Production and Characterization of Anti-Inflammatory Monascus Pigment Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cells and Media
2.3. Procedures for Monascus Cultivation
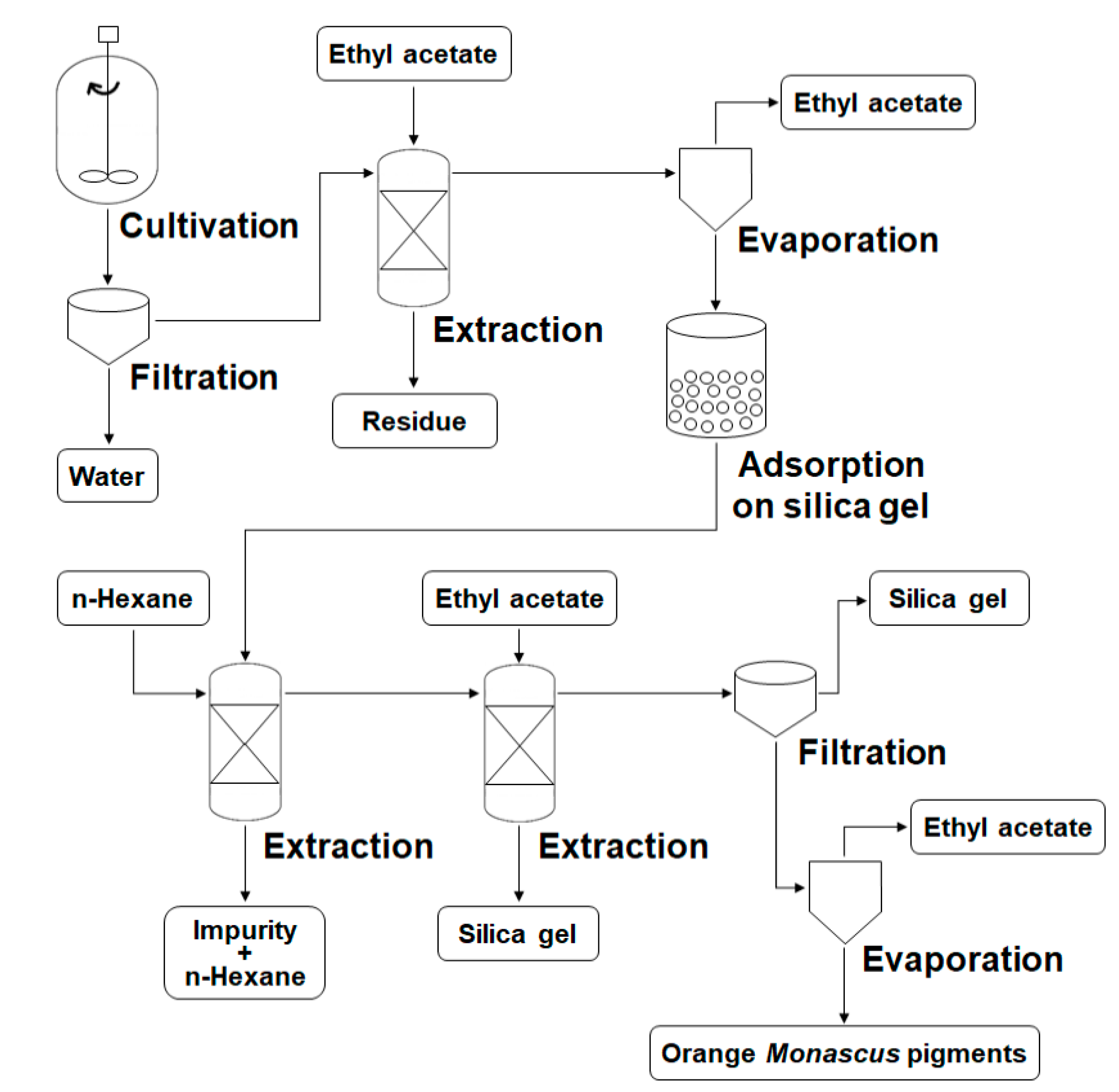
2.4. Extraction and Identification of Orange Pigments
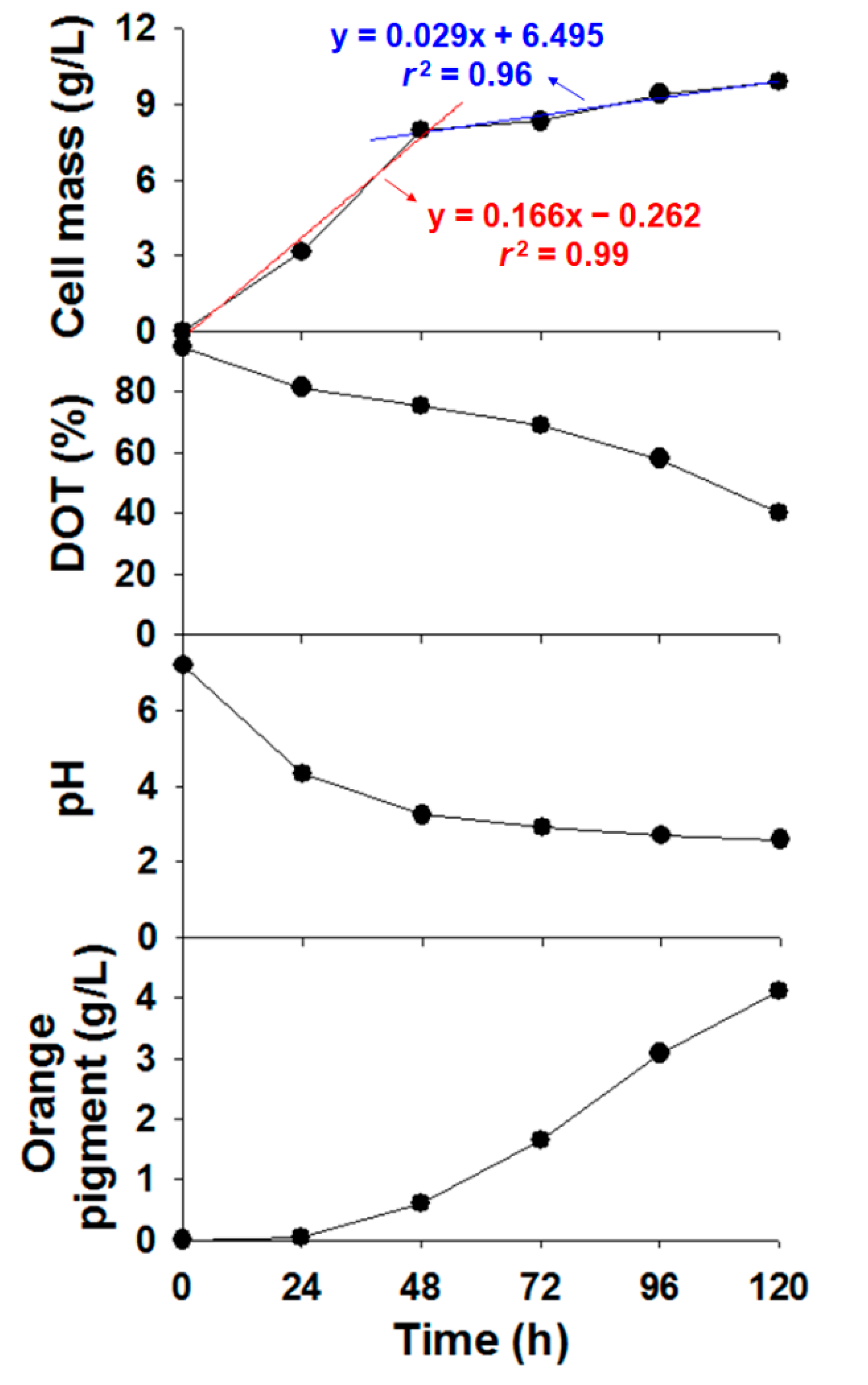
2.5. Determination of Cell Mass, Dissolved Oxygen Tension, pH, and Orange Pigment Production
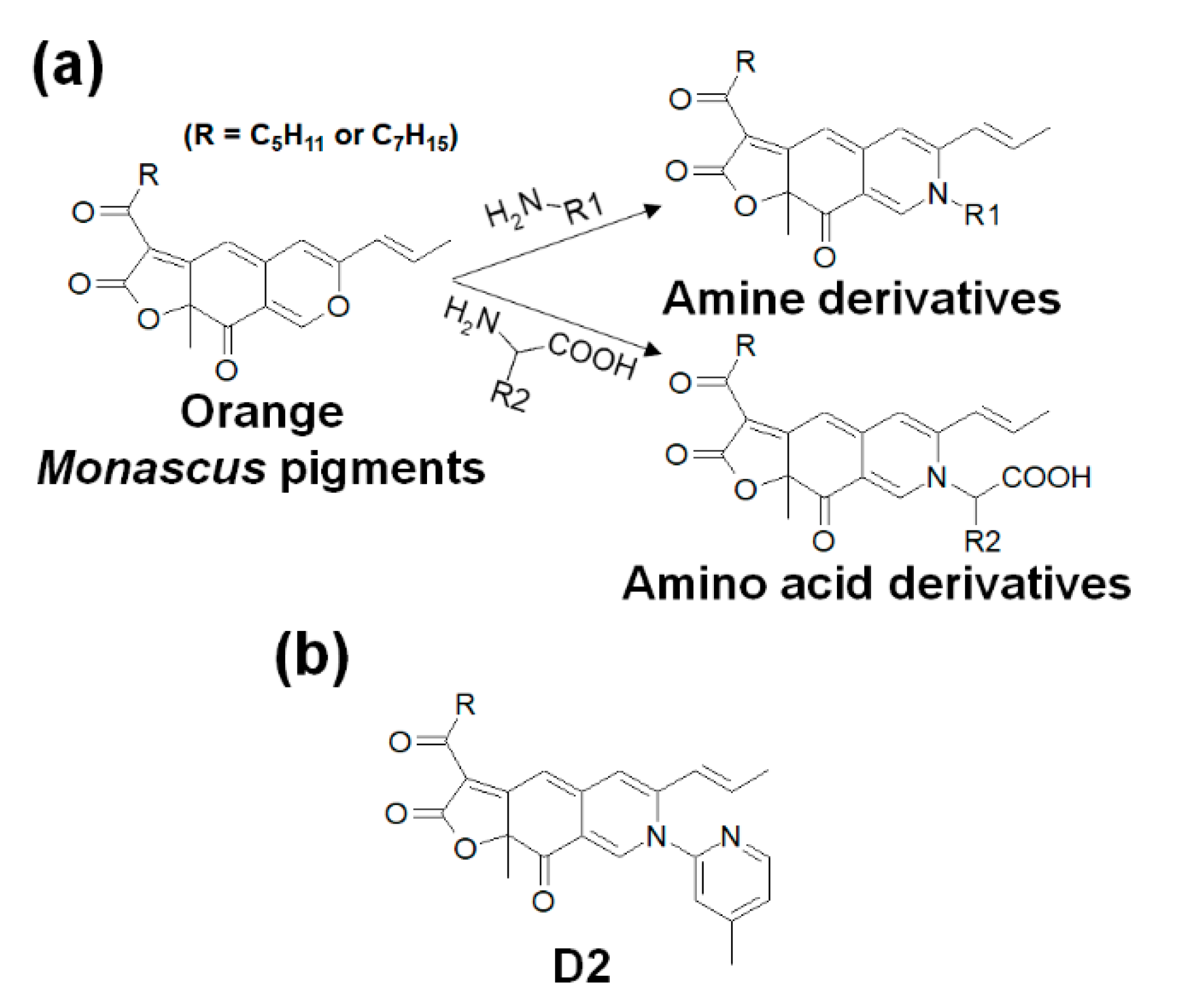
2.6. Procedures for the Synthesis of Monascus Pigment Derivatives
2.7. Measurement of NO Concentration
2.8. MTT Assay for Cell Viability
2.9. Western Blot Analysis
3. Results and Discussion
3.1. Production of Orange Monascus Pigments through Submerged Fermentation
3.2. Preparation Process for Orange Monascus Pigments and Synthesis of Derivatives
3.3. NO Production and Cytotoxicity of Monascus Pigment Derivatives in Raw 264.7 Cells
3.4. Inhibition of iNOS Expression in the Concentration of Amine Derivatives
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Xu, Z.Z.; Gao, Y.J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Chamessian, A.; Zhang, Y.Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; DuBois, R.N. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis 2015, 36, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Park, S.J.; Yun, K.S.; Kang, J.Y.; Lee, S.H. Enzymeless glucose sensor integrated with chronically implantable nerve cuff electrode for in-situ inflammation monitoring. Sens. Actuators B Chem. 2016, 222, 425–432. [Google Scholar] [CrossRef]
- Mazarakis, N.; Snibson, K.; Licciardi, P.V.; Karagiannis, T.C. The potential use of L-sulforaphane for the treatment of chronic inflammatory diseases: A review of the clinical evidence. Clin. Nutr. 2020, 39, 664–675. [Google Scholar] [CrossRef]
- Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation-nature’s way to efficiently respond to all types of challenges: Implications for understanding and managing “the epidemic” of chronic diseases. Front. Med. 2018, 5, 316. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.I.; Jain, P.; Khan, Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, S.S.; Li, Y.; Bae, C.S.; Park, K.M.; Park, D.H. Anti-inflammatory effect of Curcuma longa and Allium hookeri co-treatment via NF-κB and COX-2 pathways. Sci. Rep. 2020, 10, 5718. [Google Scholar] [CrossRef] [PubMed]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory action of glucocorticoids—New mechanisms for old drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Haß, U.; Herpich, C.; Norman, K. Anti-inflammatory diets and fatigue. Nutrients 2019, 11, 2315. [Google Scholar] [CrossRef] [PubMed]
- Kontogiorgis, C.A.; Bompou, E.M.; Ntella, M.; Berghe, W.V. Natural products from Mediterranean diet: From anti-inflammatory agents to dietary epigenetic modulators. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2010, 9, 101–124. [Google Scholar] [CrossRef]
- Hoffman, R.; Gerber, M. Food processing and the Mediterranean diet. Nutrients 2015, 7, 7925–7964. [Google Scholar] [CrossRef]
- Cheng, S.C.; Wu, Y.H.; Huang, W.C.; Pang, J.S.; Huang, T.H.; Cheng, C.Y. Anti-inflammatory property of quercetin through downregulation of ICAM-1 and MMP-9 in TNF-α-activated retinal pigment epithelial cells. Cytokine 2019, 116, 48–60. [Google Scholar] [CrossRef]
- Ahn, H.J.; You, H.J.; Park, M.S.; Li, Z.; Choe, D.; Johnston, T.V.; Ku, S.; Ji, G.E. Microbial biocatalysis of quercetin-3-glucoside and isorhamnetin-3-glucoside in Salicornia herbacea and their contribution to improved anti-inflammatory activity. RSC Adv. 2020, 10, 5339–5350. [Google Scholar] [CrossRef]
- Sreedhar, R.; Arumugam, S.; Thandavarayan, R.A.; Karuppagounder, V.; Watanabe, K. Curcumin as a therapeutic agent in the chemoprevention of inflammatory bowel disease. Drug Discov. Today 2016, 21, 843–849. [Google Scholar] [CrossRef]
- Ran, Z.; Zhang, Y.; Wen, X.; Ma, J. Curcumin inhibits high glucose-induced inflammatory injury in human retinal pigment epithelial cells through the ROS-PI3K/AKT/mTOR signaling pathway. Mol. Med. Rep. 2019, 19, 1024–1031. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Update on natural food pigments—A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Brnčić, S.R.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; Pascual-Teresa, S. A review of factors affecting anthocyanin bioavailability: Possible implications for the inter-individual variability. Foods 2020, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Cheng, M.J.; Wu, M.D.; Chen, J.J.; Chen, Y.L.; Chang, H.S. Three new constituents from the fungus of Monascus purpureus and their anti-inflammatory activity. Phytochem. Lett. 2019, 31, 242–248. [Google Scholar] [CrossRef]
- Lin, C.H.; Lin, T.H.; Pan, T.M. Alleviation of metabolic syndrome by monascin and ankaflavin: The perspective of Monascus functional foods. Food Funct. 2017, 8, 2102–2109. [Google Scholar] [CrossRef]
- Chen, W.; He, Y.; Zhou, Y.; Shao, Y.; Feng, Y.; Li, M.; Chen, F. Edible filamentous fungi from the species Monascus: Early traditional fermentations, modern molecular biology, and future genomics. Compr. Rev. Food Sci. Food Saf. 2015, 14, 555–567. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, R.; Guo, W.; Hong, J.; Li, L.; Ni, L.; Sun, J.; Liu, B.; Rao, P.; Lv, X. Monascus yellow, red and orange pigments from red yeast rice ameliorate lipid metabolic disorders and gut microbiota dysbiosis in Wistar rats fed on a high-fat diet. Food Funct. 2019, 10, 1073–1084. [Google Scholar] [CrossRef]
- Mapari, S.A.S.; Thrane, U.; Meyer, A.S. Fungal polyketide azaphilone pigments as future natural food colorants? Trends Biotechnol. 2010, 28, 300–307. [Google Scholar] [CrossRef]
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Chidambara Murthy, K.N.; Ravishankar, G.A. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality. Trends Food Sci. Technol. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Jia, L.; Tu, X.; He, K.; Wang, C.; Yin, S.; Zhou, Y.; Chen, W. Monascorubrin and rubropunctatin: Preparation and reaction characteristics with amines. Dyes Pigm. 2019, 170, 107629. [Google Scholar] [CrossRef]
- Chen, W.; Chen, R.; Liu, Q.; He, Y.; He, K.; Ding, X.; Kang, L.; Guo, X.; Xie, N.; Zhou, Y.; et al. Orange, red, yellow: Biosynthesis of azaphilone pigments in Monascus fungi. Chem. Sci. 2017, 8, 27–4925. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, J.; Huang, Y.; Xin, Q.; Wang, Z. Diversifying of chemical structure of native Monascus pigments. Front. Microbiol. 2018, 9, 3143. [Google Scholar] [CrossRef]
- Choe, D.; Jung, H.H.; Kim, D.; Shin, C.S.; Johnston, T.V.; Ku, S. In vivo evaluation of the anti-obesity effects of combinations of Monascus pigment derivatives. RSC Adv. 2020, 10, 1456–1462. [Google Scholar] [CrossRef]
- Kim, C.; Jung, H.; Kim, Y.O.; Shin, C.S. Antimicrobial activities of amino acid derivatives of monascus pigments. FEMS Microbiol. Lett. 2006, 264, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.M.; Kim, S.J.; Kim, G.W.; Rhee, J.K.; Kim, N.D.; Jung, H.; Jeun, J.; Lee, S.H.; Han, S.H.; Shin, C.S.; et al. Inhibition of hepatitis C virus replication by Monascus pigment derivatives that interfere with viral RNA polymerase activity and the mevalonate biosynthesis pathway. J. Antimicrob. Chemother. 2012, 67, 49–58. [Google Scholar] [CrossRef]
- Jeun, J.; Jung, H.; Kim, J.H.; Kim, Y.O.; Youn, S.H.; Shin, C.S. Effect of the Monascus pigment threonine derivative on regulation of the cholesterol level in mice. Food Chem. 2008, 107, 1078–1085. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.J.; Park, H.W.; Youn, S.H.; Choi, D.Y.; Shin, C.S. Development of inhibitors against lipase and α-glucosidase from derivatives of Monascus pigment. FEMS Microbiol. Lett. 2007, 276, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Choe, D.; Shin, C.S. Novel derivatives of Monascus pigment having a high CETP inhibitory activity. Nat. Prod. Res. 2014, 28, 1427–1431. [Google Scholar] [CrossRef]
- Nam, K.; Choe, D.; Shin, C.S. Antiobesity effect of a jelly food containing the L-tryptophan derivative of Monascus pigment in mice. J. Funct. Foods 2014, 9, 306–314. [Google Scholar] [CrossRef]
- Jo, D.; Choe, D.; Nam, K.; Shin, C.S. Biological evaluation of novel derivatives of the orange pigments from Monascus sp. as inhibitors of melanogenesis. Biotechnol. Lett. 2014, 36, 1605–1613. [Google Scholar] [CrossRef]
- Choe, D.; Lee, J.; Woo, S.; Shin, C.S. Evaluation of the amine derivatives of Monascus pigment with anti-obesity activities. Food Chem. 2012, 134, 315–323. [Google Scholar] [CrossRef]
- Choe, D.; Jang, H.; Jung, H.H.; Shin, C.S.; Johnston, T.V.; Kim, D.; Ku, S. In vivo anti-obesity effects of Monascus pigment threonine derivative with enhanced hydrophilicity. J. Funct. Foods 2020, 67, 103849. [Google Scholar] [CrossRef]
- Vendruscolo, F.; Schmidell, W.; de Oliveira, D.; Ninow, J.L. Kinetic of orange pigment production from Monascus ruber on submerged fermentation. Bioprocess Biosyst. Eng. 2017, 40, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.G.; Tonso, A.; Kilikian, B.V. Effect of dissolved oxygen concentration on red pigment and citrinin production by Monascus purpureus ATCC 36928. Braz. J. Chem. Eng. 2008, 25, 247–253. [Google Scholar] [CrossRef]
- Hajjaj, H.; Blanc, P.J.; Groussac, E.; Goma, G.; Uribelarrea, J.L.; Loubiere, P. Improvement of red pigment/citrinin production ratio as a function of environmental conditions by monascus ruber. Biotechnol. Bioeng. 1999, 64, 497–501. [Google Scholar] [CrossRef]
- Feng, Y.; Shao, Y.; Chen, F. Monascus pigments. Appl. Microbiol. Biotechnol. 2012, 96, 1421–1440. [Google Scholar] [CrossRef]
- Li, M.; Kang, L.; Ding, X.; Liu, J.; Liu, Q.; Shao, Y.; Molnár, I.; Chen, F. Monasone naphthoquinone biosynthesis and resistance in Monascus fungi. mBio 2020, 11, e02676-19. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Y.; Molnár, I.; Chen, F. Nature and nurture: Confluence of pathway determinism with metabolic and chemical serendipity diversifies Monascus azaphilone pigments. Nat. Prod. Rep. 2019, 36, 561–572. [Google Scholar] [CrossRef]
- Carels, M.; Shepherd, D. The effect of different nitrogen sources on pigment production and sporulation of Monascus species in submerged, shaken culture. Can. J. Microbiol. 1977, 23, 1360–1372. [Google Scholar] [CrossRef]
- Patrovsky, M.; Sinovska, K.; Branska, B.; Patakova, P. Effect of initial pH, different nitrogen sources, and cultivation time on the production of yellow or orange Monascus purpureus pigments and the mycotoxin citrinin. Food Sci. Nutr. 2019, 7, 3494–3500. [Google Scholar] [CrossRef]
- Chen, M.H.; Johns, M.R. Effect of pH and nitrogen source on pigment production by Monascus purpureus. Appl. Microbiol. Biotechnol. 1993, 40, 132–138. [Google Scholar] [CrossRef]
- Li, L.; Chen, S.; Gao, M.; Ding, B.; Zhang, J.; Zhou, Y.; Liu, Y.; Yang, H.; Wu, Q.; Chen, F. Acidic conditions induce the accumulation of orange Monascus pigments during liquid-state fermentation of Monascus ruber M7. Appl. Microbiol. Biotechnol. 2019, 103, 8393–8402. [Google Scholar] [CrossRef]
- Shi, K.; Song, D.; Chen, G.; Pistolozzi, M.; Wu, Z.; Quan, L. Controlling composition and color characteristics of Monascus pigments by pH and nitrogen sources in submerged fermentation. J. Biosci. Bioeng. 2015, 120, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Chen, G.; Pistolozzi, M.; Xia, F.; Wu, Z. Improved analysis of Monascus pigments based on their pH-sensitive Uv-Vis absorption and reactivity properties. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 1396–1401. [Google Scholar] [CrossRef]
- Agboyibor, C.; Kong, W.B.; Chen, D.; Zhang, A.M.; Niu, S.-Q. Monascus pigments production, composition, bioactivity and its application: A review. Biocatal. Agric. Biotechnol. 2018, 16, 433–447. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Bhaskar, N.; Mahendrakar, N.S. Recovery of carotenoids from shrimp waste in organic solvents. Waste Manag. 2006, 26, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Liu, L.; Huang, Y.; Zhang, X.; Wang, Z. Production of Monascus pigments as extracellular crystals by cell suspension culture. Appl. Microbiol. Biotechnol. 2018, 102, 677–687. [Google Scholar] [CrossRef]
- Zhao, G.P.; Li, Y.Q.; Yang, J.; Cui, K.Y. Antibacterial characteristics of orange pigment extracted from Monascus pigments against Escherichia coli. Czech J. Food Sci. 2016, 34, 197–203. [Google Scholar] [CrossRef]
- Lee, S.J.; McClements, D.J. Fabrication of protein-stabilized nanoemulsions using a combined homogenization and amphiphilic solvent dissolution/evaporation approach. Food Hydrocoll. 2010, 24, 560–569. [Google Scholar] [CrossRef]
- Kim, D.; Ku, S. Beneficial effects of Monascus sp. KCCM 10093 pigments and derivatives: A mini review. Molecules 2018, 23, 98. [Google Scholar] [CrossRef]
- Brannan, C.A.; Roberts, M.R. Resident microglia from adult mice are refractory to nitric oxide-inducing stimuli due to impaired NOS2 gene expression. Glia 2004, 48, 120–131. [Google Scholar] [CrossRef]
- Hsu, Y.W.; Hsu, L.C.; Liang, Y.H.; Kuo, Y.H.; Pan, T.M. New bioactive orange pigments with yellow fluorescence from Monascus-fermented dioscorea. J. Agric. Food Chem. 2011, 59, 4512–4518. [Google Scholar] [CrossRef]
- Hsu, L.C.; Liang, Y.H.; Hsu, Y.W.; Kuo, Y.H.; Pan, T.M. Anti-inflammatory properties of yellow and orange pigments from Monascus purpureus NTU 568. J. Agric. Food Chem. 2013, 61, 2796–2802. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Tokuda, H.; Yasukawa, K.; Ukiya, M.; Kiyota, A.; Sakamoto, N.; Suzuki, T.; Tanabe, N.; Nishino, H. Azaphilones, furanoisophthalides, and amino acids from the extracts of Monascus pilosus-fermented rice (red-mold rice) and their chemopreventive effects. J. Agric. Food Chem. 2005, 53, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Valls, J.; Millán, S.; Martí, M.P.; Borràs, E.; Arola, L. Advanced separation methods of food anthocyanins, isoflavones and flavanols. J. Chromatogr. A 2009, 1216, 7143–7172. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, H.; Babu, S.; Garad, S. Co-amorphous formation of high-dose zwitterionic compounds with amino acids to improve solubility and enable parenteral delivery. Mol. Pharm. 2018, 15, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Tsai, S.H.; Lin-Shiau, S.Y.; Ho, C.T.; Lin, J.K. Theaflavin-3,3′-digallate from black tea blocks the nitric oxide synthase by down-regulating the activation of NF-κB in macrophages. Eur. J. Pharmacol. 1999, 367, 379–388. [Google Scholar] [CrossRef]
- Pereira-Suárez, A.L.; Estrada-Chávez, C.; Arriaga-Díaz, C.; Espinosa-Cueto, P.; Mancilla, R. Coexpression of NRAMP1, iNOS, and nitrotyrosine in bovine tuberculosis. Vet. Pathol. 2006, 43, 709–717. [Google Scholar] [CrossRef]
- Hsu, L.C.; Hsu, Y.W.; Liang, Y.H.; Kuo, Y.H.; Pan, T.M. Anti-tumor and anti-inflammatory properties of ankaflavin and monaphilone A from Monascus purpureus NTU 568. J. Agric. Food Chem. 2011, 59, 1124–1130. [Google Scholar] [CrossRef]

| Derivative Compound | Amines | Derivative Compound | Amino Acids |
|---|---|---|---|
| D1 | 2-amino-6-methylpyridine | D22 | Serine |
| D2 | 2-amino-4-picoline | D23 | Threonine |
| D3 | (S)-(+)-2-amino-1-propanol | D24 | Cysteine |
| D4 | 5,6,7,8-tetrahydro-2-naphthylamine | D25 | Methionine |
| D5 | 2-amino-5-bromopyridine | D26 | Asparagine |
| D6 | 1-amino-4-methylpiperazine | D27 | Glutamine |
| D7 | 2-amino-4-chlorophenol | D28 | Aspartic acid |
| D8 | 2-amino-5-methylthiazole | D29 | Glutamic acid |
| D9 | 2-amino-5-chlorophenol | D30 | Lysine |
| D10 | 2-amino-4-fluorophenol | D31 | Arginine |
| D11 | 2-amino-5-iodopyridine | D32 | Histidine |
| D12 | 4-amino-4H-1,2,4-triazole | D33 | Phenylalanine |
| D13 | Methyl-d3-amine hydrochloride | D34 | Tyrosine |
| D14 | (R)-(-)-1-amino-2-propanol | D35 | Tryptophan |
| D15 | (S)-(+)-1-amino-2-propanol | D36 | Glycine |
| D16 | 5,6,7,8-tetrahydro-4H-cyclohepta[d][1,3]thiazol-2-amine | D37 | Alanine |
| D17 | Benzylamine | D38 | Valine |
| D18 | 4-methylphenethylamine | D39 | Leucine |
| D19 | 4-aminotetrahydropyran | D40 | Isoleucine |
| D20 | (R)-(-)-2-amino-1-butanol | D41 | Theanine |
| D21 | 4-phenylbutylamine |
| Amine Derivative | NO Production (%) | Cell Viability (%) | Amino Acid Derivative | NO Production (%) | Cell Viability (%) |
|---|---|---|---|---|---|
| Con | 100.0 ± 7.1 | 100.0 ± 3.8 | Con | 100.0 ± 4.9 | 100.0 ± 4.0 |
| D1 | 64.6 ± 3.4 | 53.9 ± 1.0 * | D22 | 143.0 ± 4.7 * | 94.0 ± 5.5 |
| D2 | 51.6 ± 0.9 * | 89.7 ± 8.4 | D23 | 106.9 ± 2.3 | 84.9 ± 6.4 |
| D3 | 56.2 ± 0.5 | 31.3 ± 6.7 * | D24 | 147.2 ± 6.1 * | 86.3 ± 5.6 |
| D4 | 61.2 ± 2.0 | 12.9 ± 1.6 * | D25 | 100.6 ± 3.6 | 93.8 ± 2.6 |
| D5 | 56.2 ± 3.1 * | 58.4 ± 5.1 * | D26 | 135.2 ± 6.6 * | 91.2 ± 5.0 |
| D6 | 65.1 ± 2.0 | 13.5 ± 4.7 * | D27 | 106.6 ± 18.1 | 103.2 ± 5.3 |
| D7 | 70.8 ± 1.7 | 3.5 ± 1.2 * | D28 | 126.6 ± 5.2 | 96.4 ± 15.8 |
| D8 | 48.8 ± 0.5 * | 70.9 ± 3.0 * | D29 | 103.1 ± 5.0 | 104.0 ± 6.3 |
| D9 | 66.2 ± 2.9 | 2.4 ± 1.0 * | D30 | 105.5 ± 2.2 | 112.0 ± 4.5 |
| D10 | 90.2 ± 2.5 | 2.5 ± 1.5 * | D31 | 95.8 ± 3.0 | 104.5 ± 0.8 |
| D11 | 63.6 ± 11.4 | 73.3 ± 1.1 * | D32 | 126.4 ± 4.2 * | 90.6 ± 8.8 |
| D12 | 73.3 ± 24.3 | 86.7 ± 5.4 | D33 | 97.3 ± 6.0 | 103.5 ± 4.9 |
| D13 | 73.5 ± 15.4 | 60.4 ± 8.1 | D34 | 141.7 ± 17.6 | 97.8 ± 2.1 |
| D14 | 55.6 ± 2.4 * | 23.4 ± 4.9 * | D35 | 103.0 ± 11.4 | 109.0 ± 4.5 |
| D15 | 55.9 ± 3.3 * | 63.7 ± 2.2 * | D36 | 137.3 ± 16.3 | 102.6 ± 1.0 |
| D16 | 56.8 ± 2.9 * | 16.3 ± 2.9 * | D37 | 100.9 ± 2.3 | 104.7 ± 7.0 |
| D17 | 60.3 ± 11.3 | 44.5 ± 7.3 * | D38 | 118.3 ± 4.8 | 101.3 ± 3.4 |
| D18 | 74.8 ± 5.7 | 12.0 ± 6.7 * | D39 | 100.3 ± 7.6 | 108.0 ± 2.9 |
| D19 | 56.2 ± 4.0 * | 9.6 ± 1.0 * | D40 | 136.7 ± 13.7 | 93.7 ± 4.3 |
| D20 | 58.2 ± 2.6 * | 6.3 ± 3.4 * | D41 | 84.3 ± 4.4 | 98.4 ± 3.3 |
| D21 | 66.1 ± 3.1 | 7.3 ± 3.2 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choe, D.; Song, S.M.; Shin, C.S.; Johnston, T.V.; Ahn, H.J.; Kim, D.; Ku, S. Production and Characterization of Anti-Inflammatory Monascus Pigment Derivatives. Foods 2020, 9, 858. https://doi.org/10.3390/foods9070858
Choe D, Song SM, Shin CS, Johnston TV, Ahn HJ, Kim D, Ku S. Production and Characterization of Anti-Inflammatory Monascus Pigment Derivatives. Foods. 2020; 9(7):858. https://doi.org/10.3390/foods9070858
Chicago/Turabian StyleChoe, Deokyeong, Soo Min Song, Chul Soo Shin, Tony V. Johnston, Hyung Jin Ahn, Daehwan Kim, and Seockmo Ku. 2020. "Production and Characterization of Anti-Inflammatory Monascus Pigment Derivatives" Foods 9, no. 7: 858. https://doi.org/10.3390/foods9070858
APA StyleChoe, D., Song, S. M., Shin, C. S., Johnston, T. V., Ahn, H. J., Kim, D., & Ku, S. (2020). Production and Characterization of Anti-Inflammatory Monascus Pigment Derivatives. Foods, 9(7), 858. https://doi.org/10.3390/foods9070858







