Volatile Profiling of Strawberry Fruits Cultivated in a Soilless System to Investigate Cultivar-Dependent Chemical Descriptors
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Reagents and Chemicals
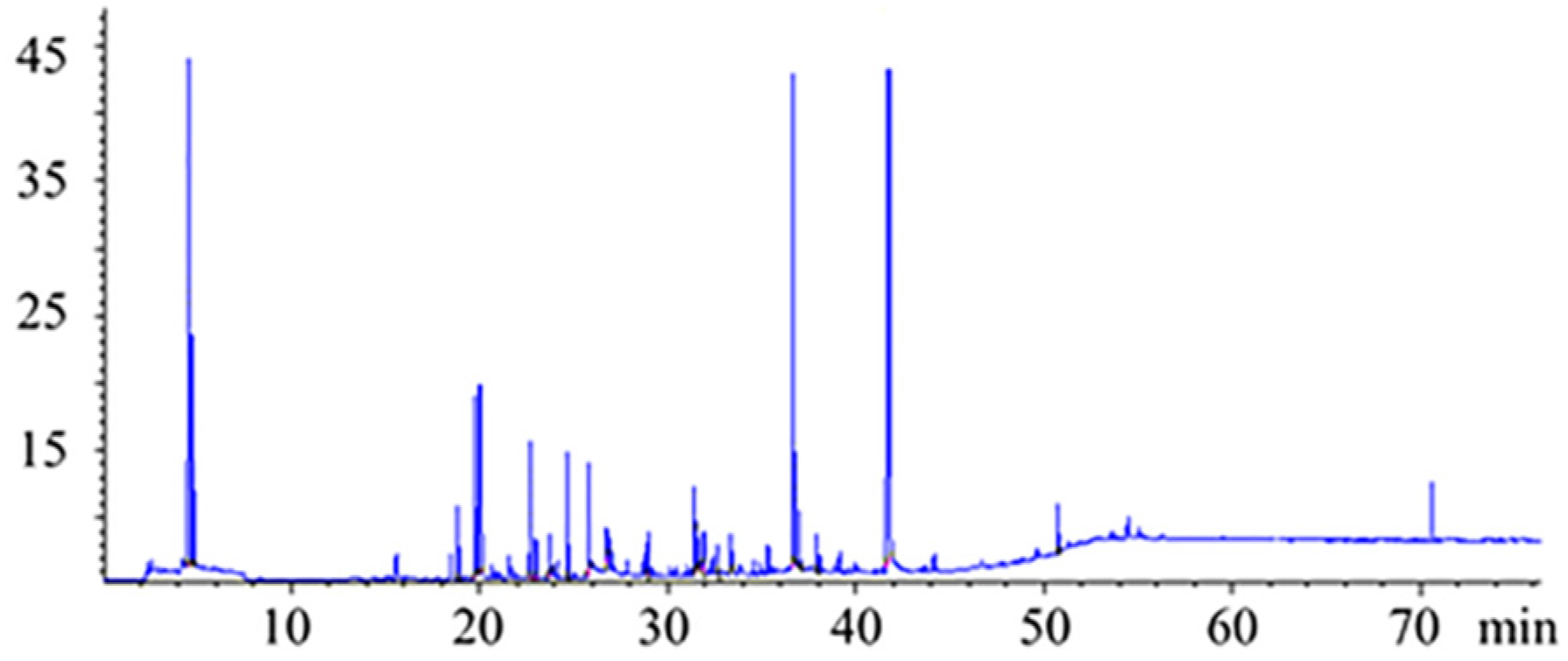
2.3. Headspace Solid-Phase Micro-Extraction Procedure
2.4. Chromatographic Analysis
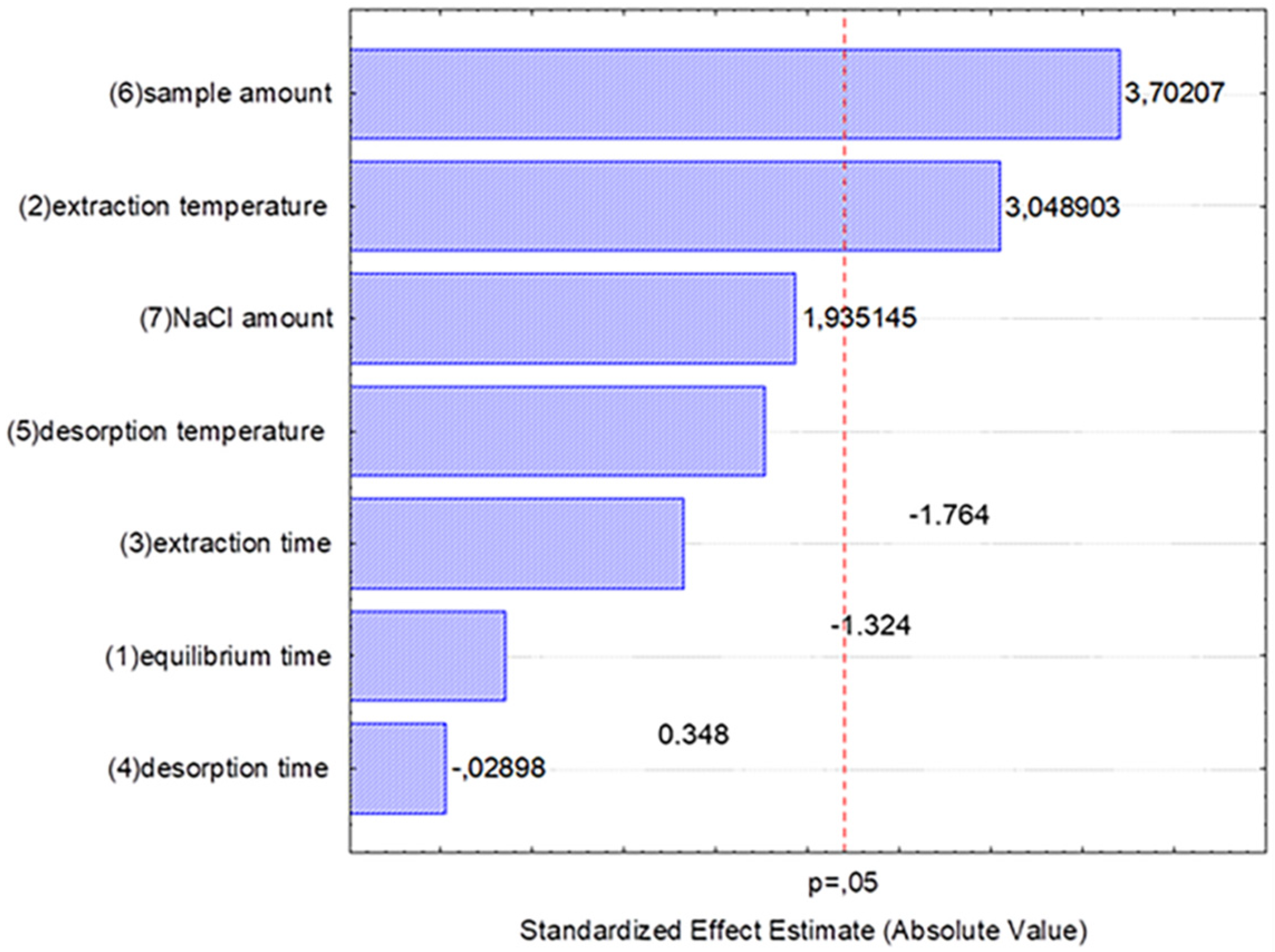
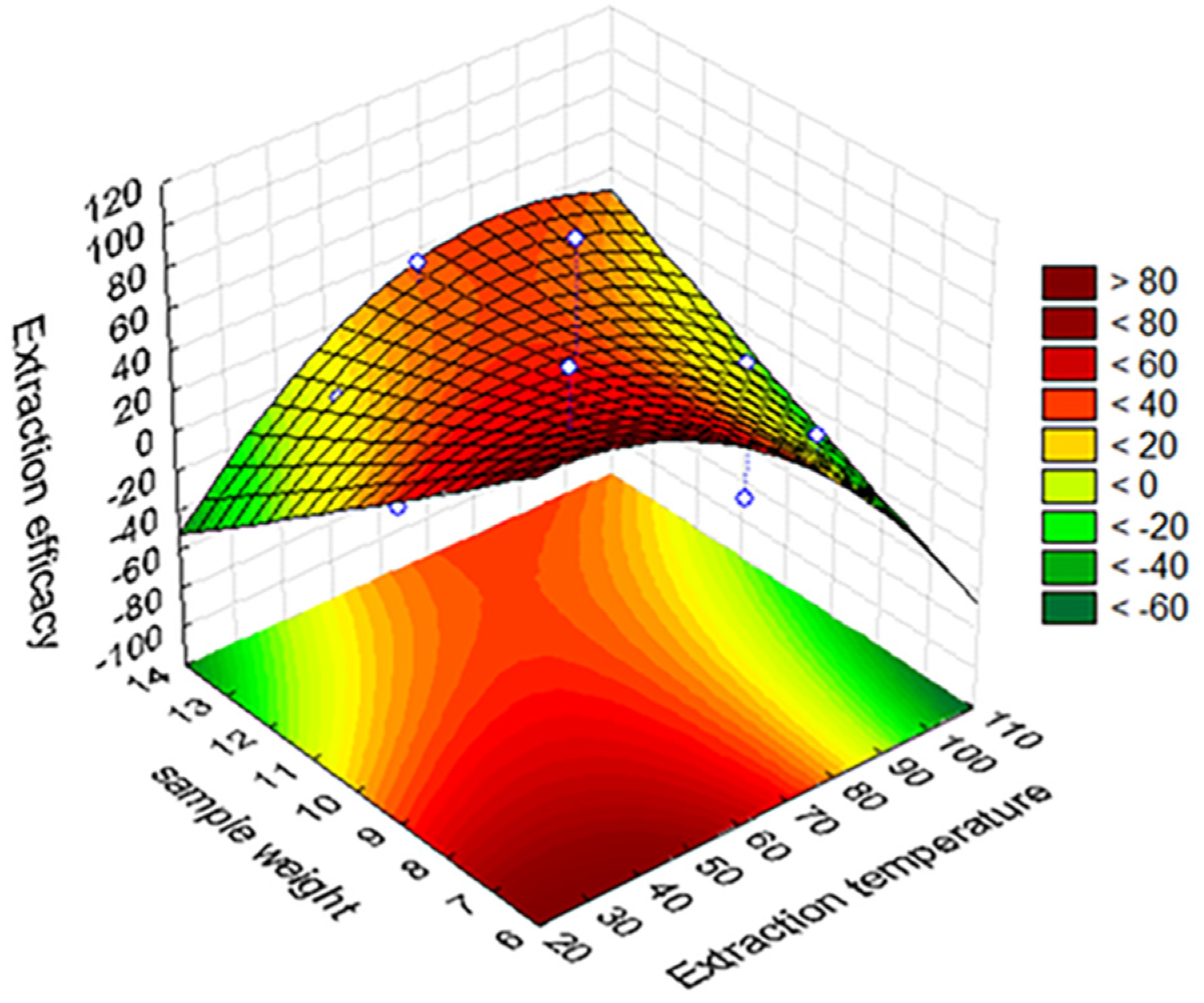
2.5. Experimental Design
2.6. Statistical Analysis
3. Results and Discussion
3.1. HS-SPME Optimization and Validation
3.2. Characterization of the Strawberry Volatile Profile
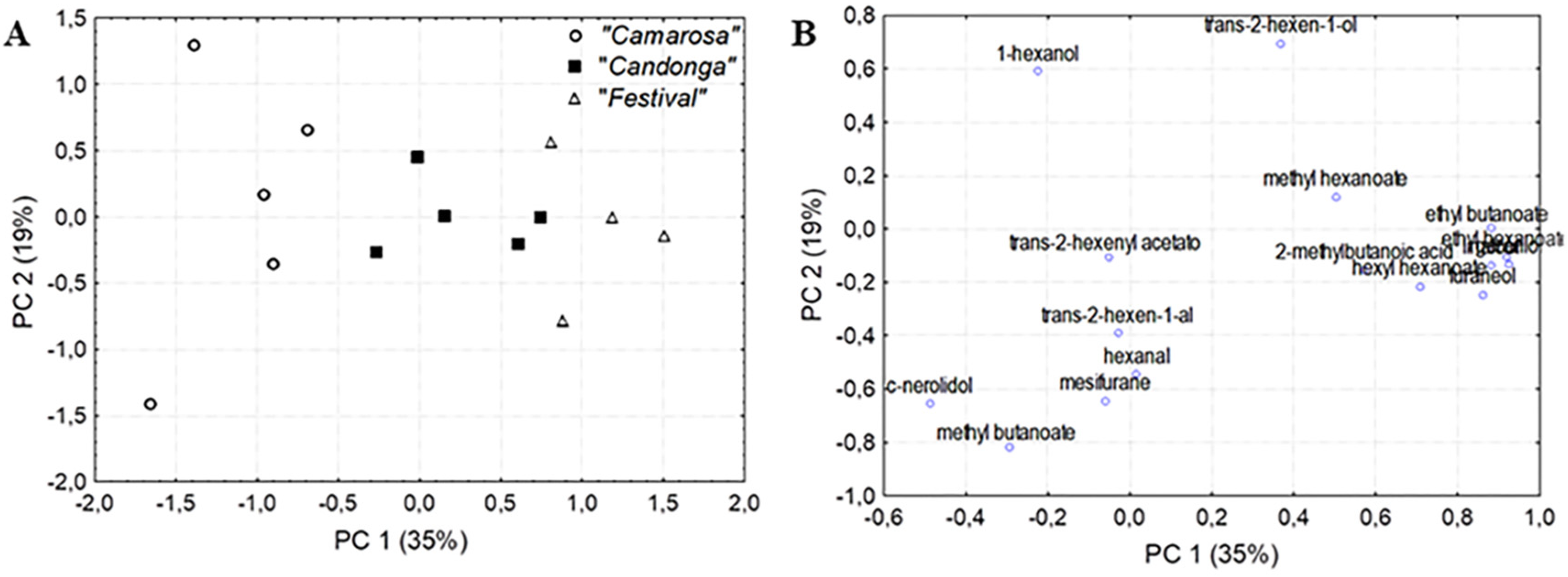
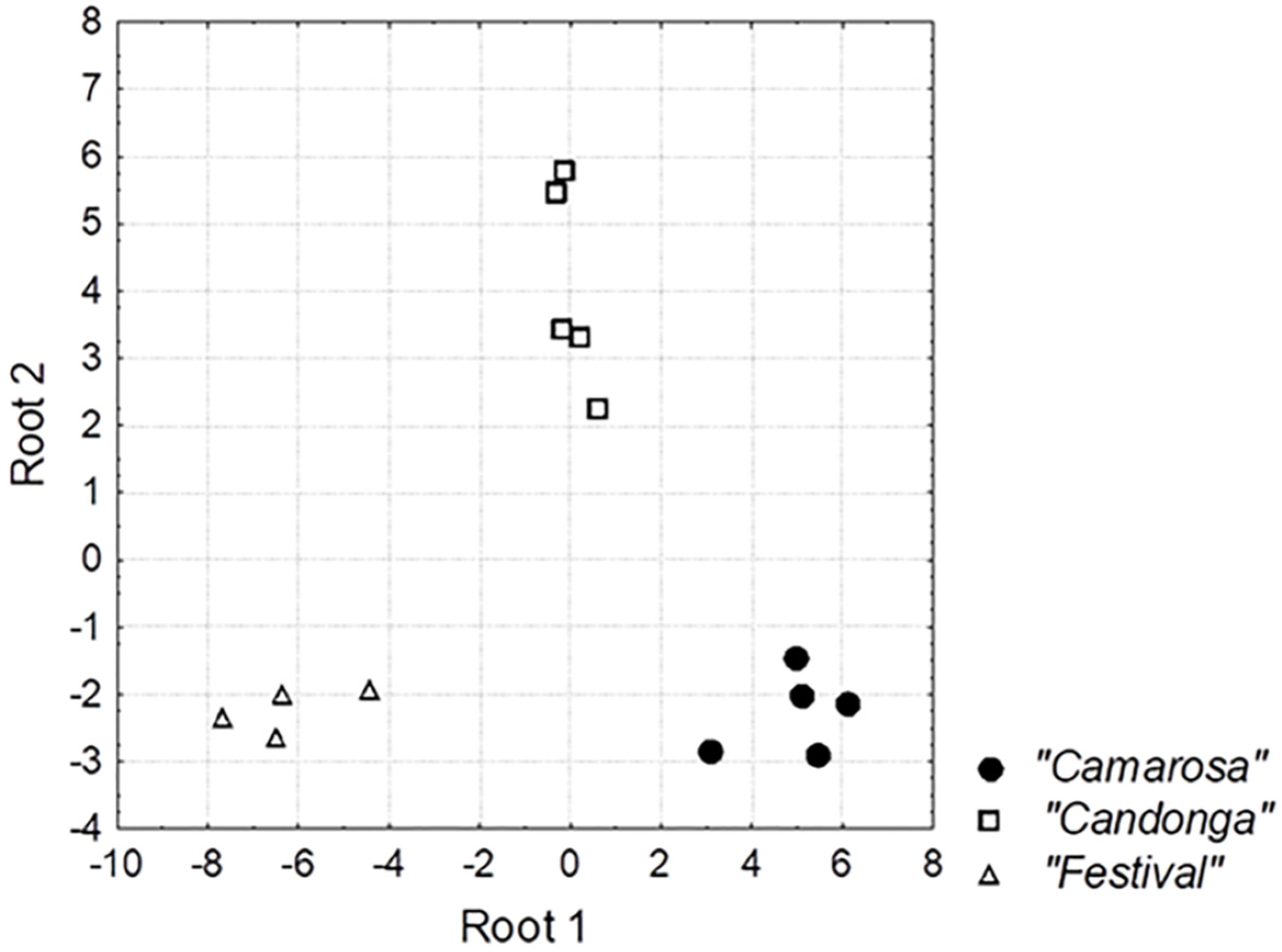
3.3. Chemometrics Approaches to Identify Chemical Descriptors of Strawberry Cultivar
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwieterman, M.L.; Colquhoun, T.A.; Jaworski, E.A.; Bartoshuk, L.M.; Gilbert, J.L.; Tieman, D.M.; Odabasi, A.Z.; Moskowitz, H.R.; Folta, K.M.; Klee, H.J.; et al. Strawberry flavor: Diverse chemical compositions, a seasonal influence, and effects on sensory perception. PLoS ONE 2014, 9, e88446. [Google Scholar] [CrossRef]
- Lu, H.; Ban, Z.; Wang, K.; Li, D.; Li, D.; Poverenov, E.; Luo, Z. Aroma volatiles, sensory and chemical attributes of strawberry (Fragaria × ananassa Duch.) achenes and receptacle. Int. J. Food Sci. Technol. 2017, 52, 2614–2622. [Google Scholar] [CrossRef]
- Schieberle, P.; Hofmann, T. Evaluation of the character impact odorants in fresh strawberry juice by quantitative measurements and sensory studies on model mixtures. J. Agric. Food Chem. 1997, 45, 227–232. [Google Scholar] [CrossRef]
- Urrutia, M.; Rambla, J.L.; Alexiou, K.G.; Granell, A.; Monfort, A. Genetic analysis of the wild strawberry (Fragaria vesca) volatile composition. Plant Physiol. Biochem. 2017, 121, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Haugeneder, A.; Trinkl, J.; Härtl, K.; Hoffmann, T.; Allwood, J.W.; Schwab, W. Answering biological questions by analysis of the strawberry metabolome. Metabolomics 2018, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.; Sanz, C. Strawberry flavor. In Handbook of Fruit and Vegetable Flavors; Hui, Y.H., Ed.; Wiley: Hoboken, NJ, USA, 2010; pp. 431–449. [Google Scholar]
- Ubeda, C.; San-Juan, F.; Concejero, B.; Callejón, R.M.; Troncoso, A.M.; Morales, M.L.; Ferreira, V.; Hernández-Orte, P. Glycosidically bound aroma compounds and impact odorants of four strawberry varieties. J. Agric. Food Chem. 2012, 60, 6095–6102. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Hong, X.; Zhao, S.; Liu, J.; Schulenburg, K.; Huang, F.C.; Franz-Oberdorf, K.; Schwab, W. Glucosylation of 4-Hydroxy-2,5-Dimethyl-3(2H)-Furanone, the key strawberry flavor compound in strawberry fruit. Plant Physiol. 2016, 171, 139–151. [Google Scholar] [CrossRef]
- Olbricht, K.; Ulrich, D.; Weiss, K.; Grafe, C. Variation in the amounts of selected volatiles in a model population of Fragaria × ananassa Duch. As influenced by harvest year. J. Agric. Food Chem. 2011, 59, 944–952. [Google Scholar] [CrossRef]
- Du, X.; Whitaker, V.; Rouseff, R. Changes in strawberry volatile sulfur compounds due to genotype, fruit maturity and sample preparation. Flavour Fragr. J. 2012, 27, 398–404. [Google Scholar] [CrossRef]
- Vandendriessche, T.; Vermeir, S.; Mayayo Martinez, C.; Hendrickx, Y.; Lammertyn, J.; Nicolaï, B.M.; Hertog, M.L.A.T.M. Effect of ripening and inter-cultivar differences on strawberry quality. LWT 2013, 52, 62–70. [Google Scholar] [CrossRef]
- Perdones, A.; Escriche, I.; Chiralt, A.; Vargas, M. Effect of chitosan-lemon essential oil coatings on volatile profile of strawberries during storage. Food Chem. 2016, 197, 979–986. [Google Scholar] [CrossRef]
- Dong, J.; Zhong, C.F.; Wang, G.; Chang, L.; Sun, J.; Sun, R.; Zhang, H.L.; Li, R.; Wei, Y.Q.; Zheng, S.Q.; et al. Comparative study on fruit volatiles of different day-neutral strawberry cultivars in autumn and winter. Sci. Agric. Sin. 2019, 52, 2309–2327. [Google Scholar]
- Ulrich, D.; Kecke, S.; Olbricht, K. What do we know about the chemistry of strawberry aroma? J. Agric. Food Chem. 2018, 66, 3291–3301. [Google Scholar] [CrossRef] [PubMed]
- Ajwa, H.A.; Klose, S.; Nelson, S.D.; Minuto, A.; Gullino, M.L.; Lamberti, F.; Lopez-Aranda, J.M. Alternatives to methyl bromide in strawberry production in the United States of America and the Mediterranean region. Phytopathol. Mediterr. 2003, 42, 220–244. [Google Scholar]
- Recamales, A.F.; López Medina, J.; Hernanz, D. Physicochemical characteristics and mineral content of strawberries grown in soil and soilless system. J. Food Qual. 2007, 30, 837–853. [Google Scholar] [CrossRef]
- Hernanz, D.; Recamales, A.F.; Meléndez-Martínez, A.J.; González-Miret, M.L.; Heredia, F.J. Assessment of the differences in the phenolic composition of five strawberry cultivars (Fragaria ananassa Duch.) grown in two different soilless system. J. Agric. Food Chem. 2007, 55, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Hernanz, D.; Recamales, A.F.; Meléndez-Martínez, A.J.; González-Miret, M.L.; Heredia, F.J. Multivariate statistical analysis of color-anthocyanin relationships in different soilless-grown strawberry genotypes. J. Agric. Food Chem. 2008, 56, 2735–2741. [Google Scholar] [CrossRef]
- Akhatou, I.; González-Domínguez, R.; Fernández-Recamales, Á. Investigation of the effect of genotype and agronomic conditions on metabolomic profiles of selected strawberry cultivars with different sensitivity to environmental stress. Plant Physiol. Biochem. 2016, 101, 14–22. [Google Scholar] [CrossRef]
- Akhatou, I.; Sayago, A.; González-Domínguez, R.; Fernández-Recamales, Á. Application of targeted metabolomics to investigate optimum growing conditions to enhance bioactive content of strawberry. J. Agric. Food Chem. 2017, 65, 9559–9567. [Google Scholar] [CrossRef]
- Akhatou, I.; Fernández-Recamales, A. Nutritional and nutraceutical quality of strawberries in relation to harvest time and crop conditions. J. Agric. Food Chem. 2014, 62, 5749–5760. [Google Scholar] [CrossRef]
- Akhatou, I.; Fernández-Recamales, Á. Influence of cultivar and culture system on nutritional and organoleptic quality of strawberry. J. Sci. Food Agric. 2014, 94, 866–875. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, R.; Sayago, A.; Akhatou, I.; Fernández-Recamales, Á. Multi-chemical profiling of strawberry as a traceability tool to investigate the effect of cultivar and cultivation conditions. Foods 2020, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Dasenaki, M.E.; Thomaidi, N.S. Quality and authenticity control of fruit juices—A review. Molecules 2019, 24, 1014. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.L.; Callejón, R.M.; Ordóñez, J.L.; Troncoso, A.M.; García-Parrilla, M.C. Comparative assessment of software for non-targeted data analysis in the study of volatile fingerprint changes during storage of a strawberry beverage. J. Chromatogr. A 2017, 1522, 70–77. [Google Scholar] [CrossRef]
- Yang, X.; Peppard, T. Solid-phase microextraction for flavor analysis. J. Agric. Food Chem. 1994, 42, 1925–1930. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Silva Junior, M.M.; Felix, C.S.A.; da Silva, D.L.F.; Santos, A.S.; Santos Neto, J.H.; de Souza, C.T.; Cruz Junior, R.A.; Souza, A.S. Multivariate optimization techniques in foods analysis. A review. Food Chem. 2019, 273, 3–8. [Google Scholar] [CrossRef]
- Pawliszyn, J. Solid Phase Microextraction: Theory and Practice; Wiley–VCH: New York, NY, USA, 1997. [Google Scholar]
- Almenar, E.; Hernández-Muñoz, P.; Gavara, R. Evolution of selected volatiles in chitosan-coated strawberries (Fragaria × ananassa) during refrigerated storage. J. Agric. Food Chem. 2009, 57, 974–980. [Google Scholar] [CrossRef]
- Vandendriessche, T.; Nicolai, B.M.; Hertog, M.L.A.T.M. Optimization of HS SPME Fast GC-MS for high-throughput analysis of strawberry aroma. Food Anal. Methods 2013, 6, 512–520. [Google Scholar] [CrossRef]
- Kafkas, E.; Kafkas, S. Identification of strawberry (Fragaria × ananassa ‘Rubygem’) volatiles using various SPME fibres by GC/MS. Acta Hortic. 2017, 1156, 689–694. [Google Scholar] [CrossRef]
- González, A.G.; Herrador, M.A. Accuracy profiles from uncertainty measurements. Talanta 2006, 70, 896–901. [Google Scholar] [CrossRef]
- Ulrich, D.; Olbricht, K. Diversity of volatile patterns in sixteen Fragaria vesca L. accessions in comparison to cultivars of Fragaria × ananassa. J. Appl. Bot. Food Qual. 2013, 86, 37–46. [Google Scholar]
- Prat, L.; Espinoza, M.I.; Agosin, E.; Silva, H. Identification of volatile compounds associated with the aroma of white strawberries (Fragaria chiloensis). J. Sci. Food Agric. 2014, 94, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Jetti, R.R.; Yang, E.; Kurnianta, A.; Finn, C.; Qian, M.C. Quantification of selected aroma-active compounds in strawberries by headspace solid-phase microextraction gas chromatography and correlation with sensory descriptive analysis. J. Food Sci. 2007, 72, 487–496. [Google Scholar] [CrossRef] [PubMed]
| Camarosa | Candonga | Festival | p-Value | |
|---|---|---|---|---|
| Esters | ||||
| methyl butanoate | 142.21 ± 14.65 a | 112.72 ± 10.59 b | 99.63 ± 7.92 b | 0.0002 |
| ethyl butanoate | 14.37 ± 1.62 a | 28.01 ± 2.31 a | 48.06 ± 3.14 b | 0.01 |
| methyl hexanoate | 24.51 ± 2.75 a | 62.31 ± 3.20 b | 57.64 ± 4.04 b | 0.0001 |
| ethyl hexanoate | ND | 19.26 ± 3.31 a | 28.48 ± 4.31 b | 0.0006 |
| hexyl hexanoate | 95.45 ± 16.59 a | 150.47 ± 24.00 a | 303.79 ± 94.39 b | 0.0063 |
| cis-3-hexenyl acetate | 40.71 ± 9.10 | 42.52 ± 8.37 | ND | NS |
| trans-2-hexenyl acetate | 24.77 ± 1.90 | 33.66 ± 3.39 | 26.44 ± 2.33 | NS |
| Aldehydes | ||||
| hexanal | 26.41 ± 9.51 | 18.24 ± 4.45 | 26.43 ± 6.54 | NS |
| trans-2-hexen-1-al | 19.75 ± 2.24 ab | 6.78 ± 0.92 a | 21.96 ± 4.44 b | 0.0055 |
| benzaldehyde | 29.21 ± 6.53 | 21.68 ± 4.85 | ND | NS |
| Alcohols | ||||
| 1-hexanol | 38.25 ± 2.10 | 21.83 ± 4.07 | 34.77 ± 2.19 | NS |
| trans-2-hexen-1-ol | 30.94 ± 12.24 | 31.96 ± 15.13 | 50.76 ± 18.35 | NS |
| benzyl alcohol | 12.68 ± 2.09 | 6.60 ± 1.47 | ND | NS |
| Terpenes | ||||
| linalool | 75.08 ± 14.63 a | 135.00 ± 21.74 b | 188.74 ± 32.02 b | 0.0053 |
| geraniol | 151.63 ± 29.96 a | 426.43 ± 35.72 b | 537.73 ± 122.01 b | 0.0001 |
| cis-nerolidol | 51.77 ± 7.60 a | 5.91 ± 1.32 b | 12.69 ± 2.46 b | 0.0001 |
| nerol | 7.59 ± 1.70 a | 7.73 ± 1.73 a | 4.33 ± 0.86 b | 0.0053 |
| Furanones | ||||
| mesifurane | 27.83 ± 6.22 | 33.84 ± 5.08 | 39.80 ± 8.12 | NS |
| furaneol | 96.56 ± 24.29 a | 210.38 ± 47.73 b | 456.97 ± 117.81 c | 0.00001 |
| Acids | ||||
| 2-methylbutanoic acid | 30.71 ± 5.67 a | 64.72 ± 16.26 b | 46.23 ± 6.14 a | 0.0017 |
| 3-methylbutanoic acid | 109.32 ± 22.48 a | 89.26 ± 15.81 a | 4.63 ± 0.92 b | 0.0001 |
| hexanoic acid | 43.01 ± 9.60 | 29.04 ± 6.42 | ND | NS |
| Lactones | ||||
| γ-nonalactone | 9.89 ± 2.21 | 11.69 ± 2.61 | ND | NS |
| Δ-decalactone | 4.83 ± 1.08 | 20.36 ± 4.55 | ND | NS |
| Varieties Investigated | Findings | Reference |
|---|---|---|
| 35 | Thirty-one volatile compounds correlated to strawberry flavor intensity, particularly esters, terpenes and furans. | [1] |
| 4 | Key odorants identified were furaneol, γ-decalactone, ethyl butanoate, ethyl hexanoate, ethyl 3-methylbutanoate, diacetyl and hexanoic acid. The aroma of Fuentepina and Candonga varieties presented mainly green notes, whereas the aromatic notes in Camarosa and Sabrina varieties were mainly sweet. | [7] |
| 12 | The most abundant volatile sulfur compounds in strawberry are methanethiol, dimethyl sulfide, dimethyl disulfide and dimethyl trisulfide, being methanethiol the predominant aromatic compound. Festival and Florida Radiance presented higher thioester concentrations., whereas Dover, Rosa Linda and Florida Belle were characterized by relatively high sulfide and low thioester concentrations. | [10] |
| 9 | Esters, such as methyl butanoate, pentyl acetate and methyl hexanoate, characterized the aroma of ripe strawberries, and allow discriminating between cultivars. | [11] |
| 16 | Great diversity of the volatile patterns in F. vesca accessions in comparison with F. × ananassa cultivars. | [33] |
| 5 | The content of volatiles varied depending on the cultivars, but in general ethyl butanoate, mesifurane, ethyl hexanoate, ethyl 3-methylbutanoate, hexyl acetate and γ -dodecalactone had the highest odor activity values. | [35] |
| Root 1 | Root 2 | ||
|---|---|---|---|
| Canonical correlation | 0.9808 | 0.9597 | |
| Eigenvalue | 25.33009 | 11.66195 | |
| Cum. Prop | 0.68474 | 1.00000 | |
| variables | F-value a | correlation of variables with roots | |
| geraniol | 23.26316 | −0.450750 | 0.217442 |
| hexyl hexanoate | 18.57001 | −0.299080 | −0.093153 |
| trans-2-hexen-1-al | 6.17330 | −0.021863 | −0.235022 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Domínguez, R.; Sayago, A.; Akhatou, I.; Fernández-Recamales, Á. Volatile Profiling of Strawberry Fruits Cultivated in a Soilless System to Investigate Cultivar-Dependent Chemical Descriptors. Foods 2020, 9, 768. https://doi.org/10.3390/foods9060768
González-Domínguez R, Sayago A, Akhatou I, Fernández-Recamales Á. Volatile Profiling of Strawberry Fruits Cultivated in a Soilless System to Investigate Cultivar-Dependent Chemical Descriptors. Foods. 2020; 9(6):768. https://doi.org/10.3390/foods9060768
Chicago/Turabian StyleGonzález-Domínguez, Raúl, Ana Sayago, Ikram Akhatou, and Ángeles Fernández-Recamales. 2020. "Volatile Profiling of Strawberry Fruits Cultivated in a Soilless System to Investigate Cultivar-Dependent Chemical Descriptors" Foods 9, no. 6: 768. https://doi.org/10.3390/foods9060768
APA StyleGonzález-Domínguez, R., Sayago, A., Akhatou, I., & Fernández-Recamales, Á. (2020). Volatile Profiling of Strawberry Fruits Cultivated in a Soilless System to Investigate Cultivar-Dependent Chemical Descriptors. Foods, 9(6), 768. https://doi.org/10.3390/foods9060768






