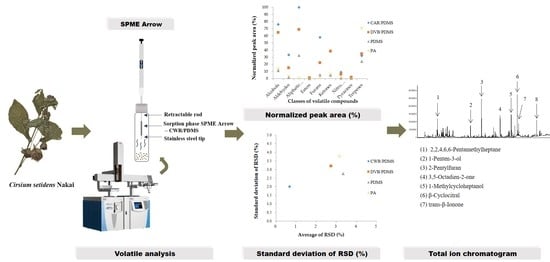
Comparison of Different Types of SPME Arrow Sorbents to Analyze Volatile Compounds in Cirsium setidens Nakai
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
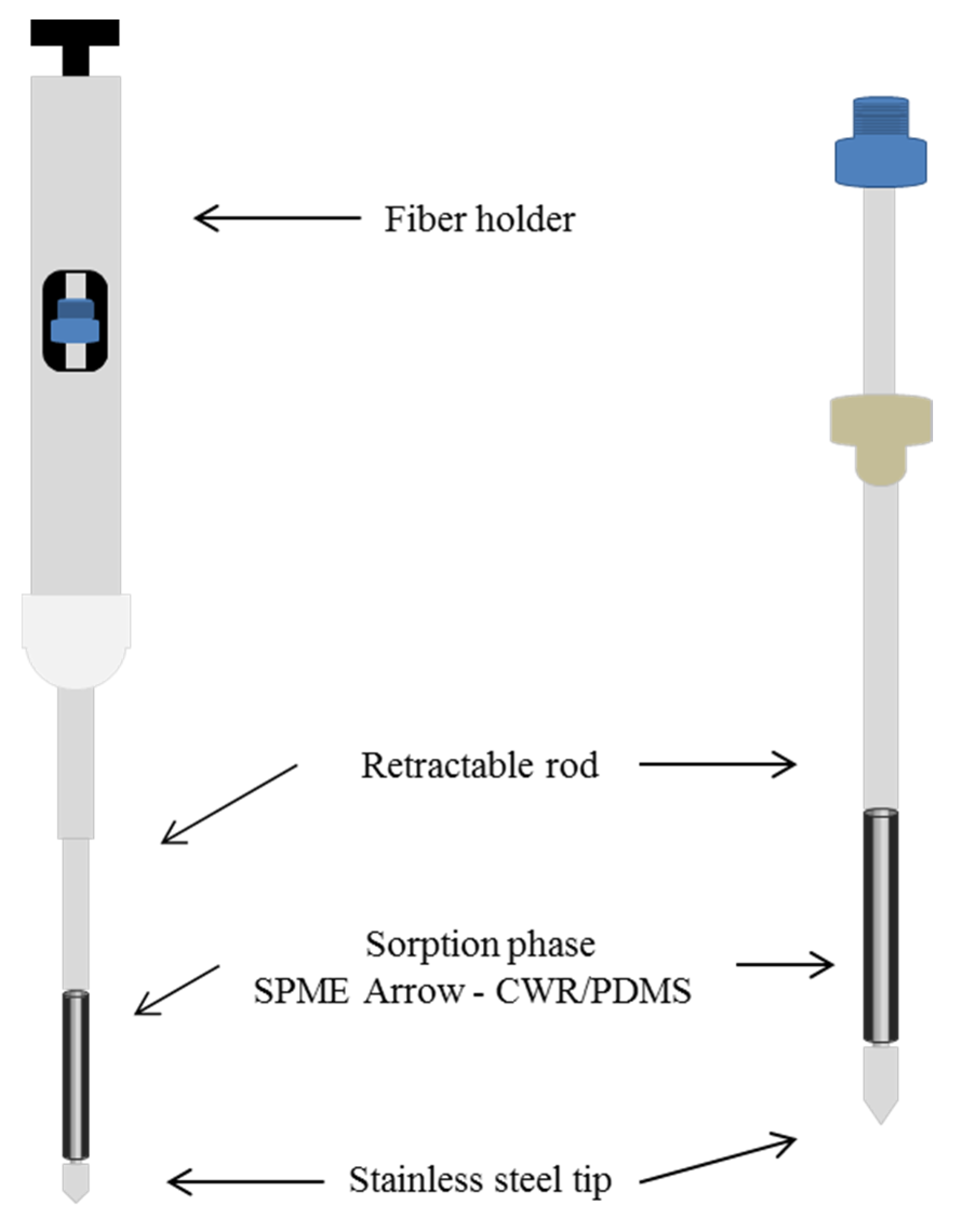
2.2. Headspace Solid Phase Microextraction Arrow Method
2.3. Gas Chromatography-Mass Spectrometry (GCMS) Analysis
2.4. Statistical Analysis
3. Results and Discussion
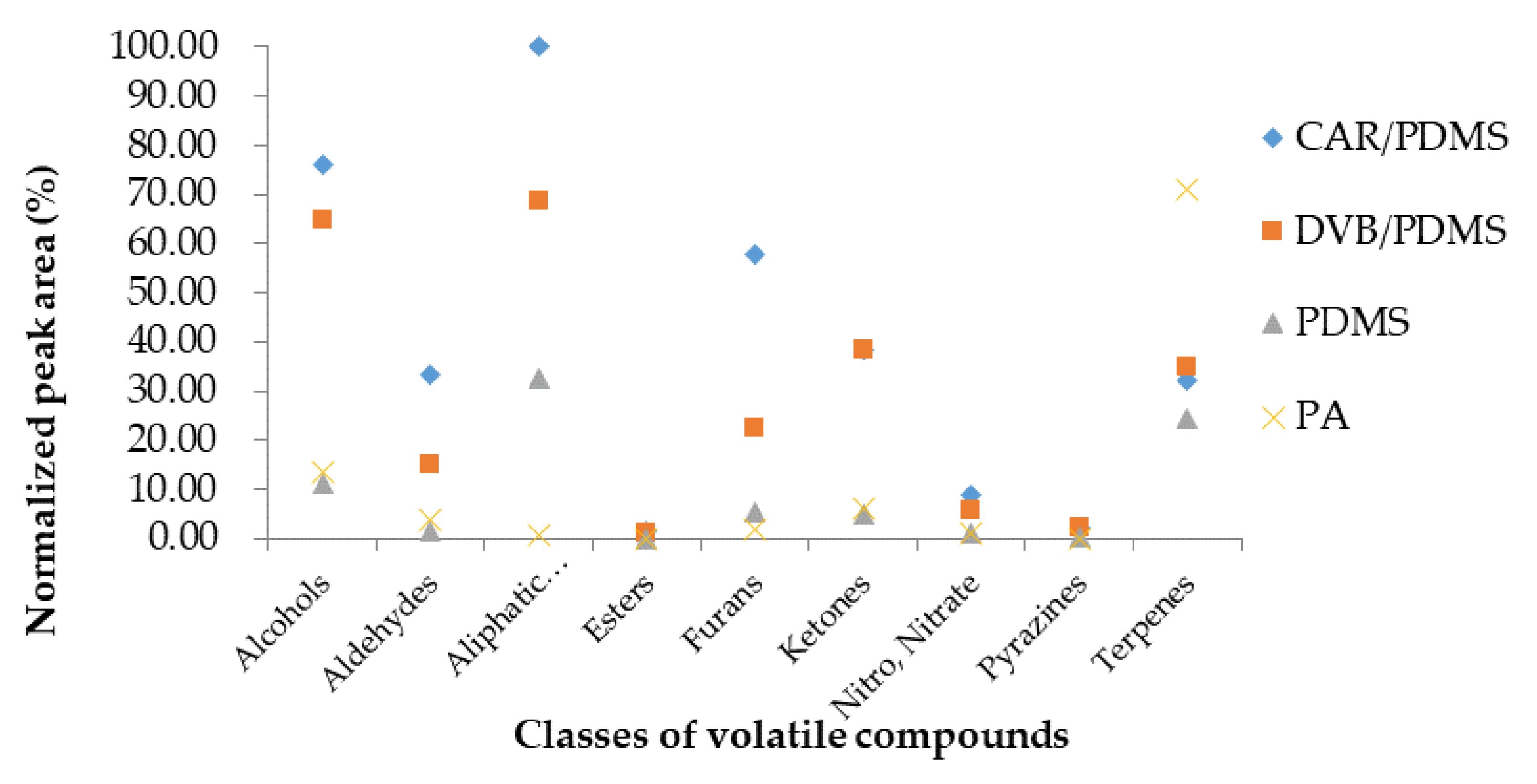
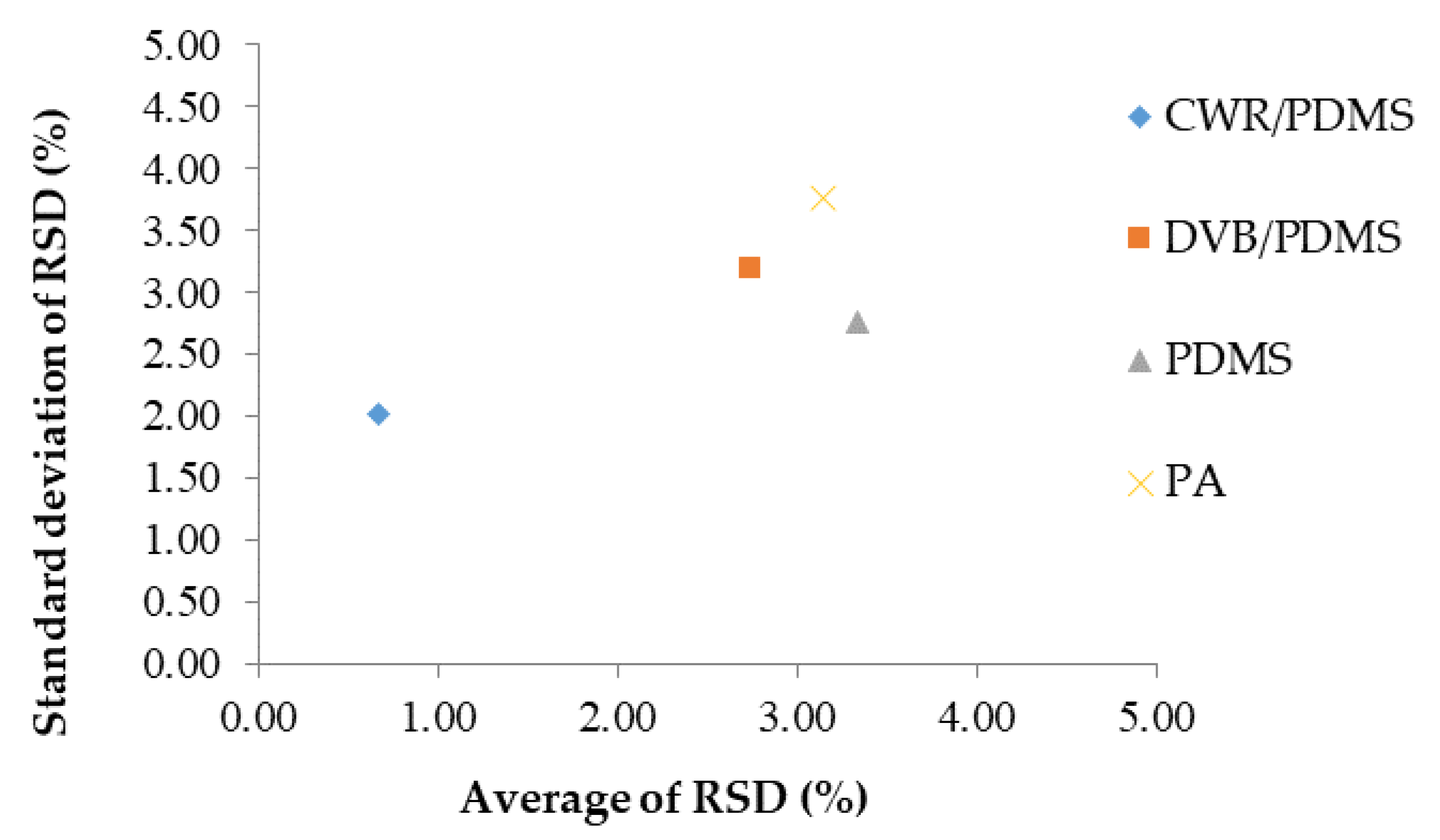
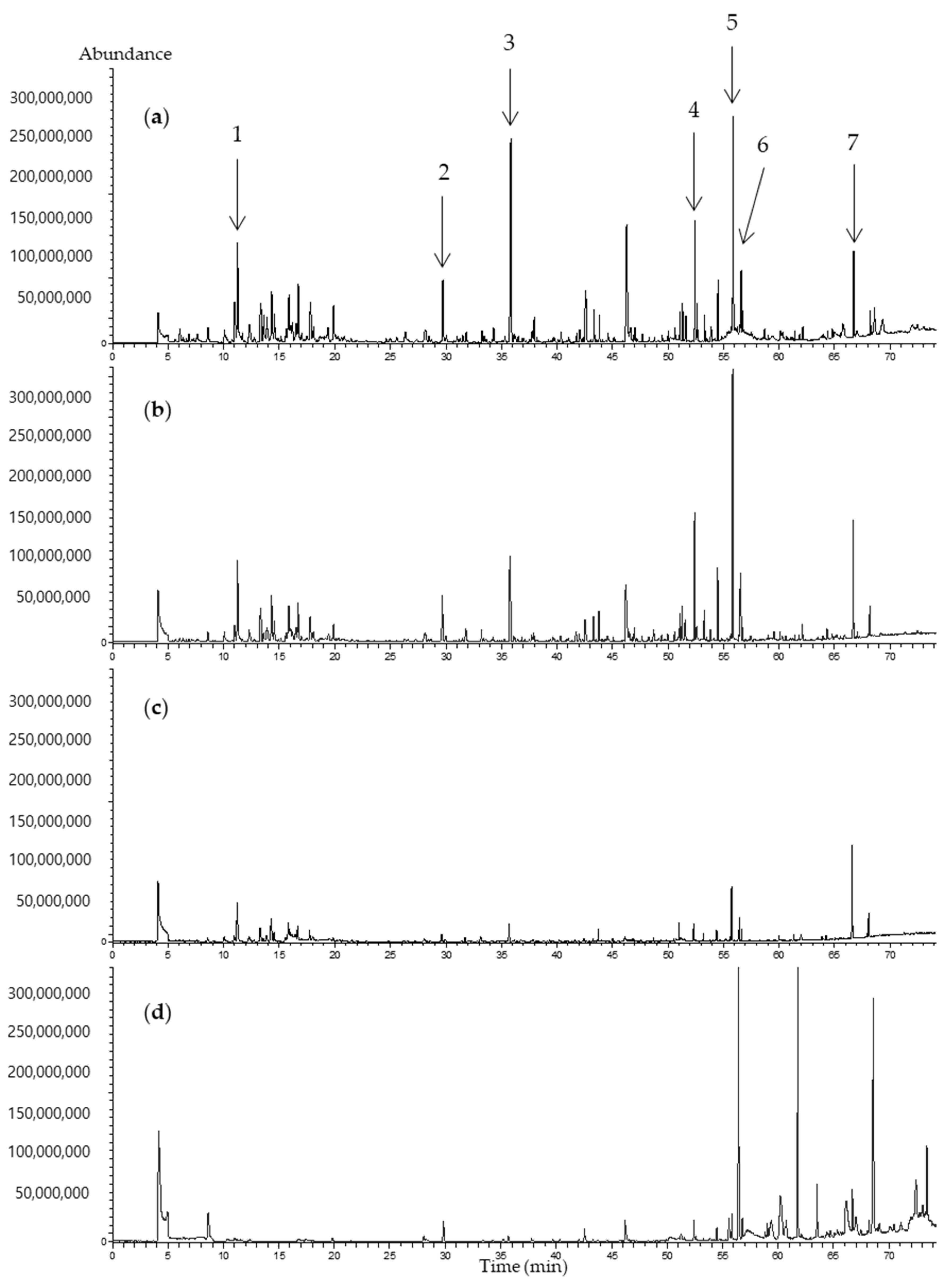
3.1. Selection of SPME Arrow Fibers
3.2. Volatile Compounds of Cirsium setidens Nakai
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, W.B.; Kwon, H.C.; Cho, O.R.; Lee, K.C.; Choi, S.U.; Baek, N.I.; Lee, K.R. Phytochemical constituens ofCirsium setidens Nakai and their cytotoxicity against human cancer cell lines. Arch. Pharm. Res. 2002, 25, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.-H.; Kim, J.H.; Kim, Y.H.; Lee, Y.J.; Lee, J.S.; Jo, J.H.; Kim, B.G.; Lim, J.K.; Lee, B.-Y. Nutritional components and physiological activities of Cirsium setidens Nakai. Korean J. Food Nutr. 2014, 43, 791–798. [Google Scholar] [CrossRef]
- Lee, S.-H.; Jin, Y.-S.; Heo, S.-I.; Shim, T.-H.; Sa, J.-H.; Choi, D.-S.; Wang, M.-H. Composition analysis and antioxidative activity from different organs of Cirsium setidens Nakai. Korean J. Food Sci. Technol. 2006, 38, 571–576. [Google Scholar]
- Kim, E.-M.; Won, S.-I. Functional composition and antioxidative activity from different organs of Native Cirsium and Carduus Genera. Korean J. Food Cook. Sci. 2009, 25, 406–414. [Google Scholar]
- Lee, S.H.; Heo, S.-I.; Li, L.; Lee, M.J.; Wang, M.-H. Antioxidant and hepatoprotective activities of Cirsium setidens NAKAI against CCl 4-induced liver damage. Am. J. Chin. Med. 2008, 36, 107–114. [Google Scholar] [CrossRef]
- Liu, S.; Luo, X.; Li, D.; Zhang, J.; Qiu, D.; Liu, W.; She, L.; Yang, Z. Tumor inhibition and improved immunity in mice treated with flavone from Cirsium japonicum DC. Int. Immunopharmacol. 2006, 6, 1387–1393. [Google Scholar] [CrossRef]
- Martínez-Vázquez, M.; Apan, T.O.R.; Lastra, A.L.; Bye, R. A comparative study of the analgesic and anti-inflammatory activities of pectolinarin isolated from Cirsium subcoriaceum and linarin isolated from Buddleia cordata. Planta Med. 1998, 64, 134–137. [Google Scholar] [CrossRef]
- Yoo, Y.-M.; Nam, J.-H.; Kim, M.-Y.; Choi, J.; Park, H.-J. Pectolinarin and pectolinarigenin of Cirsium setidens prevent the hepatic injury in rats caused by D-galactosamine via an antioxidant mechanism. Biol. Pharm. Bull. 2008, 31, 760–764. [Google Scholar] [CrossRef]
- Jeong, D.M.; Jung, H.A.; Choi, J.S. Comparative antioxidant activity and HPLC profiles of some selected Korean thistles. Arch. Pharmacal Res. 2008, 31, 28. [Google Scholar] [CrossRef]
- Yoo, S.-K.; Bae, Y.-M. Phylogenetic and chemical analyses of Cirsium pendulum and Cirsium setidens inhabiting Korea. J. Life Sci. 2012, 22, 1120–1125. [Google Scholar] [CrossRef]
- Choi, H.-S. Chemical Composition of the Essential Oil from Cirsium setidens, a Korean Medicinal Plant. Anal. Chem. Lett. 2015, 5, 94–102. [Google Scholar] [CrossRef]
- Majcher, M.; Jeleń, H. Analysis, Comparison of suitability of SPME, SAFE and SDE methods for isolation of flavor compounds from extruded potato snacks. J. Food Compos. Anal. 2009, 22, 606–612. [Google Scholar] [CrossRef]
- Sheibani, E.; Duncan, S.E.; Kuhn, D.D.; Dietrich, A.M.; O’Keefe, S.F. SDE and SPME analysis of flavor compounds in Jin Xuan Oolong tea. J. Food Sci. 2016, 81, C348–C358. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.W.; Moon, J.-K.; Shibamoto, T. Analysis and antioxidant activity of extracts from broccoli (Brassica oleracea L.) sprouts. J. Agric. Food Chem. 2015, 63, 1169–1174. [Google Scholar] [CrossRef]
- Richter, T.M.; Eyres, G.T.; Silcock, P.; Bremer, P.J. Comparison of four extraction methods for analysis of volatile hop-derived aroma compounds in beer. J. Sep. Sci. 2017, 40, 4366–4376. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Lee, Y.-Y.; Lee, K.-g.; Jang, H.W. Instrumental volatile flavor analysis of omija (Schisandra chinesis Baillon) using headspace stir-bar sorptive extraction-gas chromatography-mass spectrometry and its relationship to human sensory perceptions. J. Food Res. Int. 2019, 120, 650–655. [Google Scholar] [CrossRef]
- Kim, M.K.; Jang, H.W.; Lee, K.-G. Sensory and instrumental volatile flavor analysis of commercial orange juices prepared by different processing methods. J. Food Sci. 2018, 267, 217–222. [Google Scholar] [CrossRef]
- Perestrelo, R.; Nogueira, J.; Câmara, J. Potentialities of two solventless extraction approaches—Stir bar sorptive extraction and headspace solid-phase microextraction for determination of higher alcohol acetates, isoamyl esters and ethyl esters in wines. Talanta 2009, 80, 622–630. [Google Scholar] [CrossRef]
- Lee, J.; Shibamoto, T.; Ha, J.; Jang, H.W. Identification of volatile markers for the detection of adulterants in red ginseng (Panax ginseng) juice using headspace stir-bar sorptive extraction coupled with gas chromatography and mass spectrometry. J. Sep. Sci. 2018, 41, 2903–2912. [Google Scholar] [CrossRef]
- Miceli, N.; Cavo, E.; Ragusa, S.; Cacciola, F.; Dugo, P.; Mondello, L.; Marino, A.; Cincotta, F.; Condurso, C.; Taviano, M.F. Phytochemical characterization and biological activities of a hydroalcoholic extract obtained from the aerial parts of Matthiola incana (L.) R. BR. subsp. incana (Brassicaceae) growing wild in Sicily (Italy). Chem. Biodivers. 2019, 16, e1800677. [Google Scholar] [CrossRef]
- Condurso, C.; Mazzaglia, A.; Tripodi, G.; Cincotta, F.; Dima, G.; Lanza, C.M.; Verzera, A. Sensory analysis and head-space aroma volatiles for the characterization of capers from different geographic origin. J. Essent. Oil Res. 2016, 28, 185–192. [Google Scholar] [CrossRef]
- Herrington, J.S.; Gómez-Ríos, G.A.; Myers, C.; Stidsen, G.; Bell, D.S. Hunting Molecules in Complex Matrices with SPME Arrows: A Review. Separations 2020, 7, 12. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, W.S.; Lee, Y.Y.; Choi, Y.S.; Choi, H.; Jang, H.W. Solid-phase microextraction Arrow for the volatile organic compounds in soy sauce. J. Sep. Sci. 2019, 42, 2942–2948. [Google Scholar] [CrossRef] [PubMed]
- Song, N.-E.; Lee, J.-Y.; Lee, Y.-Y.; Park, J.-D.; Jang, H.W. Comparison of headspace–SPME and SPME-Arrow–GC–MS methods for the determination of volatile compounds in Korean salt–fermented fish sauce. Appl. Biol. 2019, 62, 16. [Google Scholar] [CrossRef]
- Nam, T.G.; Lee, J.-Y.; Kim, B.-K.; Song, N.-E.; Jang, H.W. Analyzing volatiles in brown rice vinegar by headspace solid-phase microextraction (SPME)–Arrow: Optimizing the extraction conditions and comparisons with conventional SPME. Int. J. Food Prop. 2019, 22, 1195–1204. [Google Scholar] [CrossRef]
- Helin, A.; Rönkkö, T.; Parshintsev, J.; Hartonen, K.; Schilling, B.; Läubli, T.; Riekkola, M.-L. Solid phase microextraction Arrow for the sampling of volatile amines in wastewater and atmosphere. J. Chromatogr. A 2015, 1426, 56–63. [Google Scholar] [CrossRef]
- Kremser, A.; Jochmann, M.A.; Schmidt, T.C. PAL SPME Arrow—Evaluation of a novel solid-phase microextraction device for freely dissolved PAHs in water. Anal. Bioanal. Chem. 2016, 408, 943–952. [Google Scholar] [CrossRef]
- Wang, L.-F.; Lee, J.-Y.; Chung, J.-O.; Baik, J.-H.; So, S.; Park, S.-K. Discrimination of teas with different degrees of fermentation by SPME–GC analysis of the characteristic volatile flavour compounds. J. Food Chem. 2008, 109, 196–206. [Google Scholar] [CrossRef]
- Condurso, C.; Cincotta, F.; Tripodi, G.; Verzera, A. Bioactive volatiles in Sicilian (South Italy) saffron: Safranal and its related compounds. J. Essent. Oil Res. 2017, 29, 221–227. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, H.-P.; Shao, C.-Y.; Kang, S.; Zhang, Y.; Guo, L.; Dai, W.-D.; Tan, J.-F.; Peng, Q.-H.; Lin, Z. Identification of key odorants responsible for chestnut-like aroma quality of green teas. Food Res. Int. 2018, 108, 74–82. [Google Scholar] [CrossRef]
- Champagne, E.T. Rice aroma and flavor: A literature review. Cereal Chem. 2008, 85, 445–454. [Google Scholar] [CrossRef]
- Leffingwell, J.C.; Alford, E. Volatile constituents of perique tobacco. Electron. J. Environ. Agric. Food Chem. 2005, 4, 899–915. [Google Scholar]
- Fanaro, G.B.; Duarte, R.; Araújo, M.; Purgatto, E.; Villavicencio, A. Chemistry, Evaluation of γ-radiation on green tea odor volatiles. Radiat. Phys. Chem. 2011, 80, 85–88. [Google Scholar] [CrossRef]
| Compounds | ID a | KI b | KI c | Peak Area (× 106, mean ± SD) d | |||
|---|---|---|---|---|---|---|---|
| CWR/PDMS | DVB/PDMS | PDMS | PA | ||||
| Alcohols | |||||||
| 1-Penten-3-ol | MS, KI | 1172 | 1165 | 479.54 ± 1.84 a | 333.05 ± 2.49 b | 53.74 ± 0.10 d | 153.77 ± 3.08 c |
| 1-Pentanol | MS, KI | 1260 | 1261 | 83.73 ± 2.17 a | 52.70 ± 0.09 b | ND | 16.84 ± 0.06 c |
| (Z)-2-Penten-1-ol | MS, KI | 1327 | 1325 | 369.49 ± 6.31 a | 154.93 ± 1.34 b | 15.60 ± 1.06 d | 82.52 ± 1.92 c |
| 1-Hexanol | MS, KI | 1361 | 1359 | 76.50 ± 2.12 a | 35.61 ± 1.11 b | ND | 8.60 ± 0.11 c |
| 1-Octen-3-ol | MS, KI | 1456 | 1456 | 30.88 ± 1.08 a | 26.91 ± 0.00 b | 4.66 ± 0.42 c | ND |
| 1-Methylcycloheptanol | MS | 1598 | 1160.26 ± 75.40 b | 1270.00 ± 31.02 a | 253.16 ± 4.04 c | 126.27 ± 7.96 d | |
| Aldehydes | |||||||
| 2-Methylbutanal | MS, KI | 912 | 896 | ND | 7.18 ± 0.35 | ND | ND |
| 3-Methylbutanal | MS, KI | 916 | 900 | ND | 5.96 ± 0.03 | ND | ND |
| Hexanal | MS, KI | 1078 | 1081 | 273.84 ± 6.57 a | 109.82 ± 1.32 b | ND | 23.44 ± 1.21 c |
| (E)-2-Pentenal | MS, KI | 1130 | 1130 | 33.92 ± 0.10 a | ND | ND | 5.92 ± 0.27 b |
| 2-Methyl-2-pentenal | MS, KI | 1162 | 1149 | 55.26 ± 0.85 a | 17.29 ± 0.96 b | ND | ND |
| Heptanal | MS, KI | 1186 | 1186 | 51.11 ± 0.18 a | 20.55 ± 0.65 b | ND | ND |
| 2-Hexenal | MS, KI | 1214 | 1207 | 101.18 ± 0.26 a | 32.46 ± 0.12 b | ND | 7.28 ± 0.37 c |
| (Z)-4-Heptenal | MS, KI | 1244 | 1247 | 29.54 ± 0.70 | ND | ND | ND |
| (E)-2-Heptenal | MS, KI | 1324 | 1318 | 24.33 ± 0.31 a | ND | ND | ND |
| (E)-2-Octenal | MS, KI | 1432 | 1434 | 22.83 ± 0.49 a | ND | 6.80 ± 0.04 b | ND |
| (E,E)-2,4-Heptadienal | MS, KI | 1495 | 1497 | 174.35 ± 6.61 a | 158.03 ± 3.00 b | 28.78 ± 0.55 d | 48.63 ± 1.52 c |
| Benzaldehyde | MS, KI | 1528 | 1528 | 194.50 ± 10.69 a | 81.81 ± 0.24 b | 11.01 ± 0.31 c | 18.96 ± 1.69 c |
| Aliphatic hydrocarbons | |||||||
| Octane | MS, KI | 800 | 800 | 68.36 ± 3.37 a | 12.67 ± 0.50 b | 4.80 ± 0.11 c | ND |
| 2-Ethyl-1-hexene | MS, KI | 837 | 847 | 60.74 ± 3.41 a | 12.22 ± 0.32 b | 5.45 ± 0.38 c | ND |
| 2,2,6-Trimethyloctane | MS | 935 | 154.78 ± 4.50 a | 80.45 ± 0.17 b | 37.38 ± 0.92 c | ND | |
| 2,2,4,6,6-Pentamethylheptane | MS, KI | 959 | 957 | 857.19 ± 15.49 a | 647.06 ± 5.08 b | 321.92 ± 5.27 c | 8.07 ± 0.06 d |
| 3-Methylnonane | MS, KI | 966 | 965 | 58.53 ± 2.18 a | 16.88 ± 0.04 b | 5.46 ± 0.10 c | ND |
| Decane | MS, KI | 999 | 1000 | 200.37 ± 1.59 a | 60.33 ± 0.43 b | 21.50 ± 0.07 c | ND |
| 2,3,6,7-Tetramethyloctane | MS | 1009 | 425.57 ± 6.09 a | 388.08 ± 0.87 b | 204.57 ± 0.84 c | ND | |
| 5-Ethyl-2,2,3-trimethylheptane | MS | 1031 | 387.76 ± 7.27 a | 289.75 ± 0.99 b | 158.50 ± 4.31 c | ND | |
| 2,2,7,7-Tetramethyloctane | MS | 1042 | 414.62 ± 5.55 a | 274.51 ± 5.57 b | 117.66 ± 0.12 c | ND | |
| 3-Methylundecane | MS | 1175 | 62.80 ± 3.71 a | 45.28 ± 1.63 b | 16.16 ± 0.46 c | ND | |
| Dodecane | MS | 1199 | 75.18 ± 1.07 a | 65.48 ± 1.76 b | 27.26 ± 0.08 c | ND | |
| 3-Methylene-undecane | MS | 1240 | 40.55 ± 0.15 a | 31.45 ± 0.29 b | ND | ND | |
| 3-Methyltridecane | MS | 1370 | 24.35 ± 0.25 a | 20.48 ± 0.87 b | 11.94 ± 0.52 c | ND | |
| (Z)-1,4-Hexadiene | MS | 1560 | 59.38 ± 0.89 a | 45.44 ± 0.21 b | 3.99 ± 0.22 d | 11.82 ± 0.15 c | |
| Esters | |||||||
| Ethyl hexanoate | MS, KI | 1239 | 1241 | 44.46 ± 1.73 a | 31.23 ± 0.12 b | ND | 1.96 ± 0.09 c |
| Furans | |||||||
| 2-Methylfuran | MS, KI | 868 | 876 | 51.30 ± 0.26 a | 13.97 ± 0.11 b | 5.04 ± 0.51 c | ND |
| 2-Ethylfuran | MS, KI | 954 | 960 | 316.11 ± 3.12 a | 124.42 ± 0.69 b | 48.70 ± 0.32 c | 23.52 ± 2.05 d |
| 2-Pentylfuran | MS, KI | 1234 | 1235 | 1266.54 ± 32.31 a | 487.03 ± 0.65 b | 98.17 ± 0.44 c | 29.81 ± 1.25 d |
| trans-2-(2-Pentenyl)furan | MS | 1300 | 33.72 ± 1.16 a | 20.27 ± 0.03 b | ND | ND | |
| Ketones | |||||||
| 2-Heptanone | MS, KI | 1184 | 1185 | 25.74 ± 0.13 a | 10.68 ± 0.10 b | ND | ND |
| Acetoin | MS, KI | 1283 | 1287 | 22.56 ± 0.80 a | 22.71 ± 2.19 a | ND | 12.63 ± 0.49 b |
| 2,2,6-Trimethylcyclohexanone | MS, KI | 1312 | 1307 | 50.79 ± 0.53 b | 53.09 ± 0.31 a | 11.66 ± 0.17 c | 3.39 ± 0.09 d |
| 6-Methyl-6-hepten-2-one | MS, KI | 1318 | 1320 | 72.19 ± 1.10 a | 37.42 ± 1.18 b | ND | 8.00 ± 0.15 c |
| 6-Methyl-5-heptene-2-one | MS, KI | 1340 | 1341 | 161.23 ± 1.83 a | 118.61 ± 0.30 b | 14.05 ± 0.41 c | 8.83 ± 0.10 d |
| 3,5-Octadien-2-one | MS, KI | 1524 | 1516 | 534.74 ± 0.51 a | 534.56 ± 15.23 a | 76.33 ± 0.10 c | 87.85 ± 3.52 b |
| Ketoisophorone | MS, KI | 1704 | 1708 | ND | 30.05 ± 1.63 | ND | ND |
| Nitro, Nitrate | |||||||
| 2-Nitropropane | MS | 1202 | 49.58 ± 1.11 a | 25.75 ± 0.07 b | ND | 11.16 ± 0.18 c | |
| 3-Methyl-1-butanol nitrate | MS | 1228 | 41.11 ± 0.78 a | 19.96 ± 0.12 b | ND | ND | |
| 1-Nitrobutane | MS, KI | 1291 | 1310 | 58.69 ± 0.02 a | 32.46 ± 1.16 b | 9.84 ± 1.11 c | 8.71 ± 0.30 d |
| 1-Nitrohexane | MS, KI | 1501 | 1502 | 108.95 ± 1.00 a | 90.19 ± 1.70 b | 15.65 ± 0.31 c | 15.74 ± 0.04 c |
| Pyrazines | |||||||
| Trimethylpyrazine | MS, KI | 1406 | 1413 | 24.13 ± 2.14 a | 22.86 ± 0.33 b | ND | ND |
| Tetramethylpyrazine | MS, KI | 1481 | 1467 | 39.26 ± 2.87 a | 35.71 ± 2.23 b | 10.16 ± 0.17 c | ND |
| Terpenes | |||||||
| Caryophyllene | MS, KI | 1608 | 1598 | 81.57 ± 4.56 a | 30.15 ± 2.80 b | 17.69 ± 1.67 c | ND |
| β-Cyclocitral | MS, KI | 1622 | 1600 | 304.70 ± 1.82 c | 311.87 ± 6.94 b | 106.14 ± 1.46 c | 1846.12 ± 56.83 a |
| β-Selinene | MS, KI | 1738 | 1725 | ND | ND | 23.12 ± 0.45 | ND |
| Geranylacetone | MS, KI | 1864 | 1865 | ND | 15.75 ± 0.55 a | 13.86 ± 0.62 b | ND |
| α-Ionone | MS, KI | 1868 | 1857 | ND | ND | 0.05 ± 0.00 | ND |
| trans-β-Ionone | MS, KI | 1961 | 1954 | 389.65 ± 1.50 c | 506.51 ± 24.73 a | 428.17 ± 24.52 b | 204.47 ± 24.73 d |
| β-Ionone epoxide | MS, KI | 2015 | 2002 | 150.59 ± 7.95 a | 144.34 ± 6.67 b | 108.47 ± 2.14 c | ND |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-J.; Lee, J.-Y.; Choi, Y.-S.; Sung, J.-M.; Jang, H.W. Comparison of Different Types of SPME Arrow Sorbents to Analyze Volatile Compounds in Cirsium setidens Nakai. Foods 2020, 9, 785. https://doi.org/10.3390/foods9060785
Kim S-J, Lee J-Y, Choi Y-S, Sung J-M, Jang HW. Comparison of Different Types of SPME Arrow Sorbents to Analyze Volatile Compounds in Cirsium setidens Nakai. Foods. 2020; 9(6):785. https://doi.org/10.3390/foods9060785
Chicago/Turabian StyleKim, Su-Jeong, Jun-Young Lee, Yun-Sang Choi, Jung-Min Sung, and Hae Won Jang. 2020. "Comparison of Different Types of SPME Arrow Sorbents to Analyze Volatile Compounds in Cirsium setidens Nakai" Foods 9, no. 6: 785. https://doi.org/10.3390/foods9060785
APA StyleKim, S.-J., Lee, J.-Y., Choi, Y.-S., Sung, J.-M., & Jang, H. W. (2020). Comparison of Different Types of SPME Arrow Sorbents to Analyze Volatile Compounds in Cirsium setidens Nakai. Foods, 9(6), 785. https://doi.org/10.3390/foods9060785