In Vitro Bioaccessibility and Functional Properties of Phenolic Compounds from Enriched Beverages Based on Cocoa Bean Shell
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.2.1. Beverage Ingredients
2.2.2. Beverage Formulation
2.2.3. Beverage Preparation
2.3. Sensory Evaluation
2.4. In Vitro Simulated Gastrointestinal Digestion (GID)
2.5. Analytical Determinations
2.5.1. Total Phenolic Content (TPC) Assay
2.5.2. Radical Scavenging Activity (RSA) Assay
2.5.3. In Vitro α-Glucosidase Inhibition
2.5.4. Bioactive Compound Analyses by Reverse-Phase Liquid Chromatography
2.6. Statistical Analysis
3. Results and Discussion
3.1. Comparison of the Beverages Based on Consumer Tests
3.2. Total Phenolic Content (TPC) and Radical Scavenging Activity (RSA)
3.3. Bioaccessibility of Bioactive Compounds and Functional Characteristics
3.3.1. Determination of Polyphenol and Methylxanthine Composition of Undigested and Digested Beverages through RP-HPLC-PDA.
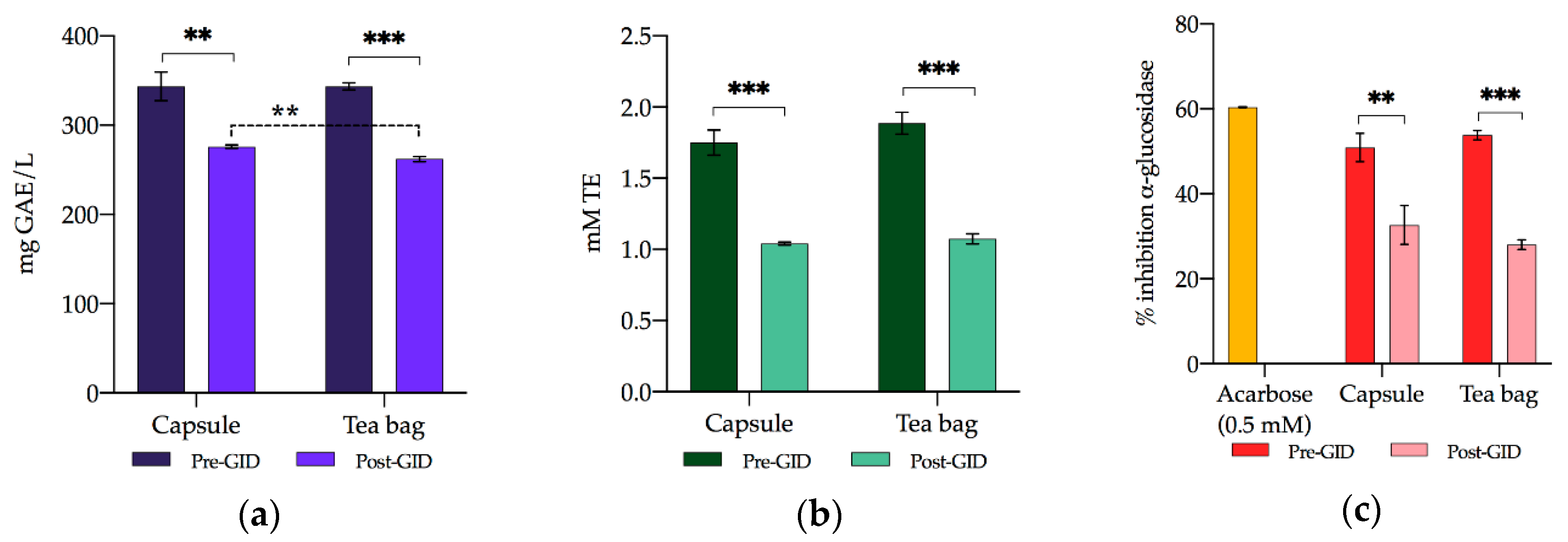
3.3.2. TPC
3.3.3. RSA
3.3.4. α-Glucosidase Inhibition Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Campos-Vega, R.; Nieto-Figueroa, K.H.; Oomah, B.D. Cocoa (Theobroma cacao L.) pod husk: Renewable source of bioactive compounds. Trends Food Sci. Technol. 2018, 81, 172–184. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Navarro, S.L.B.; Rodrigues, C.E.C. Cocoa shell and its compounds: Applications in the food industry. Trends Food Sci. Technol. 2017, 63, 103–112. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Zeppa, G.; Stévigny, C. Cocoa Bean Shell—A By-Product with Nutritional Properties and Biofunctional Potential. Nutrients 2020, 12, 1123. [Google Scholar] [CrossRef] [PubMed]
- Arlorio, M.; Coïsson, J.D.; Travaglia, F.; Varsaldi, F.; Miglio, G.; Lombardi, G.; Martelli, A. Antioxidant and biological activity of phenolic pigments from Theobroma cacao hulls extracted with supercritical CO2. Food Res. Int. 2005, 38, 1009–1014. [Google Scholar] [CrossRef]
- Mandrile, L.; Barbosa-Pereira, L.; Sorensen, K.M.; Giovannozzi, A.M.; Zeppa, G.; Engelsen, S.B.; Rossi, A.M. Authentication of cocoa bean shells by near- and mid-infrared spectroscopy and inductively coupled plasma-optical emission spectroscopy. Food Chem. 2019, 292, 47–57. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Rojo-Poveda, O.; Ferrocino, I.; Giordano, M.; Zeppa, G. Analytical dataset on volatile compounds of cocoa bean shells from different cultivars and geographical origins. Data Br. 2019, 25, 104268. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Rojo-Poveda, O.; Ferrocino, I.; Giordano, M.; Zeppa, G. Assessment of volatile fingerprint by HS-SPME/GC-qMS and E-nose for the classification of cocoa bean shells using chemometrics. Food Res. Int. 2019, 123, 684–696. [Google Scholar] [CrossRef]
- Wollgast, J.; Anklam, E. Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000, 33, 423–447. [Google Scholar] [CrossRef]
- Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Assessment of the antioxidant and antimutagenic activity of extracts from goji berry of Greek cultivation. Toxicol. Rep. 2018, 5, 251–257. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, C.; Zhang, B.; Huang, Q. The inhibitory effects of flavonoids on α-amylase and α-glucosidase. Crit. Rev. Food Sci. Nutr. 2020, 60, 695–708. [Google Scholar] [CrossRef]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.R.; Preedy, V.R.; Zibadi, S. Chocolate in Health and Nutrition; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Humana Press: Totowa, NJ, USA, 2013; ISBN 978-1-61779-802-3. [Google Scholar]
- Alminger, M.; Aura, A.-M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; McDougall, G.J.; Requena, T.; et al. In Vitro Models for Studying Secondary Plant Metabolite Digestion and Bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In Vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Quijano-Aviles, M.F.; Franco-Agurto, G.L.; Suárez-Quirumbay, K.B.; Barragán-Lucas, A.D.; Manzano-Santana, P.I. Linear programming formulation of a dairy drink made of cocoa, coffee and orange by-products. Emirates J. Food Agric. 2016, 28, 554–559. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Mateus-Reguengo, L.; Bertolino, M.; Stévigny, C.; Zeppa, G. Effects of particle size and extraction methods on cocoa bean shell functional beverage. Nutrients 2019, 11, 867. [Google Scholar] [CrossRef] [PubMed]
- Gloess, A.N.; Schönbächler, B.; Klopprogge, B.; D’Ambrosio, L.; Chatelain, K.; Bongartz, A.; Strittmatter, A.; Rast, M.; Yeretzian, C. Comparison of nine common coffee extraction methods: Instrumental and sensory analysis. Eur. Food Res. Technol. 2013, 236, 607–627. [Google Scholar] [CrossRef]
- Lim, J. Hedonic scaling: A review of methods and theory. Food Qual. Prefer. 2011, 22, 733–747. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static In Vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Guglielmetti, A.; Zeppa, G. Pulsed Electric Field Assisted Extraction of Bioactive Compounds from Cocoa Bean Shell and Coffee Silverskin. Food Bioprocess Technol. 2018, 11, 818–835. [Google Scholar] [CrossRef]
- Von Gadow, A.; Joubert, E.; Hansmann, C.F. Comparison of the Antioxidant Activity of Aspalathin with That of Other Plant Phenols of Rooibos Tea (Aspalathus linearis), α-Tocopherol, BHT, and BHA. J. Agric. Food Chem. 1997, 45, 632–638. [Google Scholar] [CrossRef]
- Ali, A.; Selamat, J.; Che Man, Y.B.; Suria, A.M. Effect of storage temperature on texture, polymorphic structure, bloom formation and sensory attributes of filled dark chocolate. Food Chem. 2001, 72, 491–497. [Google Scholar] [CrossRef]
- Iguttia, A.M.; Pereira, A.C.I.; Fabiano, L.; Silva, R.A.F.; Ribeiro, E.P. Substitution of ingredients by green coconut (Cocos nucifera L.) pulp in ice cream formulation. Procedia Food Sci. 2011, 1, 1610–1617. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Sawano, T.; Yazama, F.; Ito, H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim. Biophys. Acta Gen. Subj. 2011, 1810, 170–177. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Sanchez, L.; Caemmerer, B.; Kroh, L.W.; De Peña, M.P.; Cid, C. Extraction of coffee antioxidants: Impact of brewing time and method. Food Res. Int. 2012, 48, 57–64. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’Hiri, N.; Garcia-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Rossin, D.; Barbosa-Pereira, L.; Iaia, N.; Testa, G.; Sottero, B.; Poli, G.; Zeppa, G.; Biasi, F. A Dietary Mixture of Oxysterols Induces In Vitro Intestinal Inflammation through TLR2/4 Activation: The Protective Effect of Cocoa Bean Shells. Antioxidants 2019, 8, 151. [Google Scholar] [CrossRef]
- Mendes, T.M.N.; Murayama, Y.; Yamaguchi, N.; Sampaio, G.R.; Fontes, L.C.B.; da Silva Torres, E.A.F.; Tamura, H.; Yonekura, L. Guaraná (Paullinia cupana) catechins and procyanidins: Gastrointestinal/colonic bioaccessibility, Caco-2 cell permeability and the impact of macronutrients. J. Funct. Foods 2019, 55, 352–361. [Google Scholar] [CrossRef]
- Silva, C.P.; Sampaio, G.R.; Freitas, R.A.M.S.; Torres, E.A.F.S. Polyphenols from guaraná after In Vitro digestion: Evaluation of bioacessibility and inhibition of activity of carbohydrate-hydrolyzing enzymes. Food Chem. 2018, 267, 405–409. [Google Scholar] [CrossRef]
- Shim, S.M.; Yoo, S.H.; Ra, C.S.; Kim, Y.K.; Chung, J.O.; Lee, S.J. Digestive stability and absorption of green tea polyphenols: Influence of acid and xylitol addition. Food Res. Int. 2012, 45, 204–210. [Google Scholar] [CrossRef]
- Raab, T.; Barron, D.; Arce Vera, F.; Crespy, V.; Oliveira, M.; Williamson, G. Catechin Glucosides: Occurrence, synthesis, and stability. J. Agric. Food Chem. 2010, 58, 2138–2149. [Google Scholar] [CrossRef]
- Pešić, M.B.; Milinčić, D.D.; Kostić, A.Ž.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Popović, D.A.; et al. In Vitro digestion of meat- and cereal-based food matrix enriched with grape extracts: How are polyphenol composition, bioaccessibility and antioxidant activity affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef]
- Gültekin-Özgüven, M.; Berktaş, I.; Özçelik, B. Change in stability of procyanidins, antioxidant capacity and in-vitro bioaccessibility during processing of cocoa powder from cocoa beans. LWT Food Sci. Technol. 2016, 72, 559–565. [Google Scholar] [CrossRef]
- Rios, L.Y.; Bennett, R.N.; Lazarus, S.A.; Rémésy, C.; Scalbert, A.; Williamson, G. Cocoa procyanidins are stable during gastric transit in humans. Am. J. Clin. Nutr. 2002, 76, 1106–1110. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. Change of phenolics, carotenoids, and antioxidant capacity following simulated gastrointestinal digestion and dialysis of selected edible green leaves. Food Chem. 2018, 245, 371–379. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration Spilling the Beans: How Much Caffeine is Too Much? Available online: https://www.fda.gov/consumers/consumer-updates/spilling-beans-how-much-caffeine-too-much (accessed on 29 January 2020).
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA) Scientific Opinion on the safety of caffeine. EFSA J. 2015, 13. [CrossRef]
- Silano, V.; Bolognesi, C.; Castle, L.; Cravedi, J.; Engel, K.; Fowler, P.; Franz, R.; Grob, K.; Gürtler, R.; Husøy, T.; et al. Scientific Opinion on Flavouring Group Evaluation 49, Revision 1 (FGE.49Rev1): Xanthine alkaloids from the priority list. EFSA J. 2017, 15, 1–55. [Google Scholar] [CrossRef]
- Lester, G.E.; Lewers, K.S.; Medina, M.B.; Saftner, R.A. Comparative analysis of strawberry total phenolics via Fast Blue BB vs. Folin–Ciocalteu: Assay interference by ascorbic acid. J. Food Compos. Anal. 2012, 27, 102–107. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Cheng, C.-W.; Liang, J.-Y. Effect of esterification condensation on the Folin–Ciocalteu method for the quantitative measurement of total phenols. Food Chem. 2015, 170, 10–15. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
- Zengin, G.; Karanfil, A.; Uren, M.C.; Kocak, M.S.; Sarikurkcu, C.; Gungor, H.; Nancy Picot, C.M.; Mahomoodally, M.F. Phenolic content, antioxidant and enzyme inhibitory capacity of two Trametes species. RSC Adv. 2016, 6, 73351–73357. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (In Vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.Á.; Goya, L.; Ramos, S. Antidiabetic actions of cocoa flavanols. Mol. Nutr. Food Res. 2016, 60, 1756–1769. [Google Scholar] [CrossRef] [PubMed]
| Formulation | CBS | CB | Turmeric | Curry | Vanillin | Rooibos | Coconut | Mint | Cinnamon | Licorice |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 97 | 3 | ||||||||
| B1 | 94 | 3 | 1.5 | 1.5 | ||||||
| B2 | 91 | 3 | 3 | 3 | ||||||
| C1 | 96.6 | 3 | 0.4 | |||||||
| C2 | 96.1 | 3 | 0.9 | |||||||
| D1 | 92 | 3 | 5 | |||||||
| D2 | 87 | 3 | 10 | |||||||
| E1 | 87 | 3 | 10 | |||||||
| E2 | 77 | 3 | 20 | |||||||
| F1 | 95.5 | 3 | 1.5 | |||||||
| F2 | 94 | 3 | 3 | |||||||
| G1 | 95.4 | 3 | 0.8 | 0.8 | ||||||
| G2 | 93.8 | 3 | 1.6 | 1.6 |
| Extraction Technique | Formulation | Appearance | Odor | Taste | Flavor | Overall Liking | Purchase Interest |
|---|---|---|---|---|---|---|---|
| Capsule | A | 2672 | 2773 abc | 2777 abc | 2685 abc | 2717 ab | 3078 ab |
| B1 | 2753 | 2060 c | 1700 c | 2261 bc | 2021 b | 2050 b | |
| C2 | 2803 | 3848 a | 2629 abc | 2407 abc | 2740 ab | 2627 ab | |
| D2 | 3309 | 2577 bc | 3082 ab | 2994 abc | 2921 ab | 3090 ab | |
| E2 | 3170 | 3533 ab | 3652 a | 3607 a | 3775 a | 3635 a | |
| F1 | 2166 | 1813 c | 2343 bc | 2126 c | 2305 b | 2153 b | |
| G1 | 2435 | 2311 c | 3123 ab | 3227 ab | 2829 ab | 2674 ab | |
| Sig. | n.s. | *** | *** | ** | *** | *** | |
| Tea bag | A | 2814 | 3134 ab | 2175 b | 2149 b | 2304 bcd | 2091 b |
| B1 | 2627 | 1666 c | 2012 b | 2154 b | 1985 d | 2197 b | |
| C2 | 2027 | 3866 a | 3278 ab | 3163 ab | 3406 ab | 3689 a | |
| D2 | 2831 | 1959 bc | 2562 b | 2411 b | 2267 bcd | 2257 b | |
| E2 | 3394 | 3809 a | 3935 a | 3985 a | 3965 a | 3995 a | |
| F1 | 2802 | 1707 c | 2260 b | 2256 b | 2098 cd | 2235 b | |
| G1 | 2813 | 3166 ab | 3085 ab | 2798 b | 3282 abc | 2843 ab | |
| Sig. | n.s. | *** | *** | *** | *** | *** |
| Formulation | Capsule | Tea bag | Sig. | |
|---|---|---|---|---|
| TPC (mg GAE/L) | A | 384.98 ± 13.19 bc | 325.94 ± 26.43 c | * |
| B1 | 599.64 ± 62.14 a | 306.33 ± 12.86 c | ** | |
| C2 | 551.06 ± 50.37 a | 586.20 ± 57.27 a | n.s. | |
| D2 | 455.18 ± 24.43 b | 476.93 ± 79.30 b | n.s. | |
| E2 | 343.57 ± 15.46 c | 343.58 ± 4.77 c | n.s. | |
| F1 | 431.55 ± 65.07 b | 339.39 ± 64.64 c | n.s. | |
| G1 | 600.48 ± 25.22 a | 320.37 ± 28.43 c | *** | |
| Sig. | *** | *** | ||
| RSA (mM TE/L) | A | 1.97 ± 0.05 bc | 1.78 ± 0.12 b | n.s. |
| B1 | 2.92 ± 0.30 a | 1.70 ± 0.05 b | ** | |
| C2 | 2.01 ± 0.18 bc | 1.88 ± 0.04 b | n.s. | |
| D2 | 2.31 ± 0.11 b | 2.53 ± 0.37 a | n.s. | |
| E2 | 1.75 ± 0.08 c | 1.88 ± 0.09 b | n.s. | |
| F1 | 2.21 ± 0.27 b | 1.96 ± 0.35 b | n.s. | |
| G1 | 2.99 ± 0.25 a | 1.81 ± 0.09 b | *** | |
| Sig. | *** | * |
| Extraction Method | Capsule | Tea Bag | Sig. | |
|---|---|---|---|---|
| Formulation | E2 | E2 | ||
| Methylxanthines (mg/L of beverage) | ||||
| Theobromine | PRE | 172.09 ± 4.55 | 144.36 ± 18.83 | n.s. |
| POST | 185.81 ± 27.59 | 150.70 ± 21.29 | n.s. | |
| Sig. | n.s. | n.s. | ||
| Caffeine | PRE | 40.90 ± 2.02 | 27.57 ± 4.71 | * |
| POST | 38.11 ± 5.62 | 25.97 ± 4.08 | * | |
| Sig. | n.s. | n.s. | ||
| Polyphenols (mg/L of beverage) | ||||
| Phenolic acids | ||||
| Protocatechuic acid | PRE | 2.35 ± 0.14 | 1.93 ± 0.18 | n.s. |
| POST | 0.90 ± 0.07 | 0.72 ± 0.08 | * | |
| Sig. | *** | *** | ||
| Caffeic acid | PRE | 1.81 ± 0.14 | 1.51 ± 0.34 | n.s. |
| POST | 0.40 ± 0.04 | 0.33 ± 0.02 | n.s. | |
| Sig. | *** | ** | ||
| Flavan-3-ols | ||||
| Catechin-3-O-glucoside | PRE | 2.37 ± 0.08 | 1.94 ± 0.18 | ** |
| POST | 1.12 ± 0.13 | 1.08 ± 0.15 | * | |
| Sig. | *** | ** | ||
| Catechin | PRE | 2.04 ± 0.11 | 1.65 ± 0.15 | ** |
| POST | 2.55 ± 0.25 | 2.71 ± 0.29 | n.s. | |
| Sig. | ** | *** | ||
| Epicatechin | PRE | 4.64 ± 0.30 | 3.94 ± 1.17 | n.s. |
| POST | 0.48 ± 0.22 | 0.47 ± 0.24 | n.s. | |
| Sig. | *** | ** | ||
| Procyanidins type B | ||||
| Procyanidin B isomer (PCB) | PRE | 4.92 ± 0.56 | 4.41 ± 0.59 | n.s. |
| POST | 4.21 ± 0.34 | 3.53 ± 0.49 | n.s. | |
| Sig. | n.s. | n.s. | ||
| Procyanidin B2 (PCB2) | PRE | 6.36 ± 0.71 | 5.21 ± 1.22 | n.s. |
| POST | 2.79 ± 0.38 | 2.72 ± 0.39 | n.s. | |
| Sig. | ** | * | ||
| Flavonols | ||||
| Quercetin-3-O-glucoside | PRE | 0.24 ± 0.03 | 0.23 ± 0.05 | n.s. |
| POST | n.d. | n.d. | n/a | |
| Sig. | n/a | n/a | ||
| Quercetin-3-O-rhamnoside | PRE | 0.21 ± 0.02 | 0.18 ± 0.03 | n.s. |
| POST | n.d. | n.d. | n/a | |
| Sig. | n/a | n/a | ||
| Extraction Method | Capsule | Tea Bag | Sig. | |
|---|---|---|---|---|
| Formulation | E2 | E2 | ||
| Methylxanthines (mg/g of filling) | ||||
| Theobromine | PRE | 2.95 ± 0.08 | 4.81 ± 0.63 | ** |
| POST | 3.19 ± 0.47 | 5.02 ± 0.71 | * | |
| Sig. | n.s. | n.s. | ||
| Caffeine | PRE | 0.70 ± 0.03 | 0.92 ± 0.16 | n.s. |
| POST | 0.65 ± 0.10 | 0.87 ± 0.14 | n.s. | |
| Sig. | n.s. | n.s. | ||
| Polyphenols (μg/g of filling) | ||||
| Phenolic acids | ||||
| Protocatechuic acid | PRE | 40.35 ± 2.44 | 64.44 ± 7.53 | ** |
| POST | 15.38 ± 1.20 | 24.09 ± 2.53 | ** | |
| Sig. | *** | ** | ||
| Caffeic acid | PRE | 30.96 ± 2.42 | 50.25 ± 11.24 | * |
| POST | 6.86 ± 0.64 | 11.08 ± 0.68 | ** | |
| Sig. | *** | ** | ||
| Flavan-3-ols | ||||
| Catechin-3-O-glucoside | PRE | 40.62 ± 1.30 | 64.74 ± 5.92 | ** |
| POST | 19.27 ± 2.20 | 36.07 ± 4.98 | ** | |
| Sig. | *** | ** | ||
| Catechin | PRE | 34.93 ± 1.86 | 55.06 ± 4.90 | ** |
| POST | 43.70 ± 4.37 | 90.26 ± 9.55 | *** | |
| Sig. | ** | *** | ||
| Epicatechin | PRE | 79.58 ± 5.12 | 131.18 ± 38.99 | n.s. |
| POST | 8.25 ± 3.83 | 15.75 ± 8.04 | * | |
| Sig. | *** | ** | ||
| ProcyanidinsB | ||||
| Type B procyanidin | PRE | 84.31 ± 9.55 | 146.94 ± 19.61 | ** |
| POST | 72.10 ± 5.86 | 117.58 ± 16.40 | ** | |
| Sig. | n.s. | n.s. | ||
| Procyanidin B2 | PRE | 109.08 ± 12.18 | 173.58 ± 40.67 | * |
| POST | 47.90 ± 6.49 | 90.58 ± 12.98 | ** | |
| Sig. | ** | * | ||
| Flavonols | ||||
| Quercetin-3-O-glucoside | PRE | 4.19 ± 0.52 | 7.62 ± 1.66 | * |
| POST | n.d. | n.d. | n/a | |
| Sig. | n/a | n/a | ||
| Quercetin-3-O-rhamnoside | PRE | 3.60 ± 0.28 | 5.96 ± 0.86 | * |
| POST | n.d. | n.d. | n/a | |
| Sig. | n/a | n/a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantele, C.; Rojo-Poveda, O.; Bertolino, M.; Ghirardello, D.; Cardenia, V.; Barbosa-Pereira, L.; Zeppa, G. In Vitro Bioaccessibility and Functional Properties of Phenolic Compounds from Enriched Beverages Based on Cocoa Bean Shell. Foods 2020, 9, 715. https://doi.org/10.3390/foods9060715
Cantele C, Rojo-Poveda O, Bertolino M, Ghirardello D, Cardenia V, Barbosa-Pereira L, Zeppa G. In Vitro Bioaccessibility and Functional Properties of Phenolic Compounds from Enriched Beverages Based on Cocoa Bean Shell. Foods. 2020; 9(6):715. https://doi.org/10.3390/foods9060715
Chicago/Turabian StyleCantele, Carolina, Olga Rojo-Poveda, Marta Bertolino, Daniela Ghirardello, Vladimiro Cardenia, Letricia Barbosa-Pereira, and Giuseppe Zeppa. 2020. "In Vitro Bioaccessibility and Functional Properties of Phenolic Compounds from Enriched Beverages Based on Cocoa Bean Shell" Foods 9, no. 6: 715. https://doi.org/10.3390/foods9060715
APA StyleCantele, C., Rojo-Poveda, O., Bertolino, M., Ghirardello, D., Cardenia, V., Barbosa-Pereira, L., & Zeppa, G. (2020). In Vitro Bioaccessibility and Functional Properties of Phenolic Compounds from Enriched Beverages Based on Cocoa Bean Shell. Foods, 9(6), 715. https://doi.org/10.3390/foods9060715









