TOCOSH FLOUR (Solanum tuberosum L.): A Toxicological Assessment of Traditional Peruvian Fermented Potatoes
Abstract
1. Introduction
2. Material and Methods
2.1. Collection of Plant Material
2.2. Preparation of the Tocosh Flour
2.3. Phytochemical Analysis
2.4. Experimental Animals
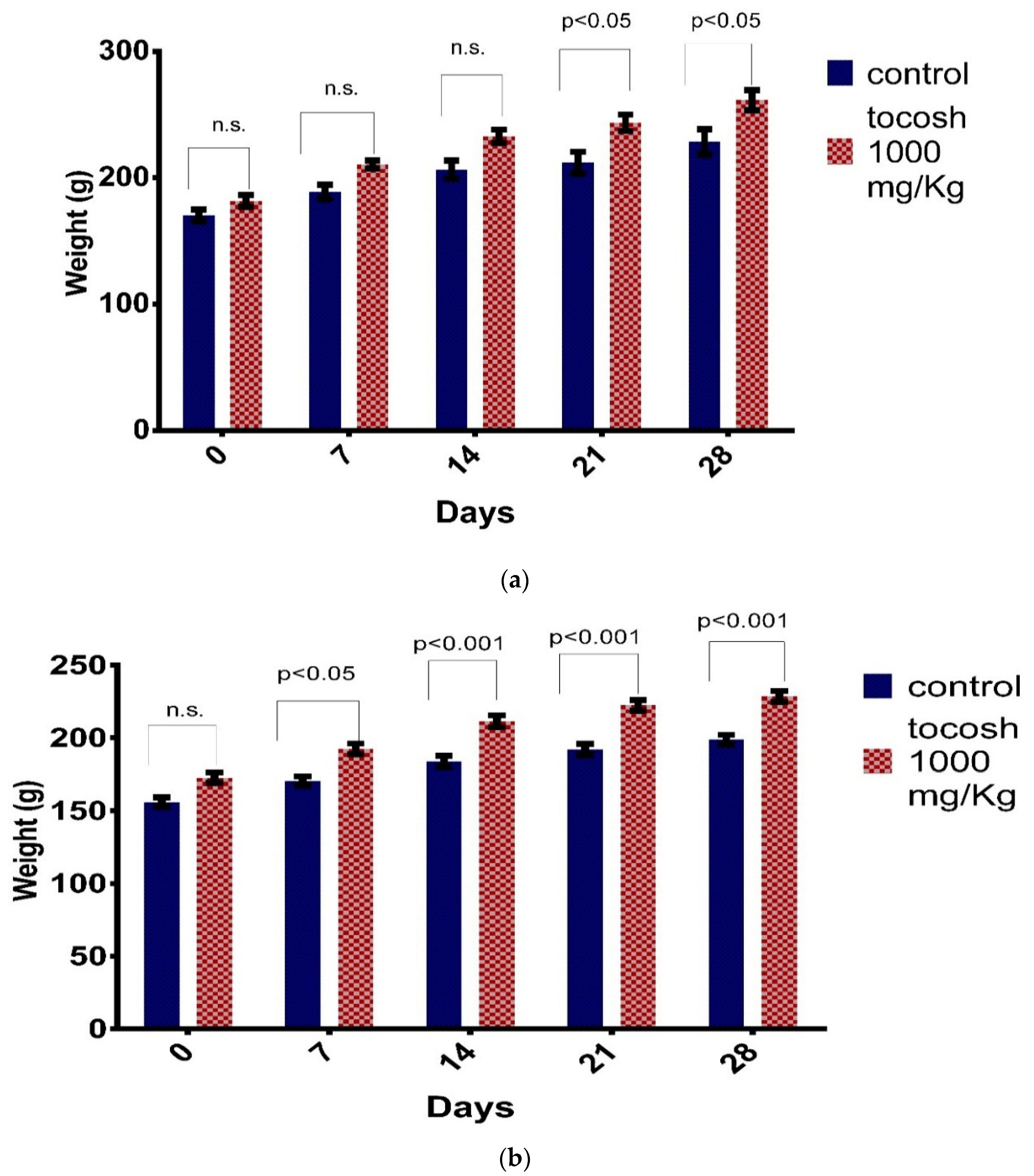
2.5. Sub-Acute Toxicity at Repeated Dose for 28 Days
2.6. Acute Oral Toxicity—Fixed-Dose Study Procedure
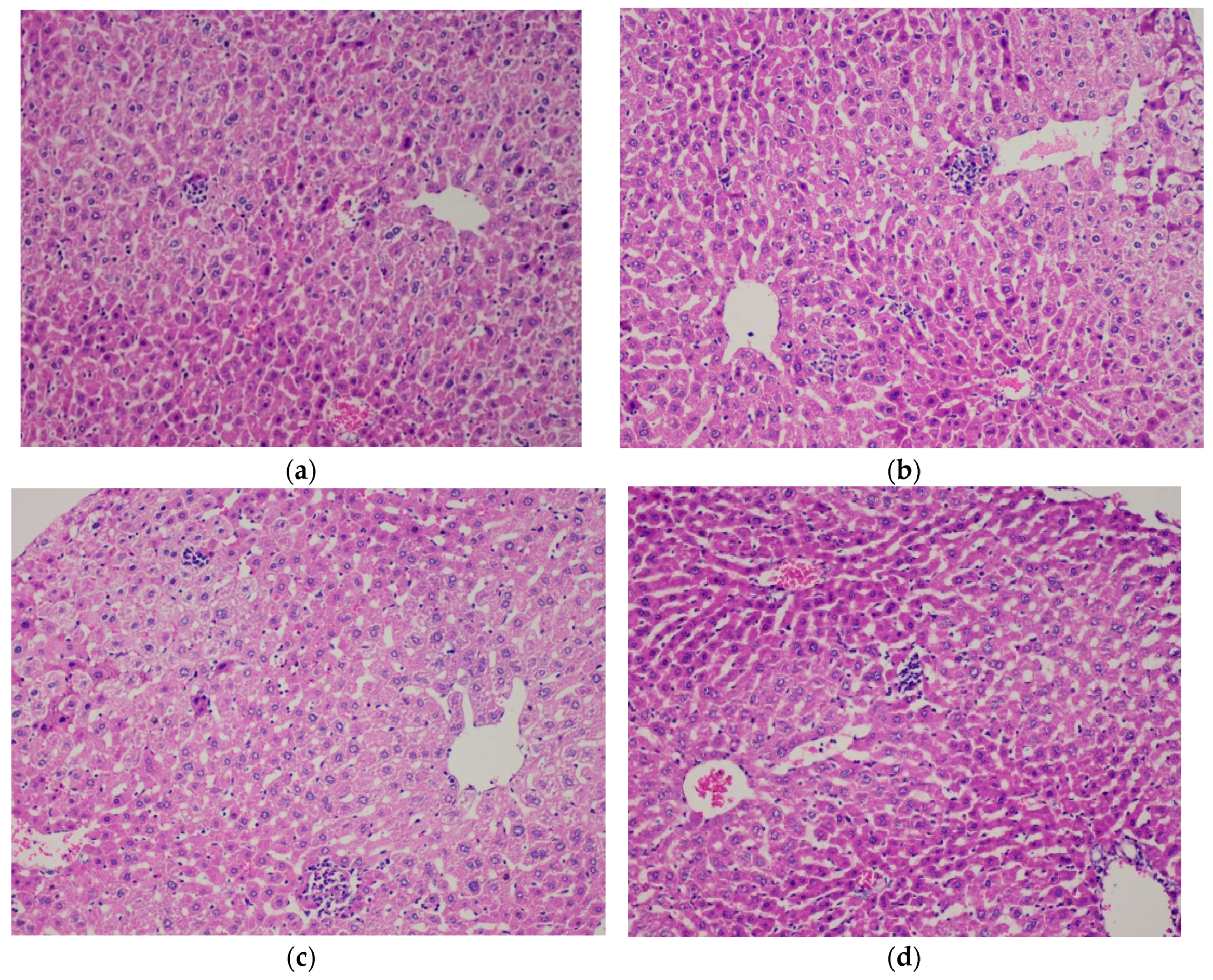
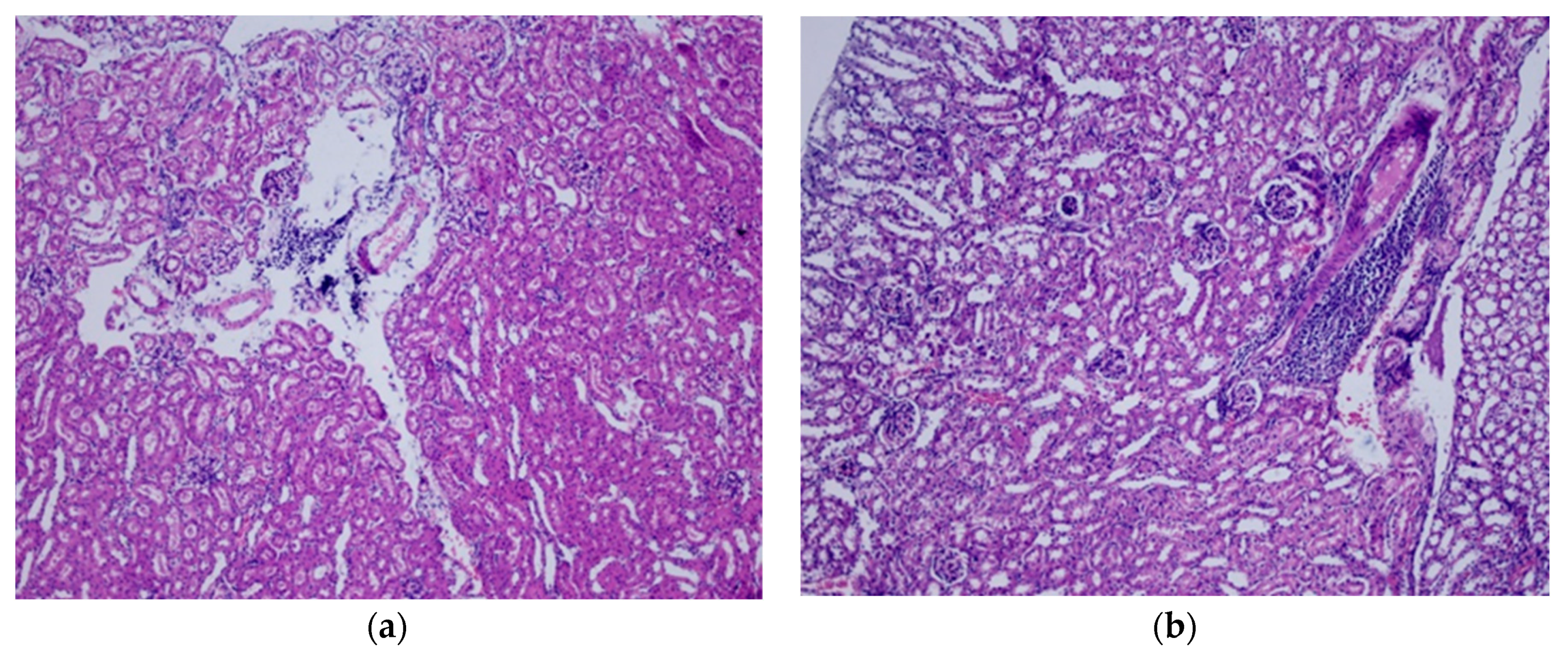
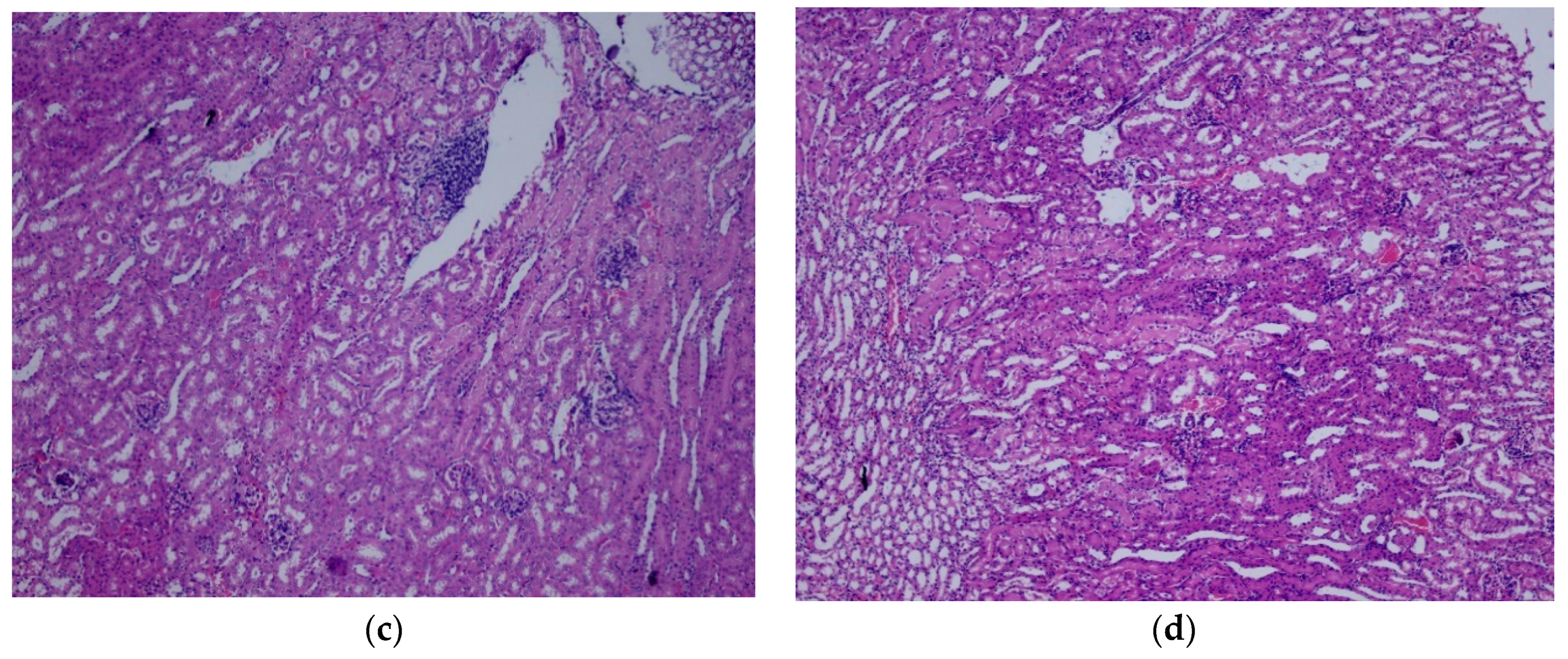
2.7. Histopathological Analysis
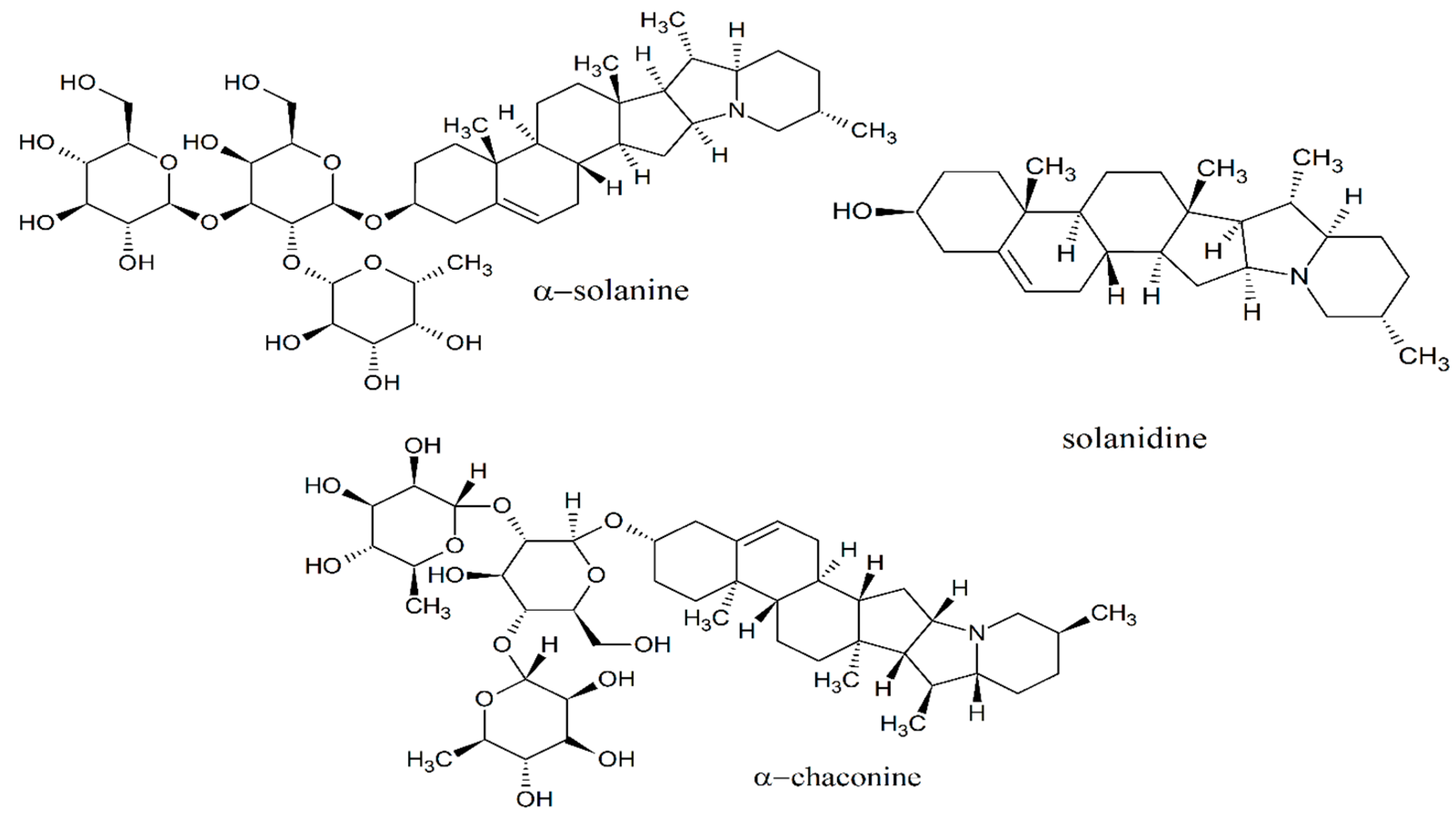
2.8. Prediction of Drug-Likeness Properties for Steroidal Glycoalkaloids: α-Solanin, α-Chaconine and Solanidine
- Permeability lower than 25: low permeability.
- Permeability between 25 and 500: medium permeability.
- Permeability higher 500: high permeability.
2.9. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
3.2. Repeated Dose Toxicity Study for 28 Days
3.3. Acute Oral Toxicity—Fixed Dose Procedure Study
3.4. Prediction of Drug-Likeness Properties for Steroidal Glycoalkaloids: α-Solanin, α-Chaconine and Solanidine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability
Conflicts of Interest
References
- Hardigan, M.A.; Laimbeer, F.P.E.; Newton, L.; Crisovan, E.; Hamilton, J.P.; Vaillancourt, B.; Wiegert-Rininger, K.; Wood, J.C.; Douches, D.S.; Farré, E.M.; et al. Genome diversity of tuber-bearing solanum uncovers complex evolutionary history and targets of domestication in the cultivated potato. Proc. Natl. Acad. Sci. USA 2017. [Google Scholar] [CrossRef] [PubMed]
- Berdugo-Cely, J.; Valbuena, R.I.; Sánchez-Betancourt, E.; Barrero, L.S.; Yockteng, R. Genetic diversity and association mapping in the colombian central collection of solanum tuberosum L. Andigenum group using SNPs markers. PLoS ONE 2017. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Milla, D.; Casas, A.; Torres-Guevara, J.; Cruz-Soriano, A. Ecological and socio-cultural factors influencing in situ conservation of crop diversity by traditional andean households in Peru. J. Ethnobiol. Ethnomed. 2011. [Google Scholar] [CrossRef] [PubMed]
- Mayta-Tovalino, F.; Sedano-Balbin, G.; Romero-Tapia, P.; Alvítez-Temoche, D.; álvarez-Paucar, M.; Gálvez-Calla, L.; Sacsaquispe-Contreras, S. Development of new experimental dentifrice of peruvian solanum tuberosum (Tocosh) fermented by water stress: Antibacterial and cytotoxic activity. J. Contemp. Dent. Pract. 2019. [Google Scholar] [CrossRef]
- Mosso, A.L.; Jimenez, M.E.; Vignolo, G.; LeBlanc, J.G.; Samman, N.C. Increasing the folate content of tuber based foods using potentially probiotic lactic acid bacteria. Food Res. Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Vignolo, G.; Todorov, S.D.; de Giori, G.S. Indigenous fermented foods and beverages produced in Latin America. In Food Intake: Regulation, Assessing and Controlling; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013. [Google Scholar]
- Langkilde, S.; Mandimika, T.; Schrøder, M.; Meyer, O.; Slob, W.; Peijnenburg, A.; Poulsen, M. A 28-day repeat dose toxicity study of steroidal glycoalkaloids, α-solanine and α-chaconine in the Syrian Golden hamster. Food Chem. Toxicol. 2009. [Google Scholar] [CrossRef]
- Barceloux, D.G. Potatoes, Tomatoes, and Solanine Toxicity (Solanum tuberosum L., Solanum lycopersicum L.). Dis. Mon. 2009, 55, 391–402. [Google Scholar] [CrossRef]
- Friedman, M. Potato glycoalkaloids and metabolites: Roles in the plant and in the diet. J. Agric. Food Chem. 2006, 54, 8655–8681. [Google Scholar] [CrossRef]
- Chen, Z.; Miller, A.R. Steroidal alkaloids in solanaceous vegetable crops. Hortic. Rev. 2010, 171–196. [Google Scholar] [CrossRef]
- Herrera-Calderon, O.; Arroyo-Acevedo, J.L.; Rojas-Armas, J.; Chumpitaz-Cerrate, V.; Figueroa-Salvador, L.; Enciso-Roca, E.; Tinco-Jayo, J.A. Phytochemical screening, total phenolic content, antioxidant and cytotoxic activity of chromolaena laevigata on human tumor cell lines. Annu. Res. Rev. Biol. 2017. [Google Scholar] [CrossRef]
- OECD/OECDE. Test No. 407: Repeated Dose 28-Day Oral Toxicity Study in Rodents; OECD Publishing: Paris, France, 2008. [Google Scholar]
- OECD. Test No. 420: Acute oral toxicity-fixed dose procedure. Oecd Guidel. Test. Chem. 2002. [Google Scholar] [CrossRef]
- Enciso-Roca, E.; Aguilar-Felices, E.J.; Tinco-Jayo, J.A.; Arroyo-Acevedo, J.L.; Herrera-Calderon, O.; Aguilar-Carranza, C.; Justil, H.G. Effects of acute and sub-acute oral toxicity studies of ethanol extract of tanacetum parthenium (L) Sch. Bip. Aerial parts in mice and rats. Annu. Res. Rev. Biol. 2017. [Google Scholar] [CrossRef]
- Butina, D.; Segall, M.D.; Frankcombe, K. Predicting ADME properties in silico: Methods and models. Drug Discov. Today 2002. [Google Scholar] [CrossRef]
- Morris, S.C.; Lee, T.H. The toxicity and teratogenicity of Solanaceae glycoalkaloids, particularly those of the potato (Solanum tuberosum): A review. Food Technol. Aust. 1984, 36, 195–202. [Google Scholar]
- Romanucci, V.; Pisanti, A.; Di Fabio, G.; Davinelli, S.; Scapagnini, G.; Guaragna, A.; Zarrelli, A. Toxin levels in different variety of potatoes: Alarming contents of α-chaconine. Phytochem. Lett. 2016. [Google Scholar] [CrossRef]
- Crawford, L.; Myhr, B. A preliminary assessment of the toxic and mutagenic potential of steroidal alkaloids in transgenic mice. Food Chem. Toxicol. 1995. [Google Scholar] [CrossRef]
- Abduh, S.B.M.; Leong, S.Y.; Agyei, D.; Oey, I. Understanding the properties of starch in potatoes (Solanum tuberosum var. Agria) after being treated with pulsed electric field processing. Foods 2009. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Schmidt, J.M.; Kristiansen, G.H.; Dalsgaard, T.K.; Larsen, L.B. Liquid chromatography mass spectrometry quantification of α-solanine, α-chaconine, and solanidine in potato protein isolates. Foods 2020. [Google Scholar] [CrossRef]
- Cui, L.; Tian, Y.; Tian, S.; Wang, Y.; Gao, F. Preparation of potato whole flour and its effects on quality of flour products: A review. Grain Oil Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Patil, B.C.; Sharma, R.P.; Salunkhe, D.K.; Salunkhe, K. Evaluation of solanine toxicity. Food Cosmet. Toxicol. 1972. [Google Scholar] [CrossRef]
- Lau, J.K.C.; Zhang, X.; Yu, J. Animal models of non-alcoholic fatty liver disease: Current perspectives and recent advances. J. Pathol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Tiniakos, D.G. Histopathology of nonalcoholic fatty liver disease. World J. Gastroenterol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Greaves, P. Histopathology of Preclinical Toxicity Studies: Interpretation and Relevance in Drug Safety Evaluation, 4th ed.; Academic Press: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Hashimoto, N.; Ito, Y.; Han, K.H.; Shimada, K.I.; Sekikawa, M.; Topping, D.L.; Bird, A.R.; Noda, T.; Chiji, H.; Fukushima, M. Potato pulps lowered the serum cholesterol and triglyceride levels in rats. J. Nutr. Sci. Vitaminol. 2006. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chiriboga, D.E.; Olendzki, B.C.; Li, W.; Leung, K.; Hafner, A.R.; Li, Y.; Ockene, I.S.; Hebert, J.R. Association between Carbohydrate Intake and Serum Lipids. J. Am. Coll. Nutr. 2006. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Panter, K.E.; Gaffield, W.; Evans, R.C.; Bunch, T.D. Effects of steroidal glycoalkaloids from potatoes (Solanum tuberosum) on in vitro bovine embryo development. Anim. Reprod. Sci. 2005. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Gao, Q.; Praticò, G.; Chen, J.; Dragsted, L.O. Biomarkers of tuber intake. Genes Nutr. 2019. [Google Scholar] [CrossRef]
- Friedman, M. Analysis of biologically active compounds in potatoes (Solanum tuberosum), tomatoes (Lycopersicon esculentum), and jimson weed (Datura stramonium) seeds. J. Chromatogr. A 2004. [Google Scholar] [CrossRef]
- Friedman, M.; Rayburn, J.R.; Bantle, J.A. Structural Relationships and Developmental Toxicity of Solanum Alkaloids in the Frog Embryo Teratogenesis Assay-Xenopus. J. Agric. Food Chem. 1992. [Google Scholar] [CrossRef]
- Connors, N.J.; Glover, R.L.; Stefan, C.; Patterson, D.; Wong, E.; Milstein, M.; Swerdlow, M.; Hoffman, R.S.; Nelson, L.S.; Smith, S.W. Biological and botanical confirmation of solanaceous glycoalkaloid poisoning by susumber berries (Solanum torvum). Clin. Toxicol. 2014. [Google Scholar] [CrossRef]
- Nigg, H.N.; Ramos, L.E.; Graham, E.M.; Sterling, J.; Brown, S.; Cornell, J.A. Inhibition of human plasma and serum butyrylcholinesterase (EC 3.1.1.8) by α-chaconine and α-solanine. Fundam. Appl. Toxicol. 1996. [Google Scholar] [CrossRef]
- Gaffield, W.; Keeler, R.F. Induction of terata in hamsters by solanidane alkaloids derived from Solanum tuberosum. Chem. Res. Toxicol. 1996. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Oqani, R.K.; Lee, J.E.; Kang, J.W.; Kim, S.Y.; Cho, E.S.; Jeong, Y.D.; Baek, J.J.; Jin, D. Il α-Solanine impairs oocyte maturation and quality by inducing autophagy and apoptosis and changing histone modifications in a pig model. Reprod. Toxicol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.P.; Gibson, J.G.; McCarroll, A.M.; McClean, G.A. Embryotoxicity of solanine and aspirin in mice. J. Reprod. Fertil. 1976. [Google Scholar] [CrossRef] [PubMed]
- Yamashoji, S.; Matsuda, T. Synergistic cytotoxicity induced by α-solanine and α-chaconine. Food Chem. 2013. [Google Scholar] [CrossRef]
- Al Sinani, S.S.S.; Eltayeb, E.A. The steroidal glycoalkaloids solamargine and solasonine in solanum plants. South. Afr. J. Bot. 2017. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016. [Google Scholar] [CrossRef]

| Organs | Control Group | Tocosh Flour Dose: 1000 mg/kg |
|---|---|---|
| Male rats | n = 5 | n = 5 |
| Brain | 0.70 ± 0.03 | 0.65 ± 0.01 |
| Heart | 0.35 ± 0.00 | 0.37 ± 0.05 |
| Lung | 0.48 ± 0.06 | 0.50 ± 0.15 |
| Liver | 2.96 ± 0.34 | 3.48 ± 0.33 * |
| Spleen | 0.18 ± 0.04 | 0.26 ± 0.05 |
| Stomach | 0.76 ± 0.03 | 0.73 ± 0.06 |
| Kidney | 0.60 ± 0.07 | 0.76 ± 0.05 |
| Testes | 1.73 ± 0.05 | 1.77 ± 0.28 |
| Female rats | n = 5 | n = 5 |
| Brain | 0.78 ± 0.10 | 0.69 ± 0.05 |
| Heart | 0.40 ± 0.01 | 0.41 ± 0.03 |
| Lung | 0.47 ± 0.12 | 0.62 ± 0.04 |
| Liver | 2.83 ± 0.02 | 3.28 ± 0.25 * |
| Spleen | 0.24 ± 0.04 | 0.26 ± 0.04 |
| Stomach | 0.62 ± 0.01 | 0.54 ± 0.04 |
| Kidney | 0.66 ± 0.03 | 0.68 ± 0.01 |
| Uterus | 0.47 ± 0.00 | 0.45 ± 0.06 |
| Parameters | Control Group | Tocosh Flour Dose: 1000 mg/kg |
|---|---|---|
| Male rats | n = 5 | n = 5 |
| AST (IU/L) | 113.00 ± 4.00 | 108.50 ± 1.50 |
| ALT (IU/L) | 69.50 ± 3.50 | 70.00 ± 1.50 |
| Alkaline Phosphatase (IU/L) | 231.00 ± 9.00 | 238.50 ± 0.50 |
| Total Bilirubin (mg/dL) | 0.10 ± 0.00 | 0.10 ± 0.00 |
| Total Protein (g/dL) | 6.95 ± 0.05 | 7.01 ± 0.05 |
| Albumin (g/dL) | 3.80 ± 0.20 | 3.95 ± 0.05 |
| Globulin (g/dL) | 3.15 ± 0.25 | 3.00 ± 0.10 |
| Total cholesterol (mg/dL) | 59.50 ± 0.50 | 60.00 ± 0.00 |
| Triglycerides (mg/dL) | 134.50 ± 8.50 | 107.00 ± 13.00 * |
| HDL (mg/dL) | 12.50 ± 0.50 | 10.60 ± 0.40 |
| LDL (mg/dL) | 20.00 ± 3.00 | 28.00 ± 3.00 * |
| Glucose (mg/dL) | 100.50 ± 0.50 | 108.50 ± 1.50 |
| Serum urea (mg/dL) | 37.50 ± 1.50 | 40.50 ± 2.50 |
| Serum creatinine (mg/dL) | 0.44 ± 0.01 | 0.44 ± 0.03 |
| Female rats | n = 5 | n = 5 |
| AST (IU/L) | 116.50 ± 0.50 | 106.00 ± 0.50 |
| ALT (IU/L) | 65.50 ± 0.50 | 63.00 ± 1.00 |
| Alkaline Phosphatase (IU/L) | 235.50 ± 5.50 | 223.00 ± 17.00 |
| Total Bilirubin (mg/dL) | 0.10 ± 0.00 | 0.10 ± 0.00 |
| Total Protein (g/dL) | 6.90 ± 0.30 | 6.90 ± 0.20 |
| Albumin (g/dL) | 3.80 ± 0.20 | 4.10 ± 0.00 |
| Globulin (g/dL) | 3.05 ± 0.05 | 2.80 ± 0.20 |
| Total cholesterol (mg/dL) | 57.00 ± 3.00 | 59.50 ± 0.50 |
| Triglycerides (mg/dL) | 139.00 ± 1.00 | 130.50 ± 10.50 |
| HDL (mg/dL) | 9.55 ± 0.25 | 8.25 ± 0.25 |
| LDL (mg/dL) | 18.00 ± 2.00 | 25.00 ± 2.00 * |
| Glucose (mg/dL) | 110.50 ± 3.50 | 113.00 ± 2.00 |
| Serum urea (mg/dL) | 35.50 ± 2.50 | 34.00 ± 0.00 |
| Serum creatinine (mg/dL) | 0.40 ± 0.02 | 0.46 ± 0.01 |
| Parameters | Control Group | Tocosh Flour Dose: 1000 mg/kg |
|---|---|---|
| Male rats | n = 5 | n = 5 |
| RBC (×106/mm3) | 7.09 ± 0.30 | 7.30 ± 0.08 |
| White blood cells (×103/mm3) | 4.14 ± 0.13 | 3.91 ± 0.17 |
| Hemoglobin (g/dL) | 14.70 ± 0.60 | 14.65 ± 0.15 |
| Hematocrit (%) | 41.80 ± 1.80 | 43.55 ± 1.45 |
| Eosinophils (%) | 1.50 ± 0.50 | 1.00 ± 0.00 |
| Basophil (%) | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Monocytes (%) | 1.00 ± 0.00 | 2.00 ± 0.00 * |
| Segmented (%) | 20.50 ± 8.50 | 17.00 ± 4.00 |
| Lymphocytes (%) | 77.00 ± 9.00 | 80.50 ± 4.50 |
| Platelets (×103/mm3) | 7.5 ± 0.26 | 7.22 ± 0.22 |
| Female rats | n = 5 | n = 5 |
| RBC (×106/mm3) | 7.00 ± 0.10 | 6.93 ± 0.22 |
| White blood cells (×103/mm3) | 4.03 ± 0.18 | 4.20 ± 0.30 |
| Hemoglobin (g/dL) | 14.65 ± 0.25 | 14.60 ± 0.10 |
| Hematocrit (%) | 42.00 ± 0.00 | 41.50 ± 0.50 |
| Eosinophils (%) | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Basophil (%) | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Monocytes (%) | 2.50 ± 0.50 | 1.50 ± 0.50 * |
| Segmented (%) | 24.00 ± 1.00 | 17.00 ± 1.00 |
| Lymphocytes (%) | 79.00 ± 1.00 | 80.50 ± 0.50 |
| Platelets (×103/mm3) | 7.3 ± 0.20 | 7.6 ± 0.35 |
| Compound | M.W. a | PSA b | n-Rot Bond (0–10) | n-ON (<10) c | n-OHNH d | Log Po/w e | LogKHSA f | Caco-2 g (nm/s) | App.MDCK (nm/s) h | % HIA i | % HOA j | Lipinski Rule of Five |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α-chaconine | 852.070 | 199.560 | 15 | 15 | 8 | −0.091 | −0.875 | <25 poor | <25 poor | <25poor | Low | 3 |
| α-solanine | 868.069 | 220.534 | 17 | 16 | 9 | −0.860 | −1.157 | <25 poor | <25 poor | <25poor | Low | 3 |
| Solanidine | 397.643 | 22.288 | 1 | 2 | 1 | 5.007 | 0.554 | 1049 | 576 | 100 | High | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velasco-Chong, J.R.; Herrera-Calderón, O.; Rojas-Armas, J.P.; Hañari-Quispe, R.D.; Figueroa-Salvador, L.; Peña-Rojas, G.; Andía-Ayme, V.; Yuli-Posadas, R.Á.; Yepes-Perez, A.F.; Aguilar, C. TOCOSH FLOUR (Solanum tuberosum L.): A Toxicological Assessment of Traditional Peruvian Fermented Potatoes. Foods 2020, 9, 719. https://doi.org/10.3390/foods9060719
Velasco-Chong JR, Herrera-Calderón O, Rojas-Armas JP, Hañari-Quispe RD, Figueroa-Salvador L, Peña-Rojas G, Andía-Ayme V, Yuli-Posadas RÁ, Yepes-Perez AF, Aguilar C. TOCOSH FLOUR (Solanum tuberosum L.): A Toxicological Assessment of Traditional Peruvian Fermented Potatoes. Foods. 2020; 9(6):719. https://doi.org/10.3390/foods9060719
Chicago/Turabian StyleVelasco-Chong, Jonas Roberto, Oscar Herrera-Calderón, Juan Pedro Rojas-Armas, Renán Dilton Hañari-Quispe, Linder Figueroa-Salvador, Gilmar Peña-Rojas, Vidalina Andía-Ayme, Ricardo Ángel Yuli-Posadas, Andres F. Yepes-Perez, and Cristian Aguilar. 2020. "TOCOSH FLOUR (Solanum tuberosum L.): A Toxicological Assessment of Traditional Peruvian Fermented Potatoes" Foods 9, no. 6: 719. https://doi.org/10.3390/foods9060719
APA StyleVelasco-Chong, J. R., Herrera-Calderón, O., Rojas-Armas, J. P., Hañari-Quispe, R. D., Figueroa-Salvador, L., Peña-Rojas, G., Andía-Ayme, V., Yuli-Posadas, R. Á., Yepes-Perez, A. F., & Aguilar, C. (2020). TOCOSH FLOUR (Solanum tuberosum L.): A Toxicological Assessment of Traditional Peruvian Fermented Potatoes. Foods, 9(6), 719. https://doi.org/10.3390/foods9060719






