Effect of Substitution of Rice Flour with Quinoa Flour on the Chemical-Physical, Nutritional, Volatile and Sensory Parameters of Gluten-Free Ladyfinger Biscuits
Abstract
1. Introduction
2. Materials and Methods
2.1. Batter Formulation
2.2. Batter and Biscuit Preparation
2.3. Flour and Biscuit Chemical-Physical and Nutritional Analysis
2.3.1. Chemical-Physical Analysis
2.3.2. Nutritional Analysis
2.4. Colour and Texture Analysis of Biscuits
2.4.1. Colour
2.4.2. Texture Analysis
2.5. Biscuit Volatile Compound Analysis
2.6. Sensory Analysis
2.7. CATA Test
2.8. Statistical Analysis
3. Results and Discussion
3.1. Flour and Biscuit Chemical-Physical and Nutritional Characteristics
3.2. Polyphenol Content and Antioxidant Activity of Flour and Biscuits
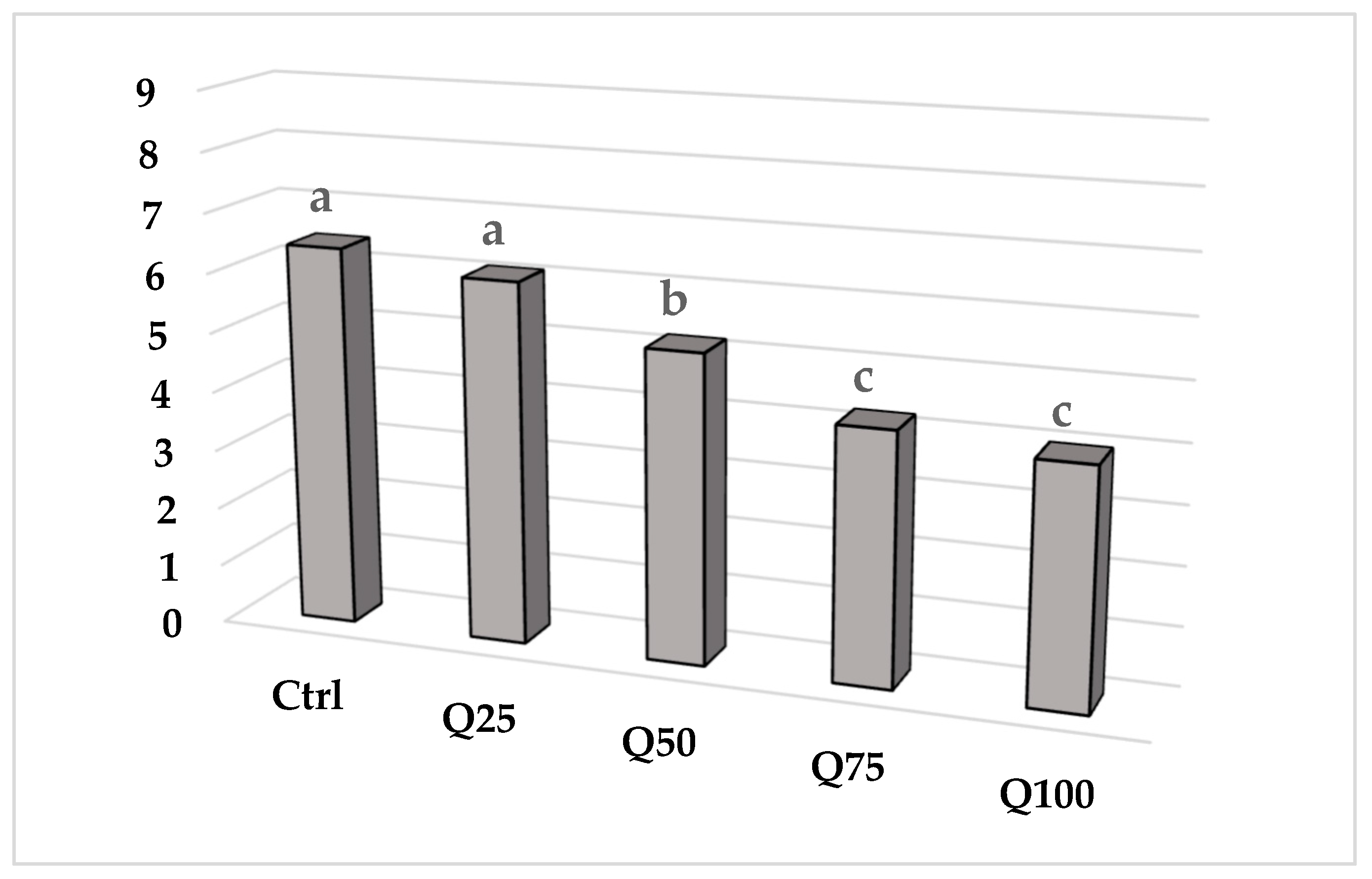
3.3. Biscuit Colour and Texture
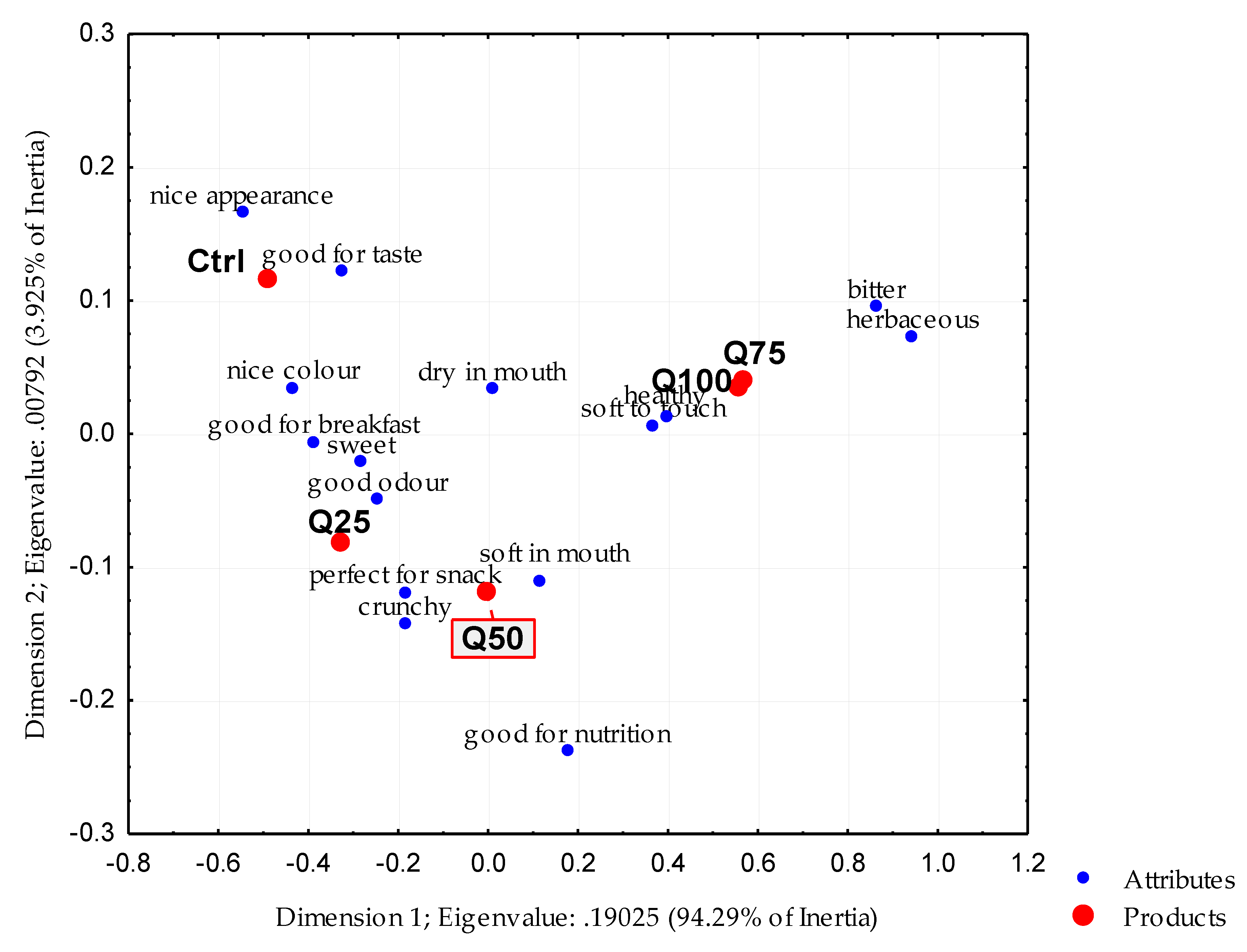
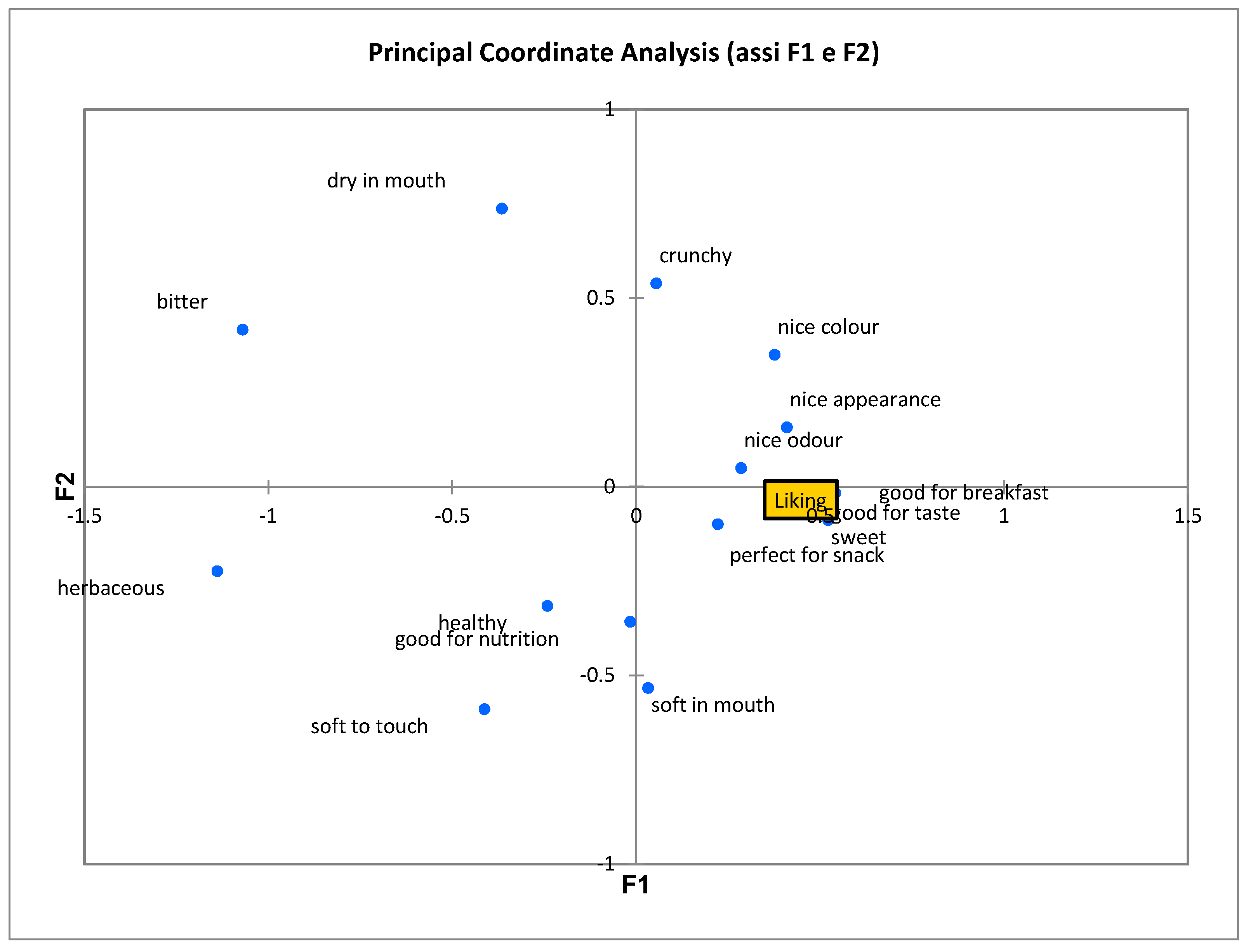
3.4. Sensory Analysis
3.5. Volatiles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pellegrini, N.; Agostoni, C. Nutritional aspects of gluten-free products. J. Sci. Food Agric. 2015, 95, 2380–2385. [Google Scholar] [CrossRef] [PubMed]
- Conte, P.; Fadda, C.; Drabińska, N.; Krupa-Kozak, U. Technological and nutritional challenges, and novelty in gluten-free breadmaking: A review. Polish J. Food Nutr. Sci. 2019, 69, 5–21. [Google Scholar] [CrossRef]
- Jnawali, P.; Kumar, V.; Tanwar, B. Celiac disease: Overview and considerations for development of gluten-free foods. Food Sci. Hum. Wellness 2016, 5, 169–176. [Google Scholar] [CrossRef]
- Naqash, F.; Gani, A.; Gani, A.; Masoodi, F.A. Gluten-free baking: Combating the challenges—A review. Trends Food Sci. Technol. 2017, 66, 98–107. [Google Scholar] [CrossRef]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef]
- Jothi, J.S.; Hashem, S.; Rana, M.R.; Rahman, M.R.T.; Shams-Ud-Din, M. Effect of Gluten-free Composite Flour on Physico-chemical and Sensory Properties of Cracker Biscuits. J. Sci. Res. 2014, 6, 521–530. [Google Scholar] [CrossRef]
- Laguna, L.; Salvador, A.; Sanz, T.; Fiszman, S.M. Performance of a resistant starch rich ingredient in the baking and eating quality of short-dough biscuits. LWT Food Sci. Technol. 2011, 44, 737–746. [Google Scholar] [CrossRef]
- Engleson, J.; Atwell, B. Gluten-free product development. Cereal Foods World 2008, 53, 180–184. [Google Scholar] [CrossRef]
- Foschia, M.; Horstmann, S.; Arendt, E.K.; Zannini, E. Nutritional therapy—Facing the gap between coeliac disease and gluten-free food. Int. J. Food Microbiol. 2016, 239, 113–124. [Google Scholar] [CrossRef]
- Di Cairano, M.; Galgano, F.; Tolve, R.; Caruso, M.C.; Condelli, N. Focus on gluten free biscuits: Ingredients and issues. Trends Food Sci. Technol. 2018, 81, 203–212. [Google Scholar] [CrossRef]
- Adebiyi, J.A.; Obadina, A.O.; Adebo, O.A.; Kayitesi, E. Comparison of nutritional quality and sensory acceptability of biscuits obtained from native, fermented, and malted pearl millet (Pennisetum glaucum) flour. Food Chem. 2017, 232, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.; Abaye, A.O.; Barbeau, W.; Thomason, W. The suitability of teff flour in bread, layer cakes, cookies and biscuits. Int. J. Food Sci. Nutr. 2013, 64, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Duta, D.E.; Culetu, A. Evaluation of rheological, physicochemical, thermal, mechanical and sensory properties of oat-based gluten free cookies. J. Food Eng. 2015, 162, 1–8. [Google Scholar] [CrossRef]
- Tavares, B.O.; da Silva, E.P.; Da Silva, V.S.N.; Soares, M.S.; Ida, E.I.; Damiani, C. Stability of gluten free sweet biscuit elaborated with rice bran, broken rice and okara. Food Sci. Technol. 2016, 36, 296–303. [Google Scholar] [CrossRef]
- Lukin, A.; Bitiutskikh, K. Investigation on the use of hemp flour in cookie production. Bulg. J. Agric. Sci. 2017, 23, 664–667. [Google Scholar]
- Chauhan, A.; Saxena, D.C.; Singh, S. Total dietary fibre and antioxidant activity of gluten free cookies made from raw and germinated amaranth (Amaranthus spp.) flour. LWT Food Sci. Technol. 2015, 63, 939–945. [Google Scholar] [CrossRef]
- Maghaydah, S.; Abdul-hussain, S.; Ajo, R.; Tawalbeh, Y.; Elsahoryi, N. Effect of lupine flour on baking characteristics of gluten free cookies. Adv. J. Food Sci. Technol. 2013, 5, 600–605. [Google Scholar] [CrossRef]
- Sakač, M.; Pestorić, M.; Mišan, A.; Nedeljković, N.; Jambrec, D.; Jovanov, P.; Banjac, V.; Torbica, A.; Hadnadev, M.; Mandić, A. Antioxidant capacity, mineral content and sensory properties of gluten-free rice and buckwheat cookies. Food Technol. Biotechnol. 2015, 53, 38–47. [Google Scholar] [CrossRef]
- Demir, M.K.; Kilinç, M. Utilization of quinoa flour in cookie production. Int. Food Res. J. 2017, 24, 2394–2401. [Google Scholar]
- Bazile, D.; Jacobsen, S.-E.; Verniau, A. The Global Expansion of Quinoa: Trends and Limits. Front. Plant. Sci. 2016, 7, 622. [Google Scholar] [CrossRef]
- Jan, K.N.; Panesar, P.S.; Singh, S. Optimization of antioxidant activity, textural and sensory characteristics of gluten-free cookies made from whole indian quinoa flour. Lebensm. Wiss. 2018, 93, 573–582. [Google Scholar] [CrossRef]
- Arneja, I.; Tanwar, B.; Chauhan, A. Nutritional composition and health benefits of golden grain of 21st century, quinoa (Chenopodium quinoa willd.): A Review. Pakistan J. Nutr. 2015, 14, 1034–1040. [Google Scholar] [CrossRef]
- Wang, S.; Opassathavorn, A.; Zhu, F. Influence of Quinoa Flour on Quality Characteristics of Cookie, Bread and Chinese Steamed Bread. J. Texture Stud. 2015, 46, 281–292. [Google Scholar] [CrossRef]
- Brito, I.L.; de Souza, E.L.; Felex, S.S.S.; Madruga, M.S.; Yamashita, F.; Magnani, M. Nutritional and sensory characteristics of gluten-free quinoa (Chenopodium quinoa Willd)-based cookies development using an experimental mixture design. J. Food Sci. Technol. 2015, 52, 5866–5873. [Google Scholar] [CrossRef] [PubMed]
- AACC International Approved Methods of Analysis. Available online: https://methods.aaccnet.org/summaries/44-15-02.aspx (accessed on 10 January 2020).
- ICC No. 104/1: Determination of ash in Cereals and Cereal Products. Available online: https://www.icc.or.at/publications/icc-standards/standards-overview/104-1-standard-method (accessed on 10 January 2020).
- ICC No. 136: Determination of Total Fat Content in Cereals and Cereal Products. Available online: https://www.icc.or.at/publications/icc-standards/standards-overview/136-standard-method (accessed on 10 January 2020).
- Carciochi, R.A.; Dimitrov, K. Optimization of antioxidant phenolic compounds extraction from quinoa (Chenopodium quinoa) seeds. J. Food Sci. Technol. 2014, 52, 4396–4404. [Google Scholar] [CrossRef]
- Žilić, S.; Hadži-Tašković Šukalović, V.; Dodig, D.; Maksimović, V.; Maksimović, M.; Basić, Z. Antioxidant activity of small grain cereals caused by phenolics and lipid soluble antioxidants. J. Cereal Sci. 2011, 54, 417–424. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Conte, P.; Del Caro, A.; Urgeghe, P.P.; Petretto, G.L.; Montanari, L.; Piga, A.; Fadda, C. Nutritional and aroma improvement of gluten-free bread: is bee pollen effective? Lebensm. Wiss. 2020, 118, 108711. [Google Scholar] [CrossRef]
- Driesener, C.; Romaniuk, J. Comparing methods of brand image measurement. Int. J. Mark. Res. 2006, 48, 681–698. [Google Scholar] [CrossRef]
- Varela, P.; Ares, G. Sensory profiling, the blurred line between sensory and consumer science. A review of novel methods for product characterization. Food Res. Int. 2012, 48, 893–908. [Google Scholar] [CrossRef]
- Jaeger, S.R.; Hunter, D.C.; Vidal, L.; Chheang, S.L.; Ares, G.; Harker, F.R. Sensory product characterization by consumers using check-all-that-apply questions: Investigations linked to term development using kiwifruit as a case study. J. Sens. Stud. 2019, 34, e12490. [Google Scholar] [CrossRef]
- Piqueras-Fiszman, B.; Jaeger, S.R. The impact of evoked consumption contexts and appropriateness on emotion responses. Food Qual. Prefer. 2014, 32, 277–288. [Google Scholar] [CrossRef]
- Miranda-Villa, P.P.; Mufari, J.R.; Bergesse, A.E.; Calandri, E.L. Effects of Whole and Malted Quinoa Flour Addition on Gluten-Free Muffins Quality. J. Food Sci. 2019, 84, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Masi, P.; Bracciale, A.; Aiello, A.; Nicolai, M.A.; Ferranti, P. Effect of added enzymes and quinoa flour on dough characteristics and sensory quality of a gluten-free bakery product. Eur. Food Res. Technol. 2018, 244, 1595–1604. [Google Scholar] [CrossRef]
- Schouteten, J.J.; De Steur, H.; Lagast, S.; De Pelsmaeker, S.; Gellynck, X. Emotional and sensory profiling by children and teenagers: A case study of the check-all-that-apply method on biscuits. J. Sens. Stud. 2017, 32, e12249. [Google Scholar] [CrossRef]
- Suárez-Estrella, D.; Torri, L.; Pagani, M.A.; Marti, A. Quinoa bitterness: Causes and solutions for improving product acceptability. J. Sci. Food Agric. 2018, 98, 4033–4041. [Google Scholar] [CrossRef]
- Plaehn, D. CATA penalty/reward. Food Qual. Prefer. 2012, 24, 141–152. [Google Scholar] [CrossRef]
- Ares, G.; Dauber, C.; Fernández, E.; Giménez, A.; Varela, P. Penalty analysis based on CATA questions to identify drivers of liking and directions for product reformulation. Food Qual. Prefer. 2014, 32, 65–76. [Google Scholar] [CrossRef]
- Macfie, H.J.; Bratchell, N.; Greenhoff, K.; Vallis, L.V. Designs To Balance the Effect of Order of Presentation and First-Order Carry-Over Effects in Hall Tests. J. Sens. Stud. 1989, 4, 129–148. [Google Scholar] [CrossRef]
- Ares, G.; Reis, F.; Oliveira, D.; Antúnez, L.; Vidal, L.; Giménez, A.; Chheang, S.L.; Hunter, D.C.; Kam, K.; Roigard, C.M.; et al. Recommendations for use of balanced presentation order of terms in CATA questions. Food Qual. Prefer. 2015, 46, 137–141. [Google Scholar] [CrossRef]
- Giménez, M.A.; Gámbaro, A.; Miraballes, M.; Roascio, A.; Amarillo, M.; Sammán, N.; Lobo, M. Sensory evaluation and acceptability of gluten-free Andean corn spaghetti. J. Sci. Food Agric. 2015, 95, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Arendt, E.K.; Zannini, E. Quinoa. In Cereal Grains for the Food and Beverage Industries, 1st ed.; Arendt, E.K., Zannini, E., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 409–438. [Google Scholar]
- Abugoch James, L.E. Quinoa (Chenopodium quinoa Willd.): Composition, chemistry, nutritional, and functional properties. Adv. Food Nutr. Res. 2009, 58, 1–31. [Google Scholar] [PubMed]
- Reguera, M.; Haros, C.M. Structure and Composition of Kernels. In Pseudocereals: Chemistry and Technology; Schoenlechner, S., Haros, C.M., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 28–48. [Google Scholar]
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.M.; Mattila, P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Jan, R.; Saxena, D.C.; Singh, S. Physico-chemical, textural, sensory and antioxidant characteristics of gluten-free cookies made from raw and germinated Chenopodium (Chenopodium album) flour. LWT Food Sci. Technol. 2016, 71, 281–287. [Google Scholar] [CrossRef]
- Habauzit, V.; Morand, C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: An update for clinicians. Ther. Adv. Chronic Dis. 2012, 3, 87–106. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=101&showFR=1. (accessed on 10 May 2020).
- Edoura-Gaena, R.B.; Allais, I.; Trystram, G.; Gros, J.B. Influence of aeration conditions on physical and sensory properties of aerated cake batter and biscuits. J. Food Eng. 2007, 79, 1020–1032. [Google Scholar] [CrossRef]
- Atef, A.A.-Z.; El-Faham, S.Y.; Wafaa, H.E. Use of Quinoa Meal to Produce Bakery Products to Celiac and Autism Stuffs. Int. J. Sci. Res. 2014, 3, 1344–1352. [Google Scholar]
- Watanabe, K.; Kawanishi-Asaoka, M.; Myojin, C.; Awata, S.; Ofusa, K.; Kodama, K. Amino acid composition, oxidative stability, and consumer acceptance of cookies made with quinoa flour. Food Sci. Technol. Res. 2014, 20, 687–691. [Google Scholar] [CrossRef]
- Frankel, E.N. Volatile lipid oxidation products. Prog. Lipid Res. 1983, 22, 1–33. [Google Scholar] [CrossRef]
- Nowak, V.; Du, J.; Charrondière, U.R. Assessment of the nutritional composition of quinoa (Chenopodium quinoa Willd.). Food Chem. 2016, 193, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Carbohydrates. In Food Chemistry; Belitz, H.D., Grosch, W., Schieberle, P., Eds.; Springer-Verlag: Berlin, Germany, 2009; pp. 245–341. [Google Scholar]
- Frankel, E.N. Lipid oxidation: Mechanisms, products and biological significance. J. Am. Oil Chem. Soc. 1984, 61, 1908–1917. [Google Scholar] [CrossRef]
| Biscuits a | Moisture | Aw | Proteins b | Lipids | Ashes | TC c | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 7.86 | ± | 0.04e | 0.556 | ± | 0.01e | 13.93 | ± | 0.03e | 5.6 | ± | 0.2d | 0.87 | ± | 0.02e | 71.84 | ± | 0.08a |
| Q25 | 9.09 | ± | 0.04c | 0.615 | ± | 0.01c | 14.25 | ± | 0.1d | 5.96 | ± | 0.02c | 0.95 | ± | 0.01d | 69.84 | ± | 0.13b |
| Q50 | 8.47 | ± | 0.03d | 0.585 | ± | 0.01d | 14.50 | ± | 0.00c | 6.12 | ± | 0.1bc | 1.07 | ± | 0.01c | 69.74 | ± | 0.15b |
| Q75 | 10.46 | ± | 0.01b | 0.684 | ± | 0.01b | 15.95 | ± | 0.03b | 6.19 | ± | 0.06ab | 1.26 | ± | 0.01b | 66.13 | ± | 0.04c |
| Q100 | 11.76 | ± | 0.02a | 0.704 | ± | 0.01a | 17.68 | ± | 0.01a | 6.35 | ± | 0.05a | 1.34 | ± | 0.02a | 62.83 | ± | 0.03d |
| Flours | ||||||||||||||||||
| QF | 12.37 | ± | 0.05 | - | 17.61 | ± | 0.01 | 4.06 | ± | 0.04 | 2.51 | ± | 0.01 | 63.45 | ± | 0.05 | ||
| RF | 14 | ± | 0.02 | - | 6.76 | ± | 0.02 | 1.3 | ± | 0.03 | 0.8 | ± | 0.05 | 77.09 | ± | 0.04 | ||
| Biscuits a | Polyphenol Fractions (mg GAE/100 g d.m.) | Flavonoids (mg CE/100 g d.m.) | Antioxidant Activity (%) * | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soluble | Insoluble b | Total | |||||||||||||
| Control | 24.4 | ± | 0.1e | 16.3 | ± | 0.5d | 40.4 | ± | 0.9e | 5.1 | ± | 0.5d | 19.5 | ± | 1.3d |
| Q25 | 51.6 | ± | 1.1d | 28.2 | ± | 1.1c | 79.8 | ± | 3.8d | 13.3 | ± | 0.1c | 39.5 | ± | 1.4c |
| Q50 | 79.8 | ± | 4.0c | 33.2 | ± | 4b | 113.0 | ± | 7.3c | 14.4 | ± | 0.4c | 66.6 | ± | 2.2b |
| Q75 | 123.4 | ± | 1.1b | 35.8 | ± | 1.1b | 159.2 | ± | 0.6b | 16.7 | ± | 0.0b | 84.3 | ± | 4.0a |
| Q100 | 142.8 | ± | 1.2a | 42.3 | ± | 1.2a | 185.1 | ± | 6.2a | 21.4 | ± | 1.4a | 84.3 | ± | 0.5a |
| Flours a | |||||||||||||||
| QF | 411 | ± | 2.1 | 52.3 | ± | 0.2 | 464.0 | ± | 10.0 | 39.1 | ± | 2.2 | 53.5 | ± | 0.1 |
| RF | 23.0 | ± | 1.1 | 17.4 | ± | 0.5 | 40.0 | ± | 4.6 | 0.9 | ± | 0.5 | 26.3 | ± | 0.2 |
| Biscuits a | Crust Colour | Texture | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | a* | b* | Hardness (N) | Slope (N/s) | Area | |||||||||||||
| Control | 76.25 | ± | 1.06a | 3.34 | ± | 0.44a | 25.26 | ± | 0.57a | 0.55 | ± | 0.22a | 0.61 | ± | 0.29a | 8.05 | ± | 1.04a |
| Q25 | 73.35 | ± | 1.21b | 4.05 | ± | 0.90a | 25.29 | ± | 1.01a | 0.51 | ± | 0.10ab | 1 | ± | 0.55a | 6.9 | ± | 0.56a |
| Q50 | 68.84c | ± | 0.97c | 5.45 | ± | 0.13b | 26.01 | ± | 0.12a | 0.4 | ± | 0.15abc | 0.53 | ± | 0.21a | 7.04 | ± | 1.03a |
| Q75 | 66.73 | ± | 0.93d | 5.93 | ± | 0.13b | 26.67 | ± | 0.11a | 0.33 | ± | 0.04bc | 0.55 | ± | 0.20a | 9.55 | ± | 3.62a |
| Q100 | 66.95 | ± | 0.92d | 4.29 | ± | 0.72a | 25.14 | ± | 0.69a | 0.2 | ± | 0.11c | 0.4 | ± | 0.18a | 8.04 | ± | 2.24a |
| CATA Attributes * | Biscuit Samples | |||||
|---|---|---|---|---|---|---|
| p-values | Control | Q25 | Q50 | Q75 | Q100 | |
| nice colour | 0.000 | 0.718c ** | 0.592c | 0.359b | 0.155a | 0.155a |
| soft to touch | 0.000 | 0.282a | 0.320ab | 0.379ab | 0.505b | 0.495b |
| sweet | 0.000 | 0.699c | 0.563bc | 0.495b | 0.243a | 0.214a |
| perfect for snack | 0.053 | 0.146a | 0.175a | 0.126a | 0.078a | 0.068a |
| crunchy | 0.054 | 0.155a | 0.165a | 0.155a | 0.058a | 0.087a |
| good for nutrition | 0.033 | 0.078a | 0.194b | 0.165ab | 0.126ab | 0.155ab |
| dry in mouth | 0.011 | 0.534b | 0.534b | 0.369a | 0.379a | 0.417a |
| good for breakfast | 0.000 | 0.602c | 0.553c | 0.330b | 0.155a | 0.155a |
| healthy | 0.044 | 0.107a | 0.136a | 0.146a | 0.223a | 0.204a |
| good for taste | 0.000 | 0.417c | 0.301bc | 0.184ab | 0.097a | 0.155ab |
| nice appearance | 0.000 | 0.631d | 0.427c | 0.214b | 0.097ab | 0.087a |
| herbaceous | 0.000 | 0.019a | 0.087a | 0.340b | 0.709c | 0.786c |
| soft in mouth | 0.099 | 0.427a | 0.583a | 0.544a | 0.466a | 0.476a |
| good odour | 0.000 | 0.466c | 0.456c | 0.330bc | 0.126a | 0.243ab |
| bitter | 0.000 | 0.029a | 0.039a | 0.126ab | 0.252bc | 0.291c |
| Must Have | Nice to Have | Does Not Influence | Does Not Harm | Must Not Have |
|---|---|---|---|---|
| nice colour | soft to touch | crunchy | dry in mouth | |
| sweet | good for nutrition | herbaceous | ||
| good for breakfast | healthy | |||
| good for taste | ||||
| nice appearance | ||||
| soft in mouth | ||||
| good odour |
| Volatile Compounds | Samples | RI | ||||
|---|---|---|---|---|---|---|
| Control | Q25 | Q50 | Q75 | Q100 | ||
| Aldehydes | ||||||
| 2-methyl-butanal | 1.13d * | 2.10b | 1.22c | 3.51a | 1.95b | 919 |
| Pentanal | 0.23a | 0.22a | 0.16b | 0.21a | 0.26a | 986 |
| Hexanal | 2.57a | 2.54a | 2.42a | 2.04b | 1.94b | 1086 |
| Heptanal | 1.85b | 2.55a | 1.72b | 2.16b | 1.80b | 1186 |
| Octanal | 2.26c | 4.30a | 2.97b | 3.71a | 3.85b | 1290 |
| Nonanal | 8.13b | 13.83a | 12.45a | 12.03a | 9.10b | 1399 |
| Benzaldehyde | 4.68b | 4.53b | 8.48b | 13.71a | 11.73a | 1555 |
| Benzene acetaldehyde | 1.41c | 1.33c | 1.64c | 3.03a | 2.50b | 1673 |
| Alcohols | ||||||
| 1-hexanol | 1.04c | 2.13b | 4.61a | 4.19a | 4.56a | 1349 |
| 1-octen-3-ol | 2.65c | 2.70a | 1.64b | 1.80b | 1.64b | 1439 |
| 1-ethynyl-cyclohexanol | 0.79b | 0.67a | 0.81a | 0.68a | 0.82a | 1643 |
| Benzyl alcohol | Nde | 0.53d | 0.89c | 1.04b | 1.36a | 1841 |
| Phenylethyl alcohol | 0.43e | 0.54d | 0.78c | 1.05b | 1.19a | 1866 |
| Terpenes | ||||||
| α-Pinene | 0.27d | 0.81c | 1.81b | 3.03a | 3.11a | 1028 |
| D-Limonene | 1.01d | 1.00d | 1.83c | 2.19b | 3.70a | 1190 |
| Nitrogen-containing derivatives | ||||||
| Methyl-pyrazine | 1.06a | 1.35a | 0.90a | 1.68a | 2.37a | 1286 |
| Pyrazine, 2,3-dimethyl- | 13.81a | 15.70a | 7.16b | 15.75a | 7.27b | 1341 |
| Others | ||||||
| Octane | 0.46c | 0.61b | 0.61b | 0.79a | 0.66ab | 800 |
| Toluene | 0.45b | 0.60a | 0.57a | 0.53a | 0.45b | 1051 |
| 2-pentyl-furan | 2.76d | 3.63c | 3.78c | 5.94b | 6.61a | 1226 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannas, M.; Pulina, S.; Conte, P.; Del Caro, A.; Urgeghe, P.P.; Piga, A.; Fadda, C. Effect of Substitution of Rice Flour with Quinoa Flour on the Chemical-Physical, Nutritional, Volatile and Sensory Parameters of Gluten-Free Ladyfinger Biscuits. Foods 2020, 9, 808. https://doi.org/10.3390/foods9060808
Cannas M, Pulina S, Conte P, Del Caro A, Urgeghe PP, Piga A, Fadda C. Effect of Substitution of Rice Flour with Quinoa Flour on the Chemical-Physical, Nutritional, Volatile and Sensory Parameters of Gluten-Free Ladyfinger Biscuits. Foods. 2020; 9(6):808. https://doi.org/10.3390/foods9060808
Chicago/Turabian StyleCannas, Michela, Simone Pulina, Paola Conte, Alessandra Del Caro, Pietro Paolo Urgeghe, Antonio Piga, and Costantino Fadda. 2020. "Effect of Substitution of Rice Flour with Quinoa Flour on the Chemical-Physical, Nutritional, Volatile and Sensory Parameters of Gluten-Free Ladyfinger Biscuits" Foods 9, no. 6: 808. https://doi.org/10.3390/foods9060808
APA StyleCannas, M., Pulina, S., Conte, P., Del Caro, A., Urgeghe, P. P., Piga, A., & Fadda, C. (2020). Effect of Substitution of Rice Flour with Quinoa Flour on the Chemical-Physical, Nutritional, Volatile and Sensory Parameters of Gluten-Free Ladyfinger Biscuits. Foods, 9(6), 808. https://doi.org/10.3390/foods9060808







