavones content in soybean genotypes with different seed coat colors. Int. Food Res. J. 2016, 23, 621–629. [Google Scholar]
- Hou, D.; Yousaf, L.; Xue, Y.; Hu, J.; Wu, J.; Hu, X.; Feng, N.; Shen, Q. Mung Bean (Vigna radiata L.): Bioactive Polyphenols, Polysaccharides, Peptides, and Health Benefits. Nutrients 2019, 11, 1238. [Google Scholar] [CrossRef]
- Zou, H.; Zhang, W.; Feng, Y.; Liang, B. Simultaneous determination of melamine and dicyandiamide in milk by UV spectroscopy coupled with chemometrics. Anal. Methods 2014, 6, 5865–5871. [Google Scholar] [CrossRef]
| Analyte | Luminosity Measurement Result | |
|---|---|---|
| L* (Mean ± SD, n = 3) | p-Value (t-Test of Coffee and Other Analytes) | |
| Coffee beans | 20.8 ± 0.8 | |
| Soybeans | 27.5 ± 0.4 | 0.00 |
| Green mung beans | 30.5 ± 1.2 | 0.00 |
| Spent coffee grounds | 18.0 ± 0.2 | 0.00 |
| Coffee beans + soybeans * | 21.4 ± 0.7 | 0.10 |
| Coffee beans + green mung beans * | 21.5 ± 0.6 | 0.07 |
| Coffee beans + spent coffee grounds * | 20.5 ± 0.4 | 0.28 |
| SP | SE | RLR | +LR | −LR | |
|---|---|---|---|---|---|
| First model | |||||
| Training set | 0.972 | 0.944 | 0.916 | 33.7 | 0.0576 |
| Test set | 0.938 | 0.875 | 0.813 | 14.1 | 0.133 |
| Second model | |||||
| Training set | 1.00 | 0.583 | 0.583 | – | 0.417 |
| Test set | 1.00 | 0.333 | 0.333 | – | 0.667 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheah, W.L.; Fang, M. HPLC-Based Chemometric Analysis for Coffee Adulteration. Foods 2020, 9, 880. https://doi.org/10.3390/foods9070880
Cheah WL, Fang M. HPLC-Based Chemometric Analysis for Coffee Adulteration. Foods. 2020; 9(7):880. https://doi.org/10.3390/foods9070880
Chicago/Turabian StyleCheah, Wai Lok, and Mingchih Fang. 2020. "HPLC-Based Chemometric Analysis for Coffee Adulteration" Foods 9, no. 7: 880. https://doi.org/10.3390/foods9070880
APA StyleCheah, W. L., & Fang, M. (2020). HPLC-Based Chemometric Analysis for Coffee Adulteration. Foods, 9(7), 880. https://doi.org/10.3390/foods9070880





