Monitoring Oxidative Status in Winemaking by Untargeted Linear Sweep Voltammetry
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Samples
2.3. Winemaking Practices
2.4. Voltammetry
2.5. Data Analysis
3. Results and Discussion
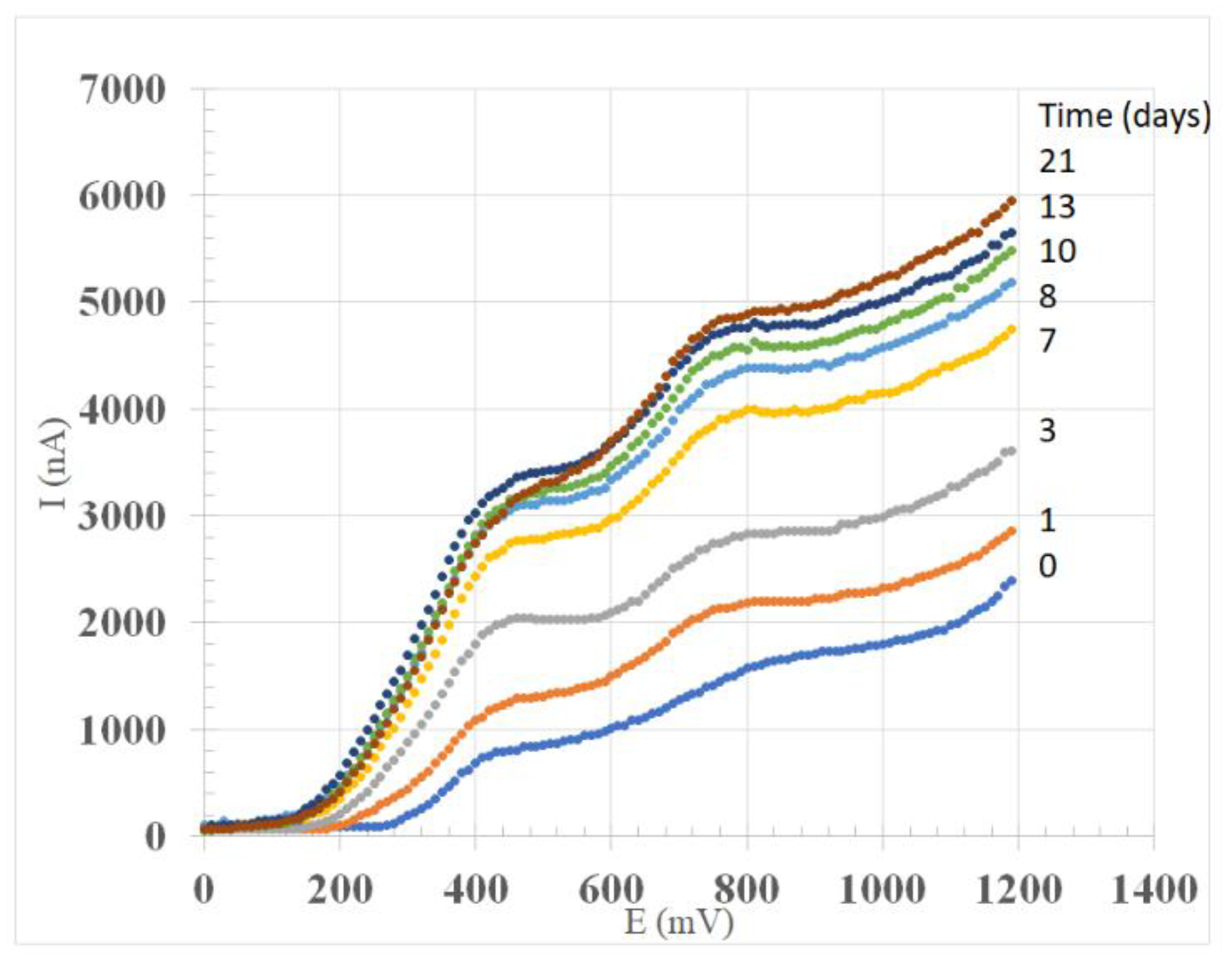
3.1. Linear Sweep Voltammetry
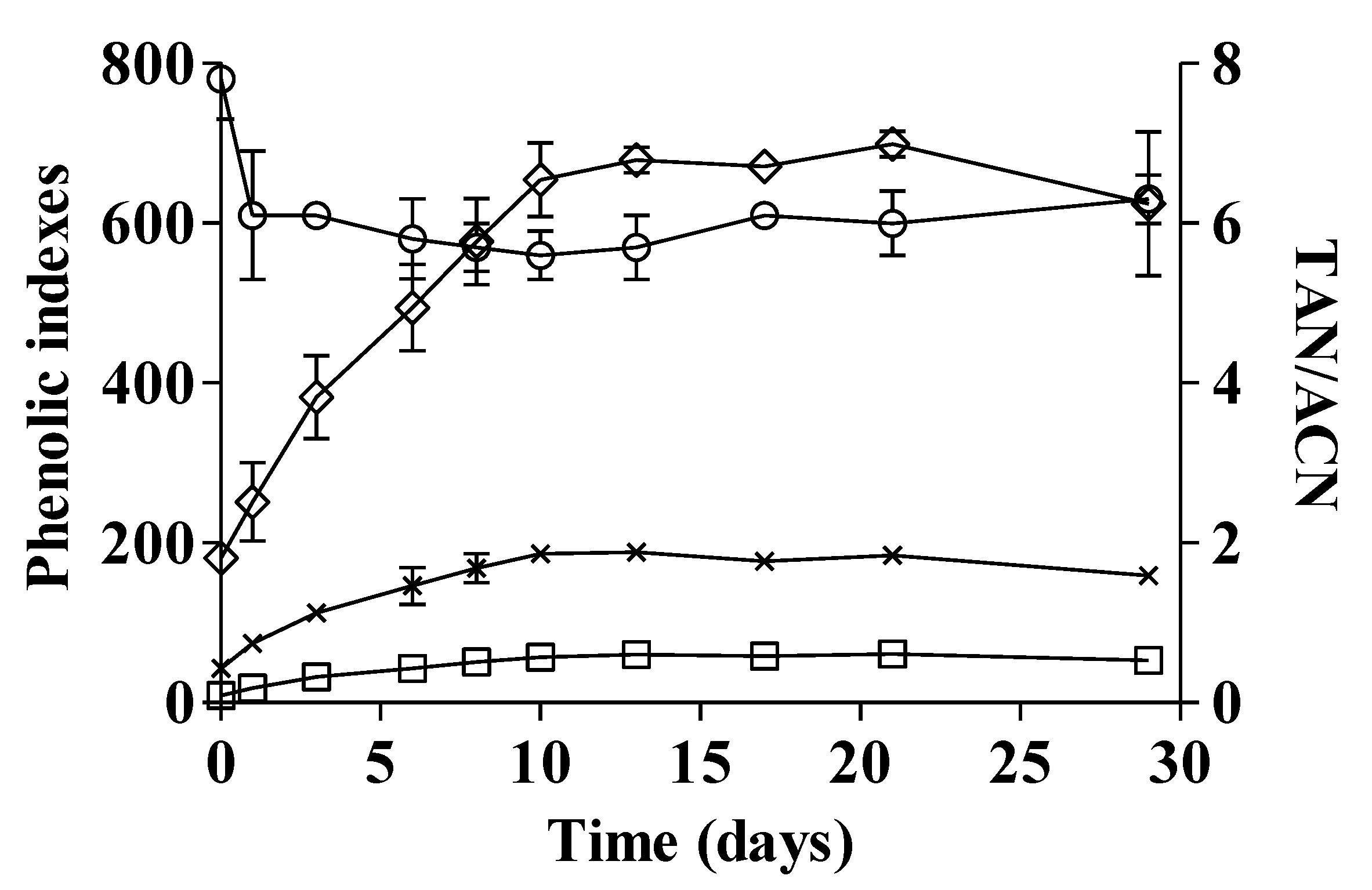
3.2. Maceration Trials
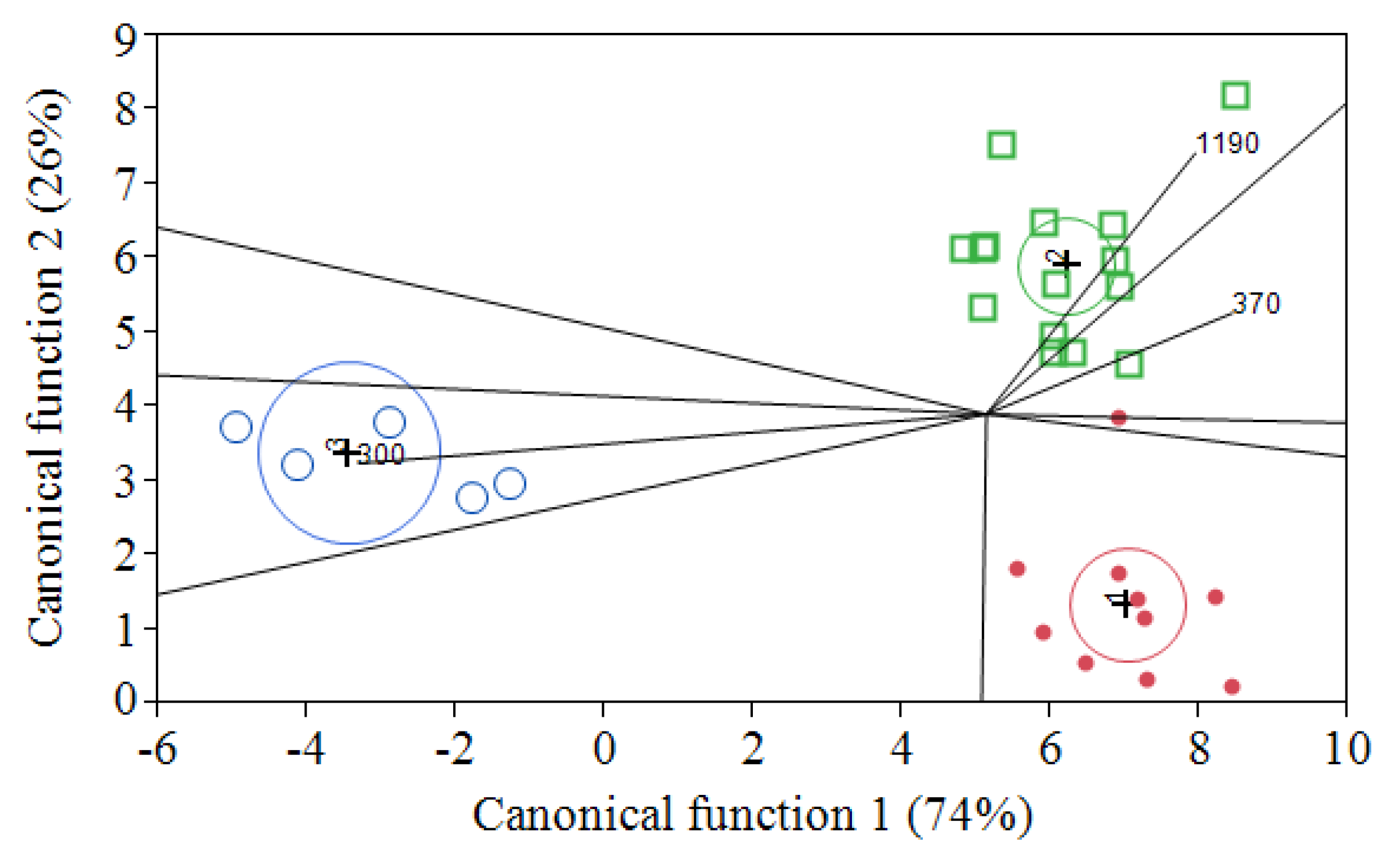
3.3. Alcoholic Fermentation Trails
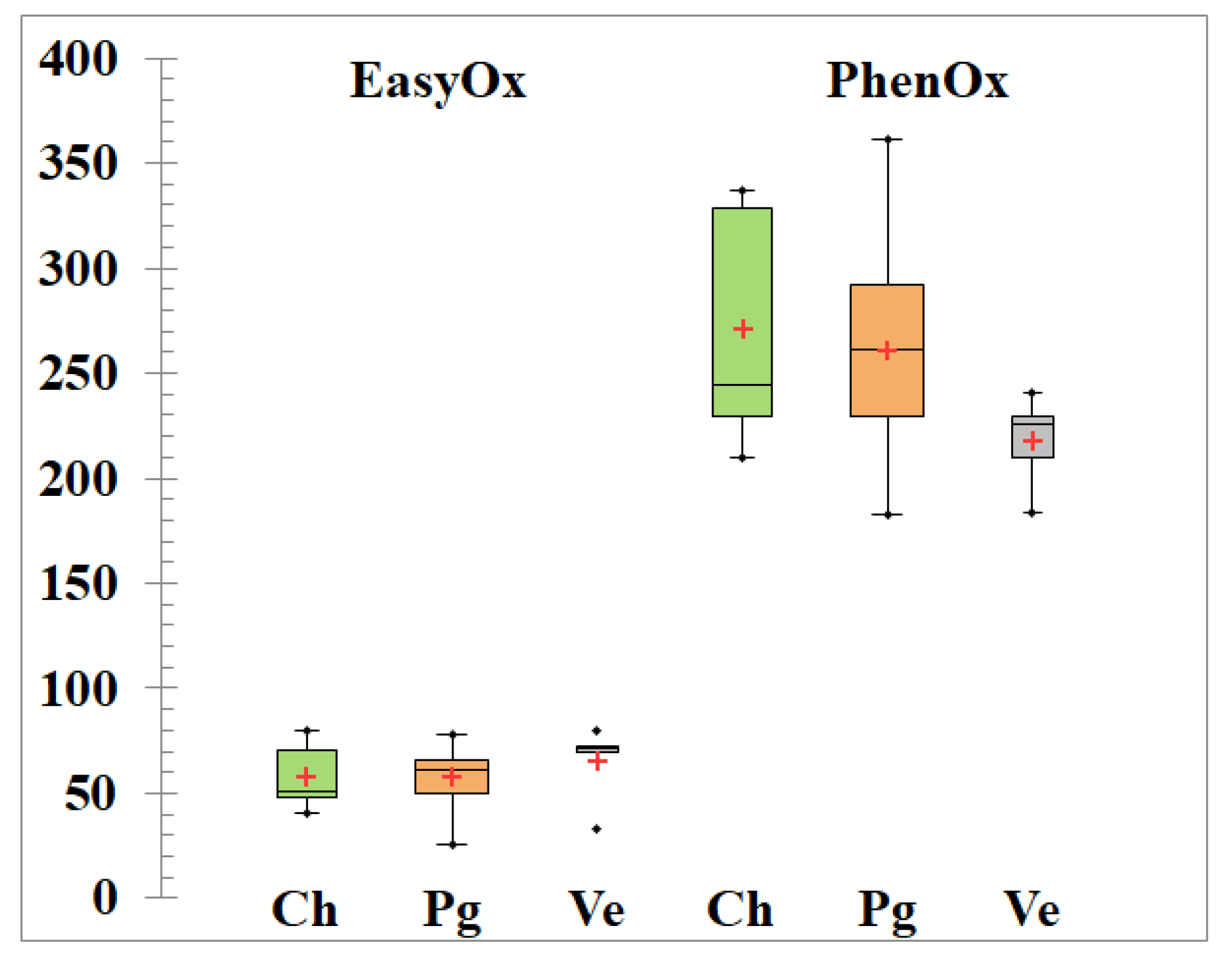
3.4. Fining Trials
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shrake, N.L.; Amirtharajah, R.; Brenneman, C.; Boulton, R.; Knoesen, A. In-line measurement of color and total phenolics during red wine fermentations using a Light-Emitting Diode sensor. Am. J. Enol. Vitic. 2014, 65, 463–470. [Google Scholar] [CrossRef]
- Killeen, D.J.; Boulton, R.; Knoesen, A. Advanced monitoring and control of redox potential in wine fermentation. Am. J. Enol. Vitic. 2018, 69, 394–399. [Google Scholar] [CrossRef]
- Castellari, M.; Simonato, B.; Tornielli, G.B.; Spinelli, P.; Ferrarini, R. Effects of different enological treatments on dissolved oxygen in wines. Ital. J. Food Sci. 2004, 16, 387–396. [Google Scholar]
- Calderón, J.F.; Del Alamo-Sanza, M.; Nevares, I.; Laurie, V.F. The influence of selected winemaking equipment and operations on the concentration of dissolved oxygen in wines. Cienc. Investig. Agrar. 2014, 41, 273–280. [Google Scholar] [CrossRef]
- Day, M.P.; Schmidt, S.A.; Smith, P.A.; Wilkes, E.N. Use and impact of oxygen during winemaking. Aust. J. Grape Wine Res. 2015, 21, 693–704. [Google Scholar] [CrossRef]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Bloom, R., Ed.; Aspen Publisher: Frederick, MD, USA, 1998. [Google Scholar]
- Stockley, C.S. Wine and health: Sulfur dioxide and the wine consumer. Aust. N. Z. Grapegrow. Winemak. 2005, 501, 73–76. [Google Scholar]
- Santos, M.C.; Nunes, C.; Saraiva, J.A.; Coimbra, M.A. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: Review of their potentialities and limitations. Eur. Food Res. Technol. 2012, 234, 1–12. [Google Scholar] [CrossRef]
- Giacosa, S.; Río Segade, S.; Cagnasso, E.; Caudana, A.; Rolle, L.; Gerbi, V. Chapter 21—SO2 in Wines: Rational Use and Possible Alternatives. In Red Wine Technology; Morata, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 309–321. [Google Scholar]
- Versari, A.; du Toit, W.; Parpinello, G.P. Oenological tannins: A review. Aust. J. Grape Wine Res. 2013, 19, 1–10. [Google Scholar] [CrossRef]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar]
- Smith, P.A.; McRae, J.M.; Bindon, K.A. Impact of winemaking practices on the concentration and composition of tannins in red wine. Aust. J. Grape Wine Res. 2015, 21, 601–614. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Singleton, V.L.; Esau, P. Phenolic Substances in Grapes and Wine, and their Significance. In Advance in Food Research; Academic Press: New York, NY, USA, 1969. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Haslam, E. Practical Polyphenolics. In From Structure to Molecular Recognition and Physiological Action; Cambridge University Press: New York, NY, USA, 1988. [Google Scholar]
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Cãmara, J.S. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Laurie, V.F. Oxidation of wine phenolics: A critical evaluation and hypotheses. Am. J. Enol. Vitic. 2006, 57, 306–313. [Google Scholar]
- Oliveira, C.M.; Silva Ferreira, A.C.; De Freitas, V.; Silva, A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2001, 44, 1115–1126. [Google Scholar] [CrossRef]
- Danilewicz, J.D. Review of oxidative processes in wine and value of reduction potentials in enology. Am. J. Enol. Vitic. 2012, 63, 1–10. [Google Scholar] [CrossRef]
- Frankel, E.N.; Meyer, A.S. The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric. 2000, 80, 1925–1941. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Craft, B.D.; Kerrihard, A.L.; Amarowicz, R.; Pegg, R.B. Phenol-based antioxidants and the in vitro methods used for their assessment. Compr. Rev. Food Sci. Food Saf. 2012, 11, 148–173. [Google Scholar] [CrossRef]
- Sochor, J.; Dobes, J.; Krystofova, O.; Ruttkay-Nedecky, B.; Babula, P.; Pohanka, M.; Jurikova, T.; Zitka, O.; Adam, V.; Klejdus, B.; et al. Electrochemistry as a tool for studying antioxidant properties. Int. J. Electrochem. Sci. 2013, 8, 8464–8489. [Google Scholar]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. A cyclic voltammetry method suitable for characterizing antioxidant properties of wine and wine phenolics. J. Agric. Food Chem. 2001, 49, 1957–1965. [Google Scholar] [CrossRef]
- Roginsky, V.; de Beer, D.; Harbertson, J.F.; Kilmartin, P.A.; Barsukova, T.; Adams, D.O. The antioxidant activity of Californian red wines does not correlate with wine age. J. Sci. Food Agric. 2006, 86, 834–840. [Google Scholar] [CrossRef]
- Makhotkina, O.; Kilmartin, P.A. Uncovering the influence of antioxidants on polyphenol oxidation in wines using an electrochemical method: Cyclic voltammetry. J. Electroanal. Chem. 2009, 633, 165–174. [Google Scholar] [CrossRef]
- Ricci, A.; Parpinello, G.P.; Teslić, N.; Kilmartin, P.A.; Versari, A. Suitability of the cyclic voltammetry measurements and DPPH• spectrophotometric assay to determine the antioxidant capacity of food-grade oenological tannins. Molecules 2019, 24, 2925. [Google Scholar] [CrossRef] [PubMed]
- Medvidović-Kosanović, M.; Šeruga, M.; Jakobek, L.; Novak, I. Electrochemical and antioxidant properties of Rutin. Collect. Czechoslov. Chem. Commun. 2010, 75, 547–561. [Google Scholar]
- Romão Sartori, E.; Fatibello-Filho, O. Simultaneous voltammetric determination of ascorbic acid and sulfite in beverages employing a glassy carbon electrode modified with carbon nanotubes within a Poly(Allylamine Hydrochloride) film. Electroanalysis 2012, 24, 627–634. [Google Scholar] [CrossRef]
- Scampicchio, M.; Lawrence, N.S.; Arecchi, A.; Mannino, S. Determination of sulfite in wine by linear sweep voltammetry. Electroanalysis 2008, 20, 444–447. [Google Scholar] [CrossRef]
- Ugliano, M. Rapid fingerprinting of white wine oxidizable fraction and classification of white wines using disposable screen printed sensors and derivative voltammetry. Food Chem. 2016, 212, 837–843. [Google Scholar] [CrossRef]
- Gonzalez, A.; Vidal, S.; Ugliano, M. Untargeted voltammetric approaches for characterization of oxidation patterns in white wines. Food Chem. 2018, 269, 1–8. [Google Scholar] [CrossRef]
- Frías, D. Seguimiento de la Maceración de Uvas Tintas Mediante PolyscanP200. 2018. Available online: https://www.vinventions.com/assets/4d4a0795-b6b2-49dd-9d05-12d7a6eb2eb8/4-daniel-frias-presentacion-polyscan-vinventions.pdf (accessed on 21 April 2020).
- Babin, B.G.; Watson, M.P. Discriminant Analysis: An Overview; Lovric, M., Ed.; International Encyclopedia of Statistical Science, Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Janeiro, P.; Oliveira Brett, A.M. Redox behavior of anthocyanins present in Vitis vinifera L. Electroanalysis 2007, 19, 1779–1786. [Google Scholar] [CrossRef]
- Aguirre, M.J.; Chen, Y.Y.; Isaacs, M.; Matsuhiro, B.; Mendoza, L.; Torres, S. Electrochemical behaviour and antioxidant capacity of anthocyanins from Chilean red wine, grape and raspberry. Food Chem. 2010, 121, 44–48. [Google Scholar] [CrossRef]
- Makhotkina, O.; Kilmartin, P.A. The use of cyclic voltammetry for wine analysis: Determination of polyphenols and free sulfur dioxide. Anal. Chim. Acta 2010, 668, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, B.; Siliani, S.; Canuti, V.; Rosi, I.; Bertuccioli, M. A kinetic study on extraction and transformation phenomena of phenolic compounds during red wine fermentation. Int. J. Food Sci. Technol. 2010, 45, 2080–2088. [Google Scholar] [CrossRef]
- Andrich, G.; Zinnai, A.; Venturi, F.; Fiorentini, R. A tentative mathematical model to describe the evolution of phenolic compounds during the maceration of Sangiovese and Merlot grapes. Ital. J. Food Sci. 2005, 17, 45–58. [Google Scholar]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar]
- Martins, R.C.; Oliveira, R.; Bento, F.; Geraldo, D.; Lopes, V.V.; Guedes de Pinho, P.; Oliveira, C.M.; Silva Ferreira, A.C. Oxidation management of white wines using cyclic voltammetry and multivariate process monitoring. J. Agric. Food Chem. 2008, 56, 12092–12098. [Google Scholar] [CrossRef] [PubMed]
- Ough, C.S. Substances extracted during skin contact with white musts. I. General wine composition and quality changes with contact time. Am. J. Enol. Vitic. 1969, 20, 93–100. [Google Scholar]
- Hernanz, D.; Recamales, Á.S.; González-Miret, M.L.; Gómez-Míguez, M.J.; Vicario, I.M.; Heredia, F.J. Phenolic composition of white wines with a prefermentative maceration at experimental and industrial scale. J. Food Eng. 2007, 80, 327–335. [Google Scholar] [CrossRef]
- Giordano, M.; Caudana, M.; Hock, M.; Zeppa, G.; Rolle, L.; Gerbi, V. First experiences of wine-making for the increasing of the varietal character of Vermentino wines. Riv. Vitic. Enol. 2012, 2, 17–28. [Google Scholar]
- Naviglio, D.; Formato, A.; Scaglione, G.; Montesano, D.; Pellegrino, A.; Villecco, F.; Gallo, M. Study of the grape cryo-maceration process at different temperatures. Foods 2018, 7, 107. [Google Scholar] [CrossRef]
- Singleton, V.L.; Draper, D.E. Adsorbents and wines. I. Selection of activated charcoals for treatment of wine. Am. J. Enol. Vitic. 1962, 13, 114–125. [Google Scholar]
- Corcho-Corral, B.; Olivares-Marín, M.; Valdes-Sánchez, E.; Fernández-González, C.; Macías-García, A.; Gómez-Serrano, V. Development of activated carbon using vine shoots (Vitis vinifera) and its use for wine treatment. J. Agric. Food Chem. 2005, 53, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Andrea-Silva, J.; Cosme, F.; Ribeiro, L.F.; Moreira, A.S.P.; Malheiro, A.C.; Coimbra, M.A.; Rosário, M.; Domingues, M.; Nunes, F.M. Origin of the pinking phenomenon of white wines. J. Agric. Food Chem. 2014, 62, 5651–5659. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N.; Waterhouse, A.L.; Teissedre, P.L. Principal phenolic phytochemicals in selected California wines and their antioxidant activity in inhibiting oxidation of human low-density lipoproteins. J. Agric. Food Chem. 1995, 43, 890–894. [Google Scholar] [CrossRef]
- Ricci, A.; Teslic, A.; Petropolus, V.-I.; Parpinello, G.P.; Versari, A. Fast analysis of total polyphenol content and antioxidant activity in wines and oenological tannins using a flow injection system with tandem diode array and electrochemical detections. Food Anal. Methods 2019, 12, 347–354. [Google Scholar] [CrossRef]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Mattioli, A.U.; Teslić, N.; Kilmartin, P.A.; Versari, A. Antioxidant activity of commercial food grade tannins exemplified in a wine model. Food Addit. Contam. Part A 2016, 33, 1761–1774. [Google Scholar] [CrossRef] [PubMed]
- Kilmartin, P.A.; Zou, H.; Waterhouse, H.L. Correlation of wine phenolic composition versus Cyclic Voltammetry response. Am. J. Enol. Vitic. 2002, 53, 294–302. [Google Scholar]
- Ugliano, M.; Wirth, J.; Begrand, S.; Dieval, J.-B.; Vidal, S. A novel voltammetric approach for rapid analysis of white grapes polyphenols and monitoring of pre-fermentative operations. Wine Vitic. J. 2016, 31, 28–31. [Google Scholar]
- Ugliano, M.; Dieval, J.-B.; Vidal, S.; Tacchini, P. Electroanalytical Method for Predicting the Oxidability of a Wine or a Grape Must. Patent US 2014/0141120 A1, 22 May 2014. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeremic, J.; Ricci, A.; Tacconi, G.; Lagarde-Pascal, C.; Parpinello, G.P.; Versari, A. Monitoring Oxidative Status in Winemaking by Untargeted Linear Sweep Voltammetry. Foods 2020, 9, 728. https://doi.org/10.3390/foods9060728
Jeremic J, Ricci A, Tacconi G, Lagarde-Pascal C, Parpinello GP, Versari A. Monitoring Oxidative Status in Winemaking by Untargeted Linear Sweep Voltammetry. Foods. 2020; 9(6):728. https://doi.org/10.3390/foods9060728
Chicago/Turabian StyleJeremic, Jelena, Arianna Ricci, Gabriele Tacconi, Christine Lagarde-Pascal, Giuseppina Paola Parpinello, and Andrea Versari. 2020. "Monitoring Oxidative Status in Winemaking by Untargeted Linear Sweep Voltammetry" Foods 9, no. 6: 728. https://doi.org/10.3390/foods9060728
APA StyleJeremic, J., Ricci, A., Tacconi, G., Lagarde-Pascal, C., Parpinello, G. P., & Versari, A. (2020). Monitoring Oxidative Status in Winemaking by Untargeted Linear Sweep Voltammetry. Foods, 9(6), 728. https://doi.org/10.3390/foods9060728






