Liquid Chromatographic Determination of Biogenic Amines in Fish Based on Pyrene Sulfonyl Chloride Pre-Column Derivatization
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Instrumentation
2.3. Fish Samples
2.4. Method Development
2.4.1. Extraction of Biogenic Amines
2.4.2. Optimization of Derivatization of Biogenic Amines–Final Protocol
2.4.3. HPLC-UV-FLD Analysis
2.4.4. LC-MS Analysis
2.5. Method Validation
3. Results and Discussion
3.1. Liquid Chromatographic Separation
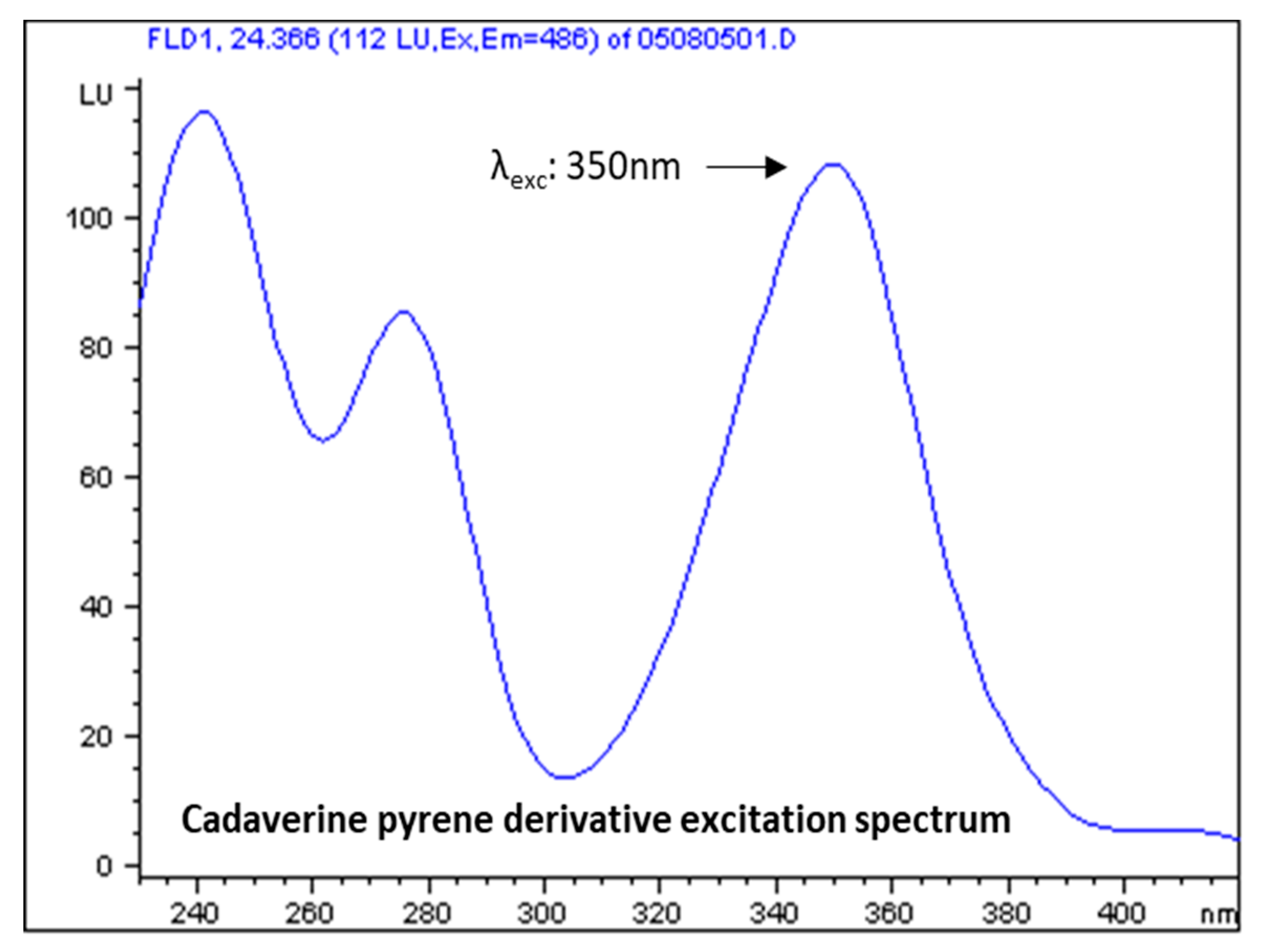
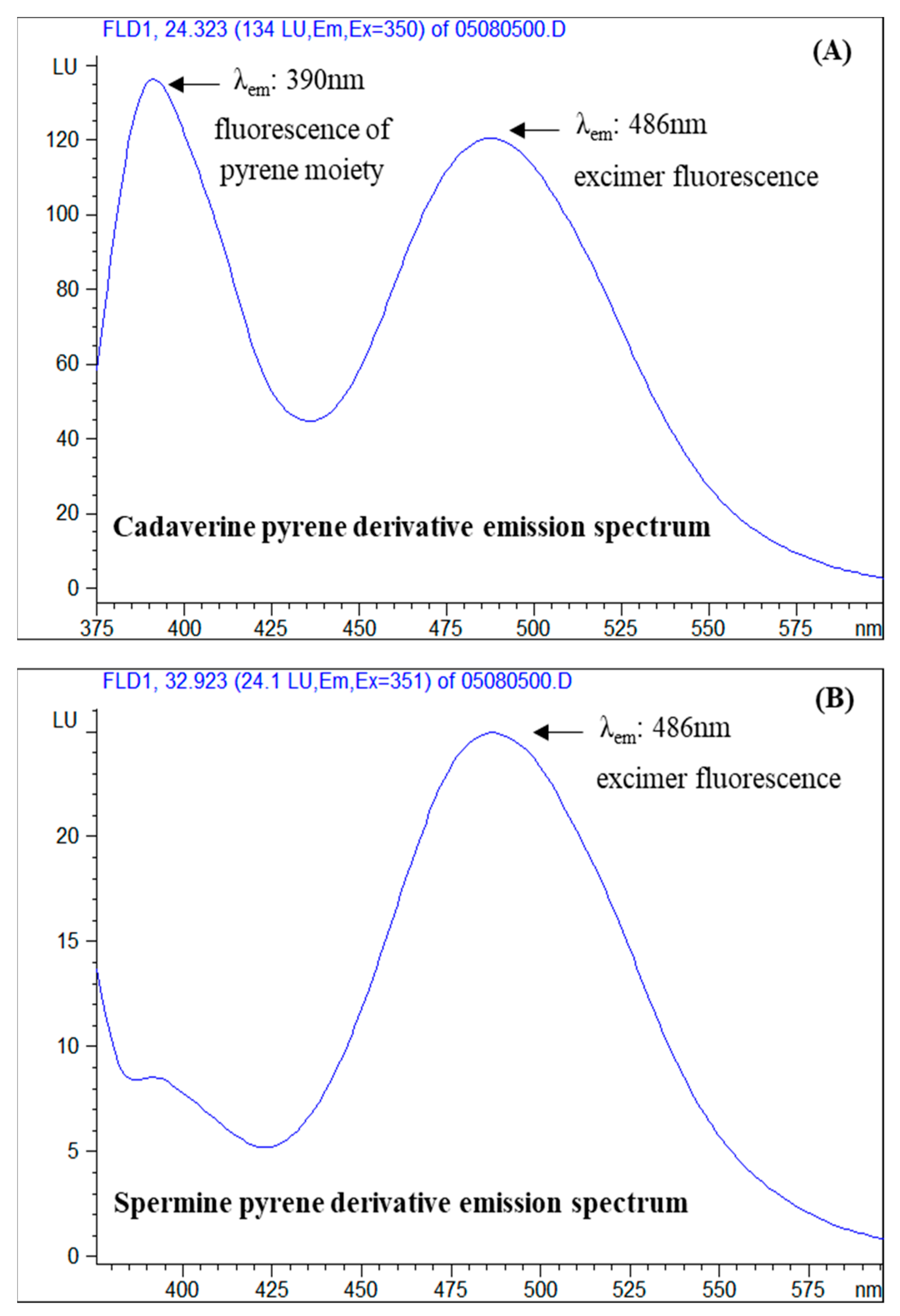
3.2. Selection of Excitation and Emission Wavelengths
3.3. Optimization of the Derivatization Procedure
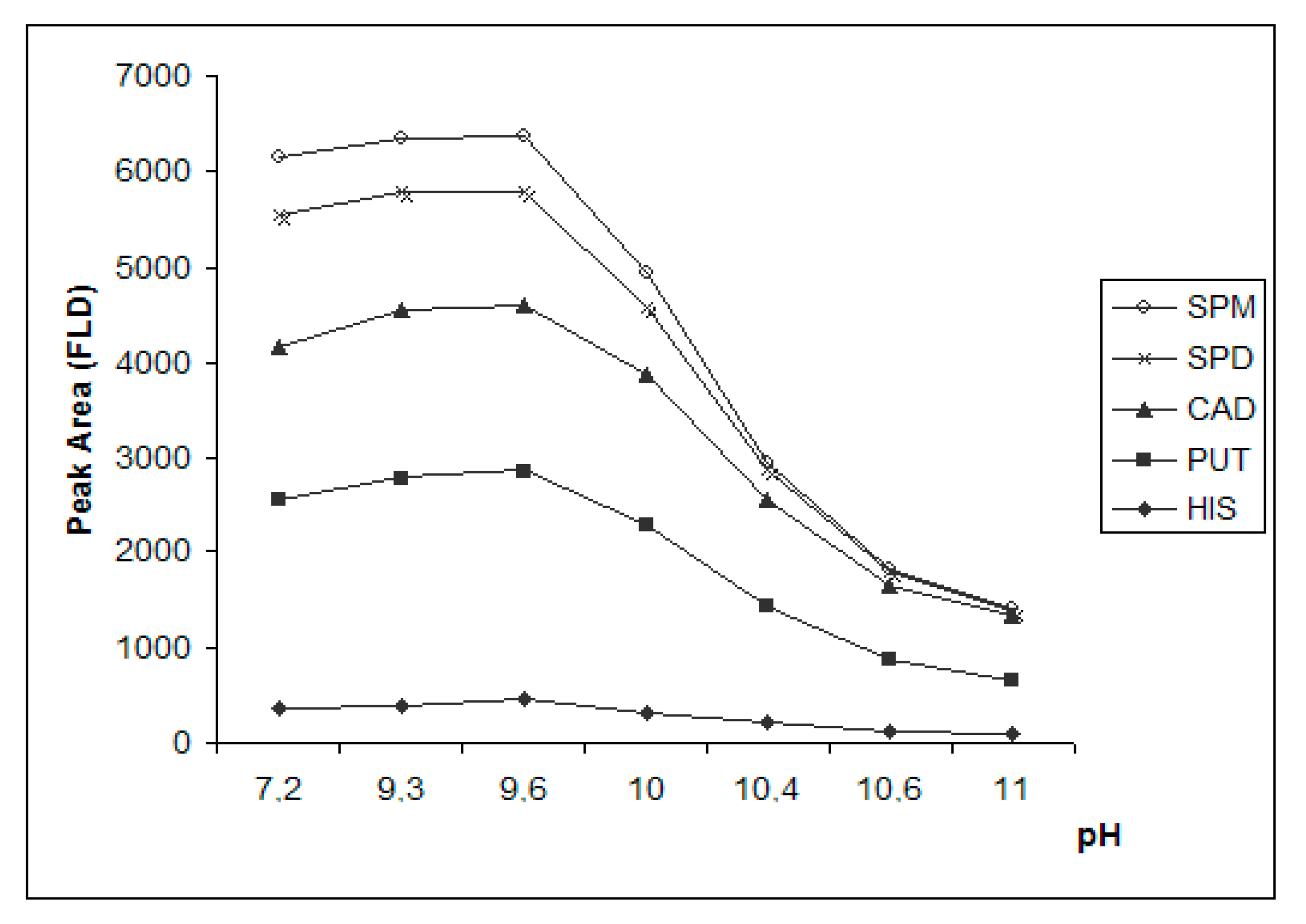
3.3.1. Effect of pH
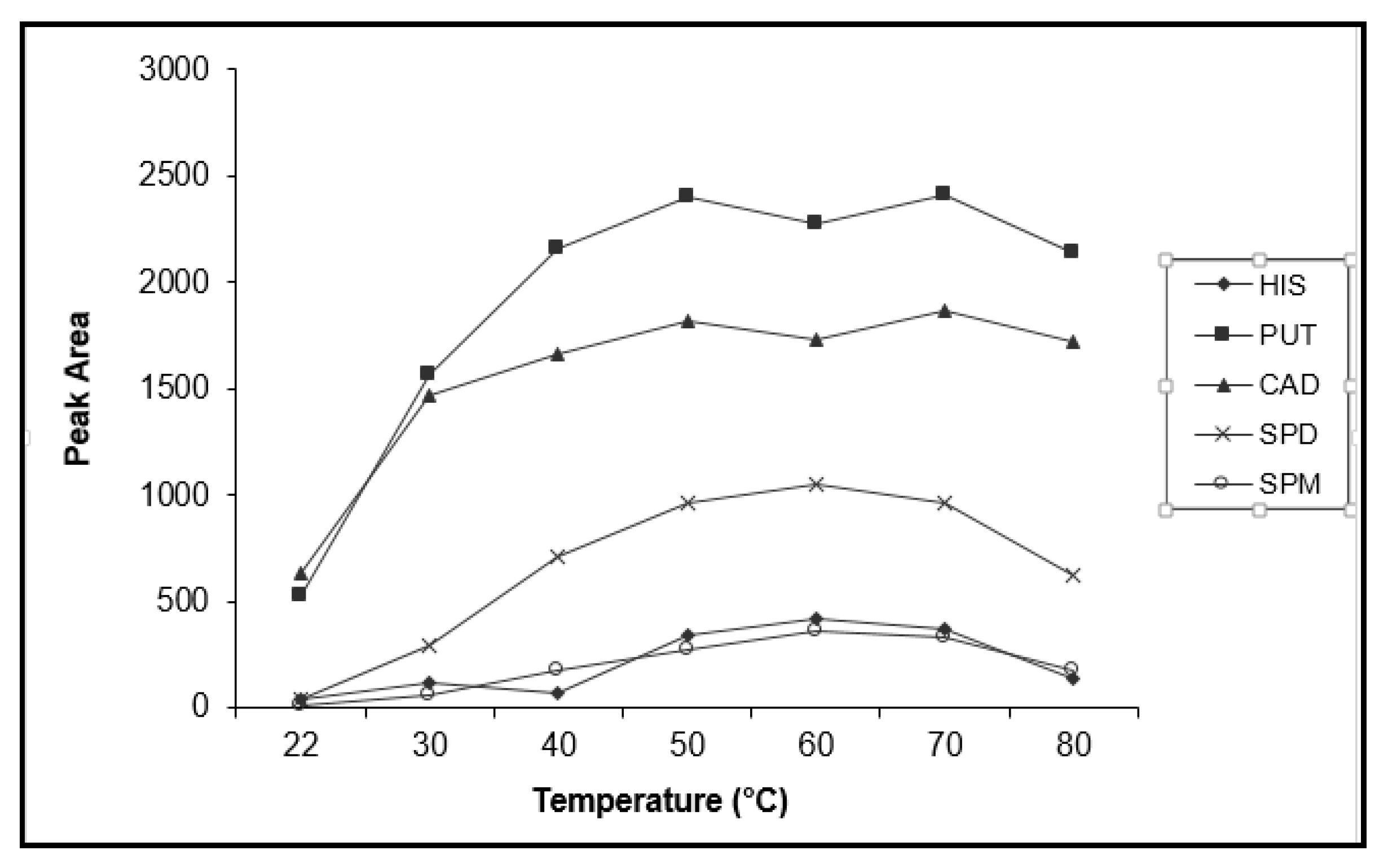
3.3.2. Effect of Temperature and Time
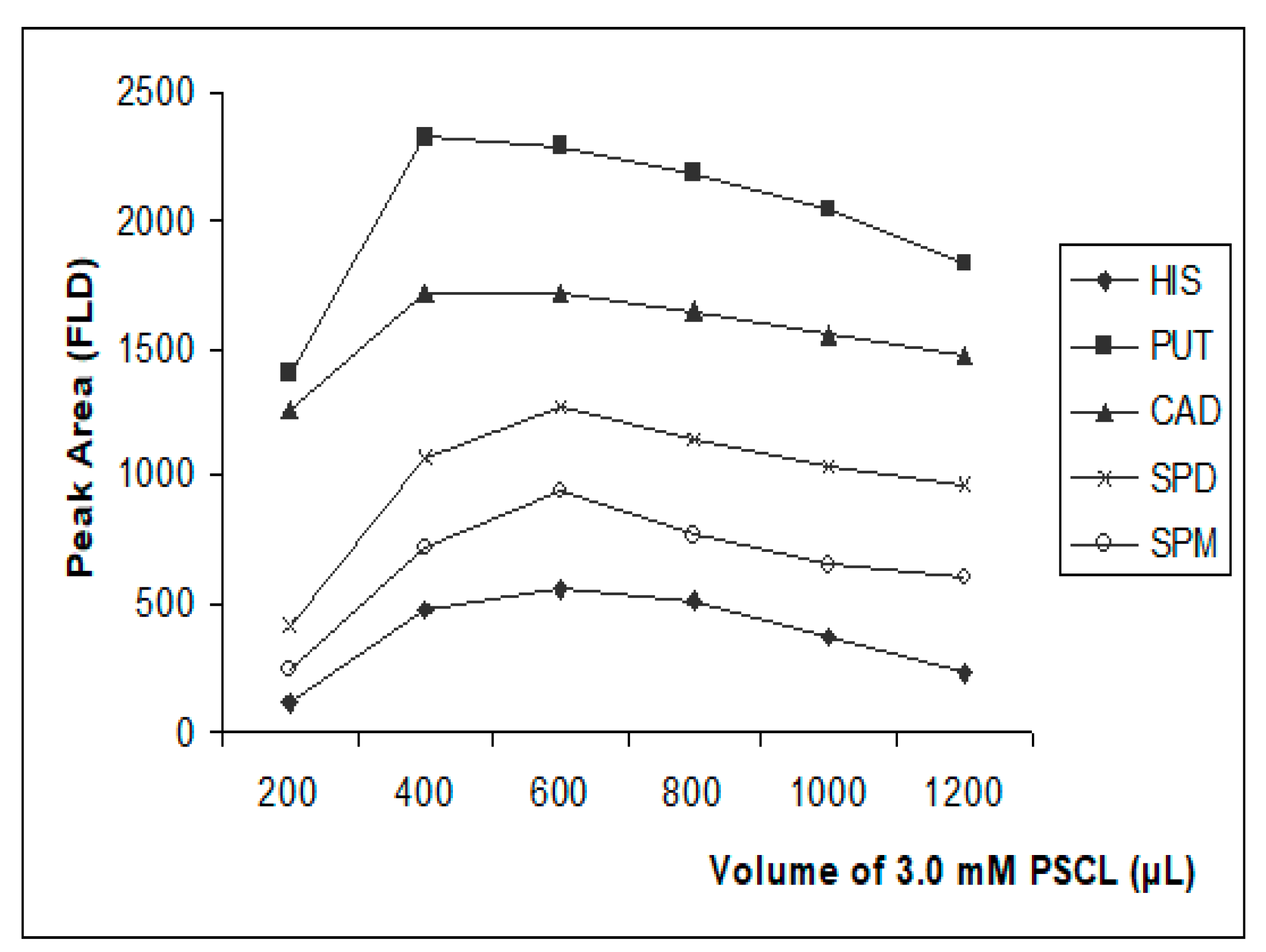
3.3.3. Effect of Derivatization Reagent Amount
3.4. Stability of the Derivatives
3.5. LC-MS Identification of Biogenic Amine Derivatives
3.6. Method Performance
3.7. Concentration Levels of Biogenic Amines in Fish Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baixas-Nogueras, S.; Bover-Cid, S.; Veciana-Nogués, M.T.; Mariné-Font, A.; Vidal-Carou, M.C. Biogenic Amine Index for Freshness Evaluation in Iced Mediterranean Hake (Merluccius merluccius). J. Food Prot. 2005, 68, 2433–2438. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of Biogenic Amines on Food Quality and Safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Doeun, D.; Davaatseren, M.; Chung, M. Biogenic amines in foods. Food Sci. Biotechnol. 2017, 26, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific Opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef]
- Mietz, J.L. Chemical quality index of canned tuna as determined by high-pressure liquid chromatography. J. Food Sci. 1977, 42, 155–158. [Google Scholar] [CrossRef]
- Shalaby, A.R. Significance of biogenic amines to food safety and human health. Food Res. Int. 1996, 29, 7–675. [Google Scholar] [CrossRef]
- Bulushi, I.A.; Poole, S.; Deeth, H.C.; Dykes, G.A. Biogenic Amines in Fish: Roles in Intoxication, Spoilage, and Nitrosamine Formation—A Review. Crit. Rev. Food Sci. Nutr. 2009, 49, 369–377. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 338, 1–26.
- Fish and Fishery Products Hazards and Controls Guidance. Available online: https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/Seafood/ucm2018426.htm (accessed on 17 March 2020).
- Zhang, N.; Wang, H.; Zhang, Z.X.; Deng, Y.H.; Zhang, H.S. Sensitive determination of biogenic amines by capillary electrophoresis with a new fluorogenic reagent 3-(4-fluorobenzoyl)-2-quinolinecarboxaldehyde. Talanta 2008, 76, 791–797. [Google Scholar] [CrossRef]
- An, D.; Chen, Z.; Zheng, J.; Chen, S.; Wang, L.; Huang, Z.; Weng, L. Determination of biogenic amines in oysters by capillary electrophoresis coupled with electrochemiluminescence. Food Chem. 2015, 168, 1–6. [Google Scholar] [CrossRef]
- Daniel, D.; Dos Santos, V.B.; Vidal, D.T.; do Lago, C.L. Determination of biogenic amines in beer and wine by capillary electrophoresis-tandem mass spectrometry. J. Chromatogr. A 2015, 1416, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Plotka-Wasylka, J.; Simeonov, V.; Namiesnik, J. An in situ derivatization - dispersive liquid-liquid microextraction combined with gas-chromatography - mass spectrometry for determining biogenic amines in home-made fermented alcoholic drinks. J. Chromatogr. A 2016, 1453, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Faria, M.A.; Fernandes, J.O. Gas chromatography-mass spectrometry assessment of amines in Port wine and grape juice after fast chloroformate extraction/derivatization. J. Agric. Food Chem. 2011, 59, 8742–8753. [Google Scholar] [CrossRef] [PubMed]
- De Borba, B.M.; Rohrer, J.S. Determination of biogenic amines in alcoholic beverages by ion chromatography with suppressed conductivity detection and integrated pulsed amperometric detection. J. Chromatogr. A 2007, 1155, 22–30. [Google Scholar] [CrossRef]
- Tao, Z.; Sato, M.; Han, Y.; Tan, Z.; Yamaguchi, T.; Nakano, T. A simple and rapid method for histamine analysis in fish and fishery products by TLC determination. Food Control. 2011, 22, 1154–1157. [Google Scholar] [CrossRef]
- Dadáková, E.; Křížek, M.; Pelikánová, T. Determination of biogenic amines in foods using ultra-performance liquid chromatography (UPLC). Food Chem. 2009, 116, 365–370. [Google Scholar] [CrossRef]
- Costa, M.P.; Balthazar, C.F.; Rodrigues, B.L.; Lazaro, C.A.; Silva, A.C.; Cruz, A.G.; Conte Junior, C.A. Determination of biogenic amines by high-performance liquid chromatography (HPLC-DAD) in probiotic cow’s and goat’s fermented milks and acceptance. Food Sci. Nutr. 2015, 3, 172–178. [Google Scholar] [CrossRef]
- Liu, S.J.; Xu, J.J.; Ma, C.L.; Guo, C.F. A comparative analysis of derivatization strategies for the determination of biogenic amines in sausage and cheese by HPLC. Food Chem. 2018, 266, 275–283. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Ye, D.Q.; Zhu, B.Q.; Wu, G.F.; Duan, C.Q. Rapid HPLC analysis of amino acids and biogenic amines in wines during fermentation and evaluation of matrix effect. Food Chem. 2014, 163, 6–15. [Google Scholar] [CrossRef]
- Mayer, H.K.; Fiechter, G.; Fischer, E. A new ultra-pressure liquid chromatography method for the determination of biogenic amines in cheese. J. Chromatogr. A 2010, 1217, 3251–3257. [Google Scholar] [CrossRef]
- Lázaro, C.A.; Conte-Júnior, C.A.; Cunha, F.L.; Mársico, E.T.; Mano, S.B.; Franco, R.M. Validation of an HPLC Methodology for the Identification and Quantification of Biogenic Amines in Chicken Meat. Food Anal. Method 2013, 6, 1024–1032. [Google Scholar] [CrossRef]
- Jia, S.; Ryu, Y.; Kwon, S.W.; Lee, J. An in situ benzoylation-dispersive liquid-liquid microextraction method based on solidification of floating organic droplets for determination of biogenic amines by liquid chromatography-ultraviolet analysis. J. Chromatogr. A 2013, 1282, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Redruello, B.; Ladero, V.; Cuesta, I.; Alvarez-Buylla, J.R.; Martin, M.C.; Fernandez, M.; Alvarez, M.A. A fast, reliable, ultra high performance liquid chromatography method for the simultaneous determination of amino acids, biogenic amines and ammonium ions in cheese, using diethyl ethoxymethylenemalonate as a derivatising agent. Food Chem. 2013, 139, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- De Mey, E.; Drabik-Markiewicz, G.; De Maere, H.; Peeters, M.C.; Derdelinckx, G.; Paelinck, H.; Kowalska, T. Dabsyl derivatisation as an alternative for dansylation in the detection of biogenic amines in fermented meat products by reversed phase high performance liquid chromatography. Food Chem. 2012, 130, 1017–1023. [Google Scholar] [CrossRef]
- Kang, L.; You, J.; Sun, Z.; Wang, C.; Ji, Z.; Gao, Y.; Suo, Y.; Li, Y. LC Determination of Trace Biogenic Amines in Foods Samples with Fluorescence Detection and MS Identification. Chromatographia 2011, 73, 43–50. [Google Scholar] [CrossRef]
- Tahmouzi, S.; Khaksar, R.; Ghasemlou, M. Development and validation of an HPLC-FLD method for rapid determination of histamine in skipjack tuna fish (Katsuwonus pelamis). Food Chem. 2011, 126, 756–761. [Google Scholar] [CrossRef]
- Wu, H.; Li, G.; Liu, S.; Ji, Z.; Zhang, Q.; Hu, N.; Suo, Y.; You, J. Simultaneous Determination of Seven Biogenic Amines in Foodstuff Samples Using One-Step Fluorescence Labeling and Dispersive Liquid–Liquid Microextraction Followed by HPLC-FLD and Method Optimization Using Response Surface Methodology. Food Anal. Method 2014, 8, 685–695. [Google Scholar] [CrossRef]
- Triki, M.; Jiménez-Colmenero, F.; Herrero, A.M.; Ruiz-Capillas, C. Optimisation of a chromatographic procedure for determining biogenic amine concentrations in meat and meat products employing a cation-exchange column with a post-column system. Food Chem. 2012, 130, 1066–1073. [Google Scholar] [CrossRef]
- Kelly, M.T.; Blaise, A.; Larroque, M. Rapid automated high performance liquid chromatography method for simultaneous determination of amino acids and biogenic amines in wine, fruit and honey. J. Chromatogr. A 2010, 1217, 7385–7392. [Google Scholar] [CrossRef]
- Sánchez, J.A.; Ruiz-Capillas, C. Application of the simplex method for optimization of chromatographic analysis of biogenic amines in fish. Eur. Food Res. Technol. 2011, 234, 285–294. [Google Scholar] [CrossRef]
- Zhao, Q.X.; Xu, J.; Xue, C.H.; Sheng, W.J.; Gao, R.C.; Xue, Y.; Li, Z.J. Determination of Biogenic Amines in Squid and White Prawn by High-Performance Liquid Chromatography with Postcolumn Derivatization. J. Agric. Food Chem. 2007, 55, 3083–3088. [Google Scholar] [CrossRef] [PubMed]
- Zotou, A.; Notou, M. Enhancing Fluorescence LC Analysis of Biogenic Amines in Fish Tissues by Precolumn Derivatization with Naphthalene-2,3-dicarboxaldehyde. Food Anal. Method 2012, 6, 89–99. [Google Scholar] [CrossRef]
- Li, G.; Dong, L.; Wang, A.; Wang, W.; Hu, N.; You, J. Simultaneous determination of biogenic amines and estrogens in foodstuff by an improved HPLC method combining with fluorescence labeling. LWT Food Sci. Technol. 2014, 55, 355–361. [Google Scholar] [CrossRef]
- Marks Rupp, H.S.; Anderson, C.R. Determination of putrescine and cadaverine in seafood (finfish and shellfish) by liquid chromatography using pyrene excimer fluorescence. J. Chromatogr. A 2005, 1094, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Lozanov, V.; Petrov, S.; Mitev, V. Simultaneous analysis of amino acid and biogenic polyamines by high-performance liquid chromatography after pre-column derivatization with N-(9-fluorenylmethoxycarbonyloxy)succinimide. J. Chromatogr. A 2004, 1025, 201–208. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Kłodzińska, E.; Namieśnik, J.; Płotka-Wasylka, J. Literature update of analytical methods for biogenic amines determination in food and beverages. TrAC Trends Anal. Chem. 2018, 98, 128–142. [Google Scholar] [CrossRef]
- Ochi, N. Simultaneous determination of eight underivatized biogenic amines in salted mackerel fillet by ion-pair solid-phase extraction and volatile ion-pair reversed-phase liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2019, 1601, 115–120. [Google Scholar] [CrossRef]
- Kaufmann, A.; Maden, K. Easy and Fast Method for the Determination of Biogenic Amines in Fish and Fish Products with Liquid Chromatography Coupled to Orbitrap Tandem Mass Spectrometry. J. AOAC Int. 2018, 101, 336–341. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, Y.; Zhou, Y.; Li, G.H.; Yang, W.Z.; Feng, X.S. A review of pretreatment and analytical methods of biogenic amines in food and biological samples since 2010. J. Chromatogr. A 2019, 1605, 360361. [Google Scholar] [CrossRef]
- Nishikawa, H.; Tabata, T.; Kitani, S. Simple Detection Method of Biogenic Amines in Decomposed Fish by Intramolecular Excimer Fluorescence. Food Nutr. Sci. 2012, 3, 1020–1026. [Google Scholar] [CrossRef]
- Nohta, H.; Satozono, H.; Koiso, K.; Yoshida, H.; Ishida, J.; Yamaguchi, M. Highly Selective Fluorometric Determination of Polyamines Based on Intramolecular Excimer-Forming Derivatization with a Pyrene Labeling Reagent. Anal. Chem. 2000, 72, 4199–4204. [Google Scholar] [CrossRef] [PubMed]
- Yoshitake, T.; Ichinose, F.; Yoshida, H.; Todoroki, K.; Kehr, J.; Inoue, O.; Nohta, H.; Yamaguchi, M. A sensitive and selective determination method of histamine by HPLC with intramolecular excimer-forming derivatization and fluorescence detection. Biomed. Chromatogr. 2003, 17, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Todoroki, K.; Ishii, Y.; Miyauchi, C.; Palee, A.; Min, J.Z.; Inoue, K.; Suzuki, K.; Toyo’oka, T. An easy-to-use excimer fluorescence derivatization reagent, 2-chloro-4-methoxy-6-(4-(pyren-4-yl)butoxy)-1,3,5-triazine, for use in the highly sensitive and selective liquid chromatography analysis of histamine in Japanese soy sauces. Anal. Chim. Acta 2015, 880, 145–151. [Google Scholar] [CrossRef] [PubMed]
- DeSilva, K.H.; Vest, F.B.; Kames, H.T. Pyrene Sulphonyl Chloride as a Reagent for Quantitation of Oestrogens in Human Serum Using HPLC with Conventional and Laser-Induced Fluorescence Detection. Biomed. Chromatogr. 1996, 10, 318–324. [Google Scholar] [CrossRef]
- Paiva, B.T.; Tominaga, M.; Paiva, M.C. Ionization of Histamine, N-Acetylhistamine, and Their Iodinated Derivatives. J. Med. Chem. 1970, 13, 689–692. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cadaverine#section=Dissociation-Constants (accessed on 8 April 2020).
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/1_4-Diaminobutane#section=pKa (accessed on 8 April 2020).
- DRUGBANK. Available online: https://www.drugbank.ca/drugs/DB03566 (accessed on 8 April 2020).
- DRUGBANK. Available online: https://www.drugbank.ca/drugs/DB00127 (accessed on 8 April 2020).
- Ly, D.; Mayrhofer, S.; Schmidt, J.-M.; Zitz, U.; Domig, K.J. Biogenic Amine Contents and Microbial Characteristics of Cambodian Fermented. Foods 2020, 9, 198. [Google Scholar] [CrossRef]
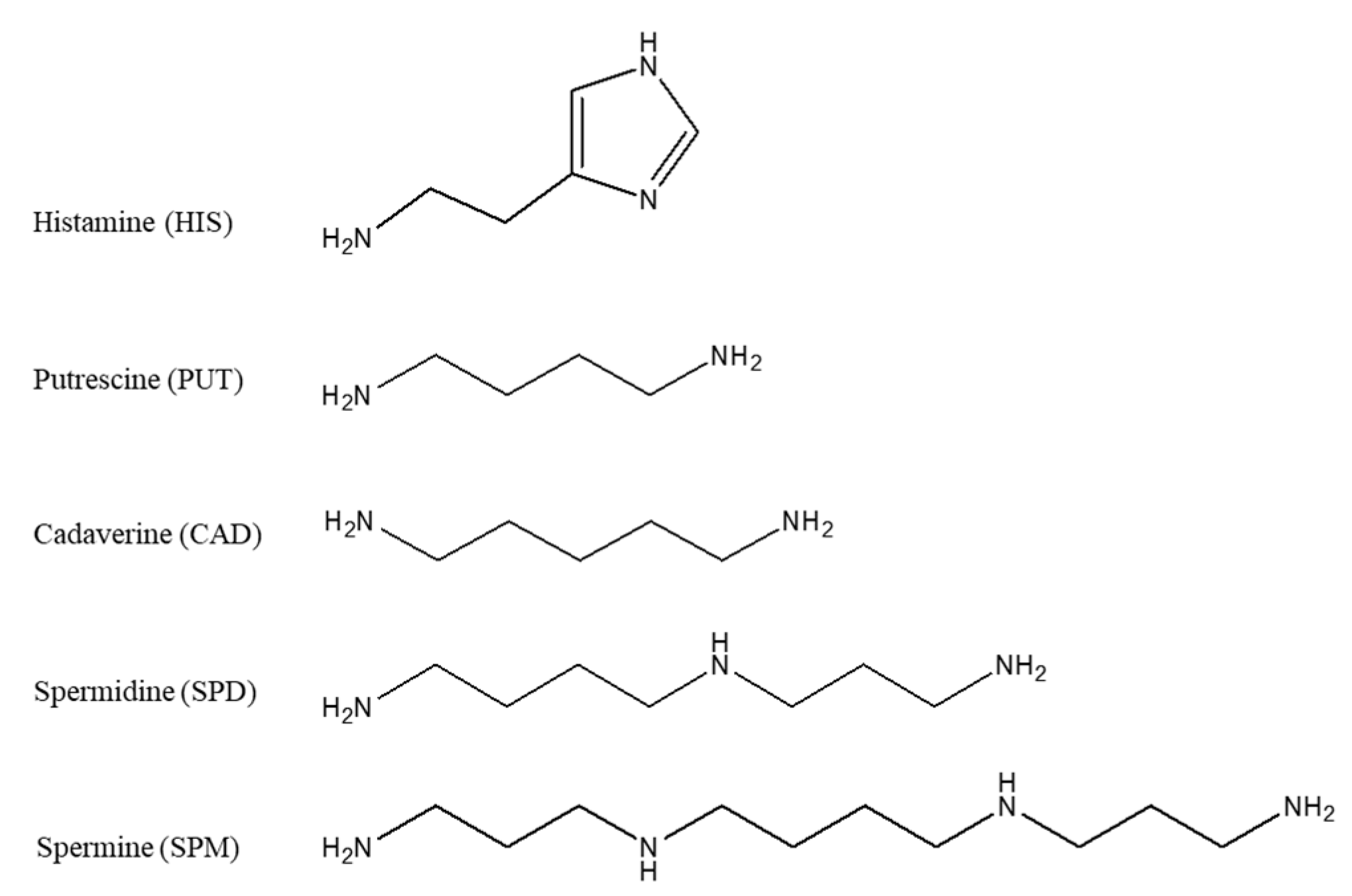
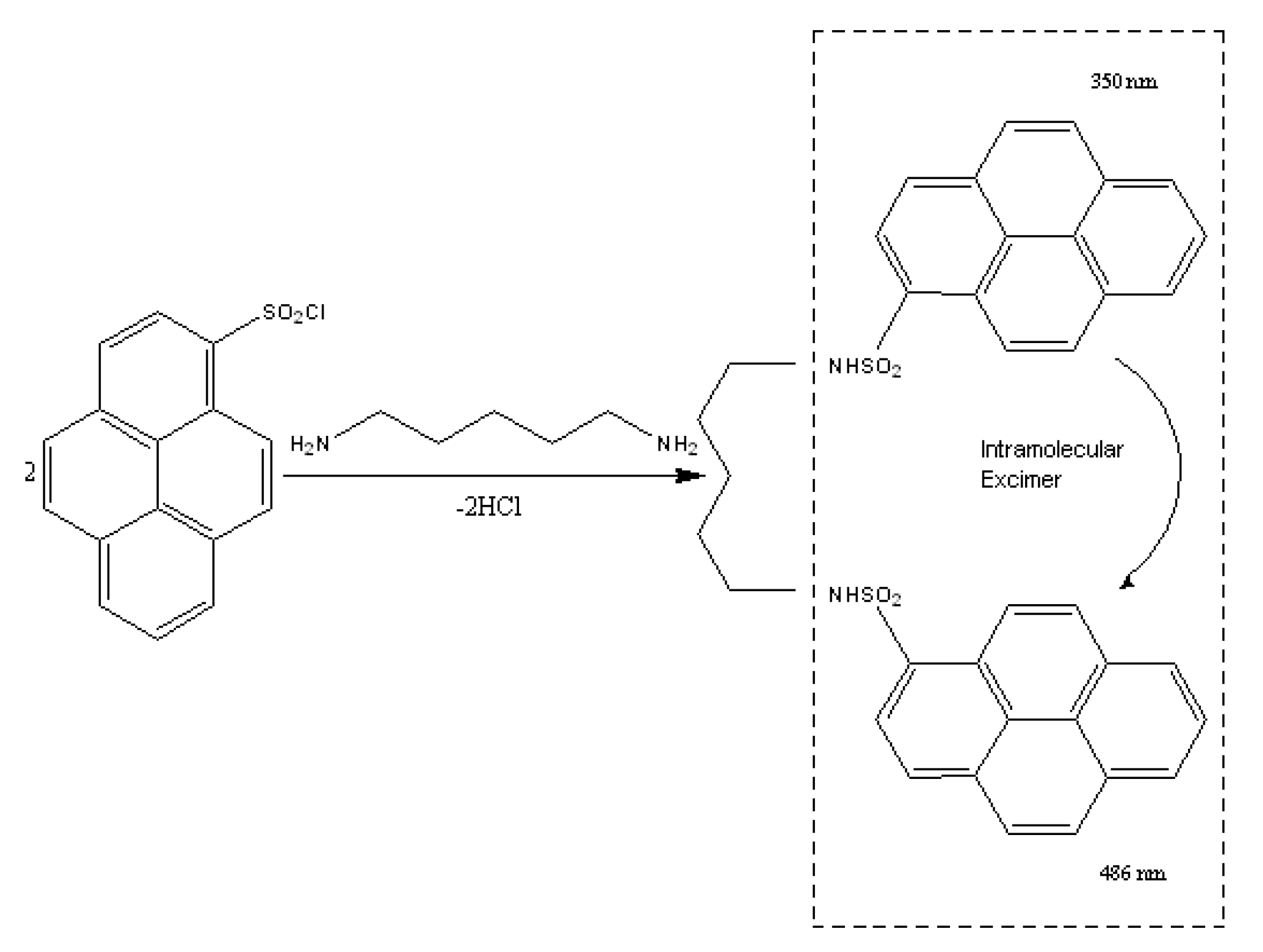
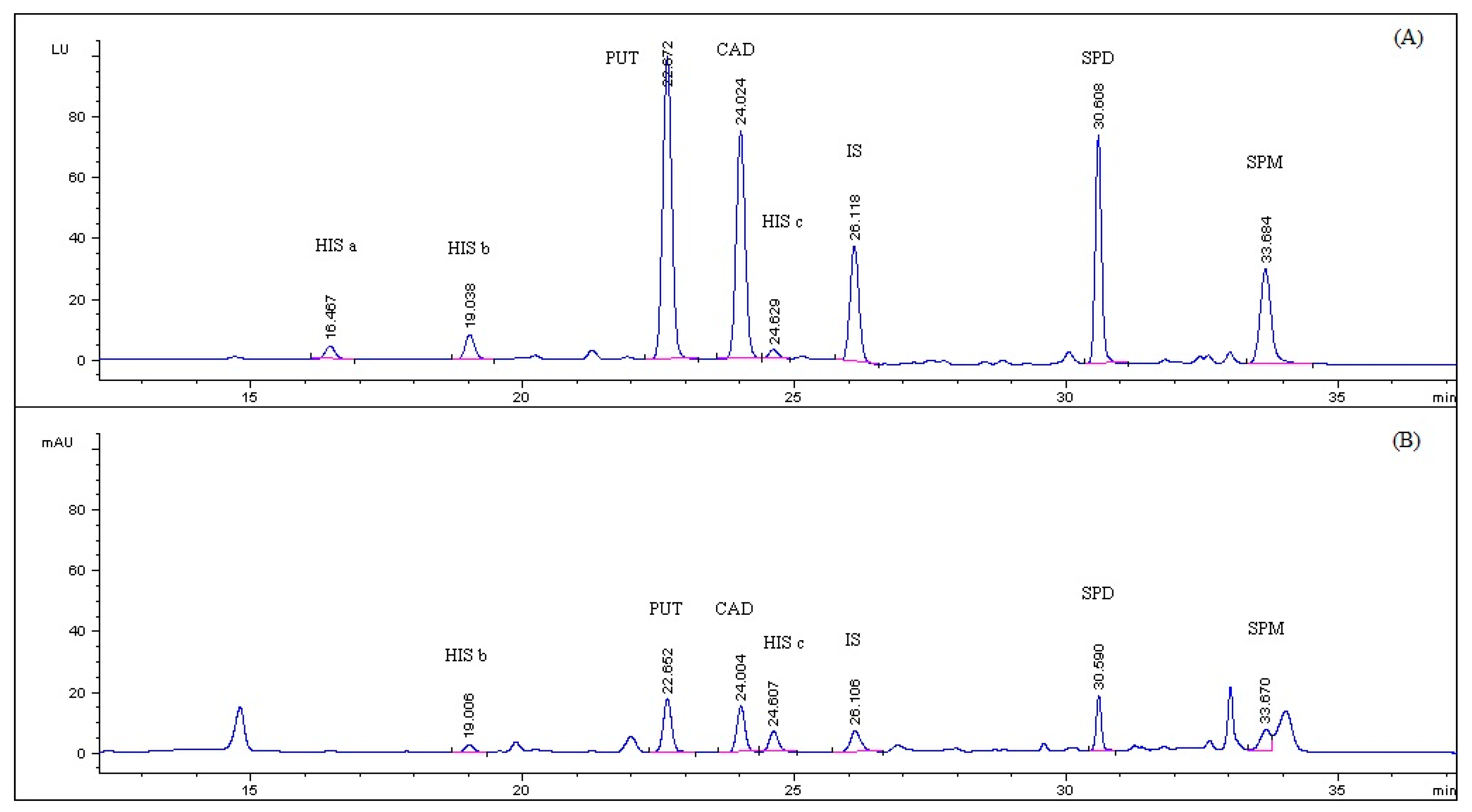
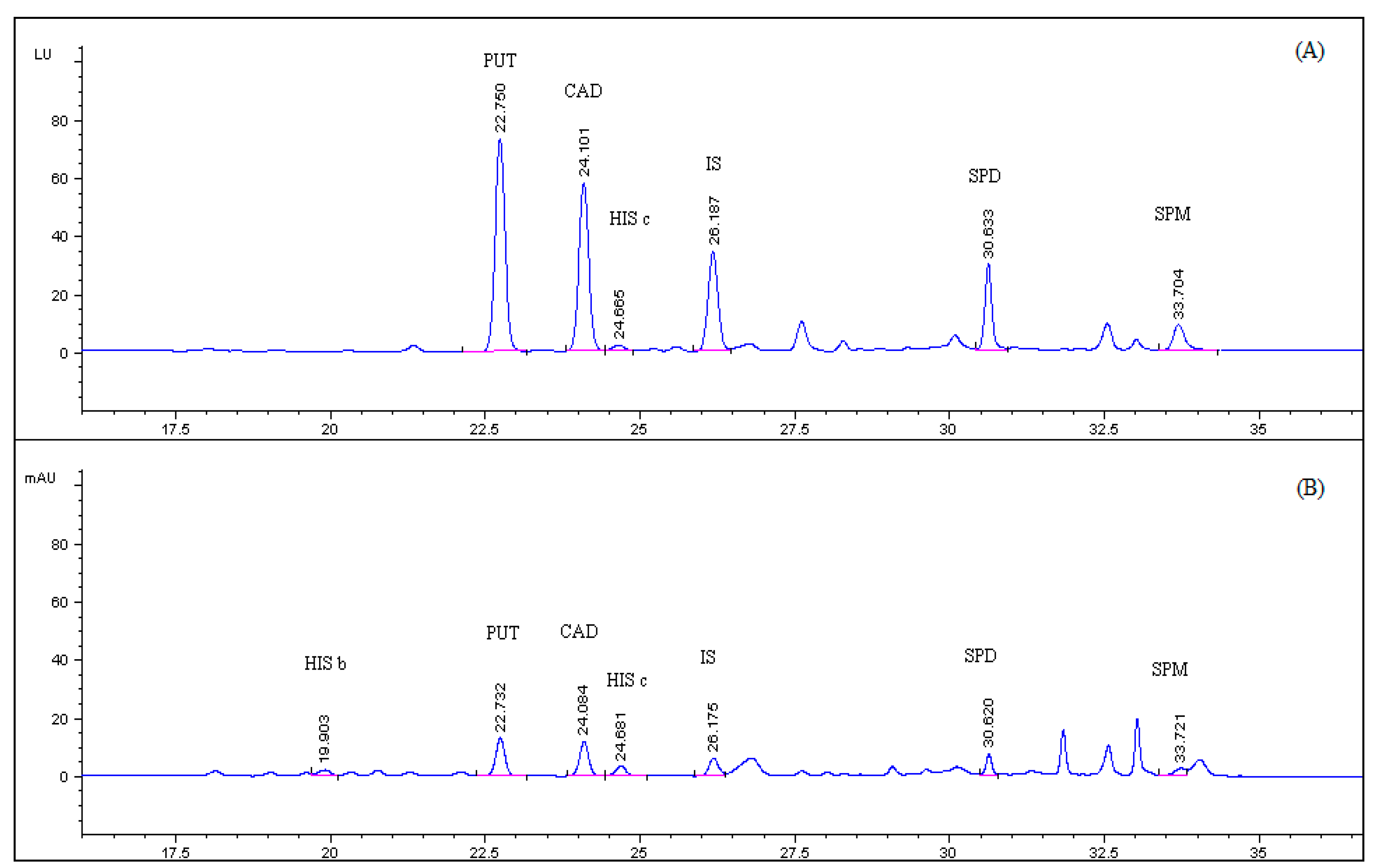
| PUT | CAD | HIS c | SPD | SPM | |
|---|---|---|---|---|---|
| Retention Time (min) | 22.7 | 24.1 | 24.6 | 30.6 | 33.7 |
| Width at 50% of Peak Height (min) | 0.25 | 0.23 | 0.19 | 0.15 | 0.21 |
| Asymmetry Factor | 0.92 | 0.95 | 0.98 | 0.87 | 0.88 |
| Theoretical Plates (N) ×103 | 45 | 61 | 94 | 231 | 143 |
| Resolution | 1.7 | 1.7 | 2.0 | 2.5 | 2.4 |
| Pyrene Derivatives | MW (g/mol) | [M + H]+ | [M+Na]+ | [2M+Na]+ | No of Pyrene Moieties |
|---|---|---|---|---|---|
| Putrescine | 615.8 | 617.8 | 639.8 | 1256.5 | 2 |
| Cadaverine | 630.9 | 631.9 | 653.9 | 1284.6 | 2 |
| Histamine | 639.8 | 640.8 | 662.8 | - | 2 |
| 1,7-diaminoheptane (I.S) | 658.9 | 659.8 | 681.8 | 1340.6 | 2 |
| Spermidine | 938.2 | - | 961.2 | - | 3 |
| Spermine | 1259.5 | 1260.5 | - | - | 4 |
| Calibration Curve | R | LODinstr (μg mL−1) | LOQinstr (μg mL−1) | ||
|---|---|---|---|---|---|
| PUT | UV | y = (352.6 ± 6.9) × C + (32 ± 30) | 0.998 | 0.03 | 0.09 |
| FLD | y = (2148 ± 111) × C + (111 ± 114) | 0.992 | 0.03 | 0.09 | |
| CAD | UV | y = (315.8 ± 3.0) × C + (9 ± 13) | 0.9995 | 0.02 | 0.07 |
| FLD | y = (1350 ± 55) × C + (240 ± 125) | 0.993 | 0.02 | 0.06 | |
| SPD | UV | y = (221.0 ± 8.4) × C + (38 ± 36) | 0.993 | 0.04 | 0.10 |
| FLD | y = (1178 ± 45) × C + (35 ± 46) | 0.996 | 0.03 | 0.10 | |
| SPM | UV | y = (168.7 ± 4.8) × C + (29 ± 21) | 0.996 | 0.07 | 0.20 |
| FLD | y = (959 ± 14) × C + (-21 ± 31) | 0.9992 | 0.05 | 0.15 | |
| HIS | UV | y = (180.1 ± 3.6) × C + (30 ± 16) | 0.998 | 0.08 | 0.20 |
| FLD | y = (352 ± 16) × C + (37 ± 35) | 0.992 | 0.10 | 0.30 | |
| Calibration Curve | R | LOD (mg kg−1) | LOQ (mg kg−1) | ||
|---|---|---|---|---|---|
| PUT | UV | y = (239.7 ± 3.3) × C + (-9.6 ± 3.3) | 0.9992 | 0.2 | 0.6 |
| FLD | y = (1458 ± 22) × C + (-67 ± 22) | 0.9991 | 0.2 | 0.6 | |
| CAD | UV | y = (237.4 ± 2.0) × C + (1.7 ± 2.0) | 0.9997 | 0.1 | 0.3 |
| FLD | y = (1286 ± 16) × C + (-11 ± 16) | 0.9994 | 0.2 | 0.5 | |
| SPD | UV | y = (58.6 ± 2.4) × C + (-0.8 ± 2.4) | 0.993 | 0.5 | 1.6 |
| FLD | y = (301 ± 10) × C + (-18 ± 10) | 0.995 | 0.4 | 1.3 | |
| SPM | UV | y = (20.51 ± 0.82) × C + (1.99 ± 0.81) | 0.994 | 0.5 | 1.6 |
| FLD | y = (113.4 ± 4.6) × C + (16.3 ± 4.5) | 0.994 | 0.5 | 1.6 | |
| HIS | UV | y = (118.1 ± 5.2) × C + (-20.0 ± 6.3) | 0.996 | 0.7 | 2.1 |
| FLD | y = (134.3 ± 8.4) × C + (-46.8 ± 10.1) | 0.992 | 1.0 | 3.1 | |
| LODs (mg kg−1) | PUT | CAD | SPD | SPM | HIS | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| UV | FLD | UV | FLD | UV | FLD | UV | FLD | UV | FLD | |
| Tuna | 0.3 | 0.3 | 0.4 | 0.3 | 0.7 | 0.5 | 0.6 | 1.0 | 0.7 | 0.6 |
| Mackerel | 1.0 | 1.4 | 0.5 | 0.3 | 0.6 | 0.6 | 1.2 | 0.5 | 0.2 | 0.7 |
| Anchovy | 0.6 | 0.8 | 0.5 | 1.0 | 0.5 | 0.5 | 0.5 | 0.6 | 0.5 | 0.1 |
| Anchovy Marinated | 0.6 | 0.7 | 0.8 | 0.7 | 1.0 | 0.5 | 1.1 | 0.5 | 0.8 | 0.5 |
| Sea bass | 0.2 | 0.2 | 0.1 | 0.2 | 0.5 | 0.4 | 0.5 | 0.5 | 0.7 | 1.0 |
| Sardines | 0.6 | 0.7 | 0.6 | 0.8 | 0.3 | 0.2 | 0.4 | 1.1 | 0.2 | 0.4 |
| Instrumental Precision 0.5 μg mL−1, %RSD, n = 5 | Method Intra-Day Assay %RSD, n = 6 | Method Inter-Day Assay %RSD, n = 6, k = 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UV | UV | FLD | FLD | UV | UV | FLD | FLD | UV | UV | FLD | FLD | |
| PA | F | PA | F | PA | F | PA | F | PA | F | PA | F | |
| PUT | 2.3 | 0.1 | 2.6 | 0.4 | 8.2 | 9.3 | 10 | 8.2 | 8.9 | 12 | 9.6 | 15 |
| CAD | 2.3 | 0.3 | 2.7 | 0.5 | 8.2 | 9.1 | 7.4 | 6.1 | 9.5 | 12 | 11 | 16 |
| SPD | 2.4 | 0.3 | 2.7 | 0.4 | 12 | 8.7 | 12 | 8.8 | 14 | 13 | 21 | 15 |
| SPM | 1.8 | 0.7 | 2.8 | 0.5 | 13 | 15 | 14 | 11 | 12 | 19 | 14 | 23 |
| HIS | 4.2 | 3.9 | 4.2 | 2.3 | 10 | 9.4 | 11 | 12 | 11 | 9.4 | 10 | 14 |
| UV | FLD | |||||
|---|---|---|---|---|---|---|
| Compound | Absolute Recovery (%) | Relative Recovery % (±SD) | Absolute Recovery (%) | Relative Recovery % (±SD) | ||
| PUT | 68 | 88 | (±12) | 68 | 86 | (±15) |
| CAD | 75 | 102 | (±2.6) | 95 | 98 | (±2.1) |
| SPD | 27 | 98 | (±12) | 26 | 88 | (±6.6) |
| SPM | 12 | 112 | (±8.0) | 12 | 114 | (±6.5) |
| HIS | 66 | 72 | (±13) | 38 | 67 | (±11) |
| Content of BAs (mg kg−1) | PUT | CAD | SPD | SPM | HIS | Sum of BAs |
|---|---|---|---|---|---|---|
| Tuna | ND | <0.9 | 2.4 | 7.6 | ND | 10.9 |
| Mackerel | 40.1 | 0.9 | 3.6 | 2.5 | 2.5 | 49.6 |
| Anchovy | 2.4 | 3.0 | <1.5 | <1.8 | 5.8 | 14.5 |
| Anchovy Marinated | 3.8 | 5.6 | <1.5 | <1.5 | <1.5 | 13.9 |
| Sea bass | <0.6 | ND | 1.4 | 2.1 | ND | 4.1 |
| Sardines | 2.7 | 10.8 | 8.1 | 9.6 | 3.7 | 34.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plakidi, E.S.; Maragou, N.C.; Dasenaki, M.E.; Megoulas, N.C.; Koupparis, M.A.; Thomaidis, N.S. Liquid Chromatographic Determination of Biogenic Amines in Fish Based on Pyrene Sulfonyl Chloride Pre-Column Derivatization. Foods 2020, 9, 609. https://doi.org/10.3390/foods9050609
Plakidi ES, Maragou NC, Dasenaki ME, Megoulas NC, Koupparis MA, Thomaidis NS. Liquid Chromatographic Determination of Biogenic Amines in Fish Based on Pyrene Sulfonyl Chloride Pre-Column Derivatization. Foods. 2020; 9(5):609. https://doi.org/10.3390/foods9050609
Chicago/Turabian StylePlakidi, Elvira S., Niki C. Maragou, Marilena E. Dasenaki, Nikolaos C. Megoulas, Michael A. Koupparis, and Nikolaos S. Thomaidis. 2020. "Liquid Chromatographic Determination of Biogenic Amines in Fish Based on Pyrene Sulfonyl Chloride Pre-Column Derivatization" Foods 9, no. 5: 609. https://doi.org/10.3390/foods9050609
APA StylePlakidi, E. S., Maragou, N. C., Dasenaki, M. E., Megoulas, N. C., Koupparis, M. A., & Thomaidis, N. S. (2020). Liquid Chromatographic Determination of Biogenic Amines in Fish Based on Pyrene Sulfonyl Chloride Pre-Column Derivatization. Foods, 9(5), 609. https://doi.org/10.3390/foods9050609






