Garlic, Onion, and Cinnamon Essential Oil Anti-Biofilms’ Effect against Listeria monocytogenes
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Culture
2.2. Essential Oils
2.3. Identification of the Essential Oil Components
2.4. Agar Disk Diffusion Assay
2.5. Determination of Minimal Inhibitory Concentration (MIC)
2.6. Inhibition of Initial Cell Attachment
2.7. Inhibition of Preformed Biofilm
2.8. Biofilm Biomass (Crystal Violet Assay)
2.9. Statistical Analysis of Quantitative Data
3. Results
3.1. Chemical Composition of the Essential Oils
3.2. Antimicrobial Activity
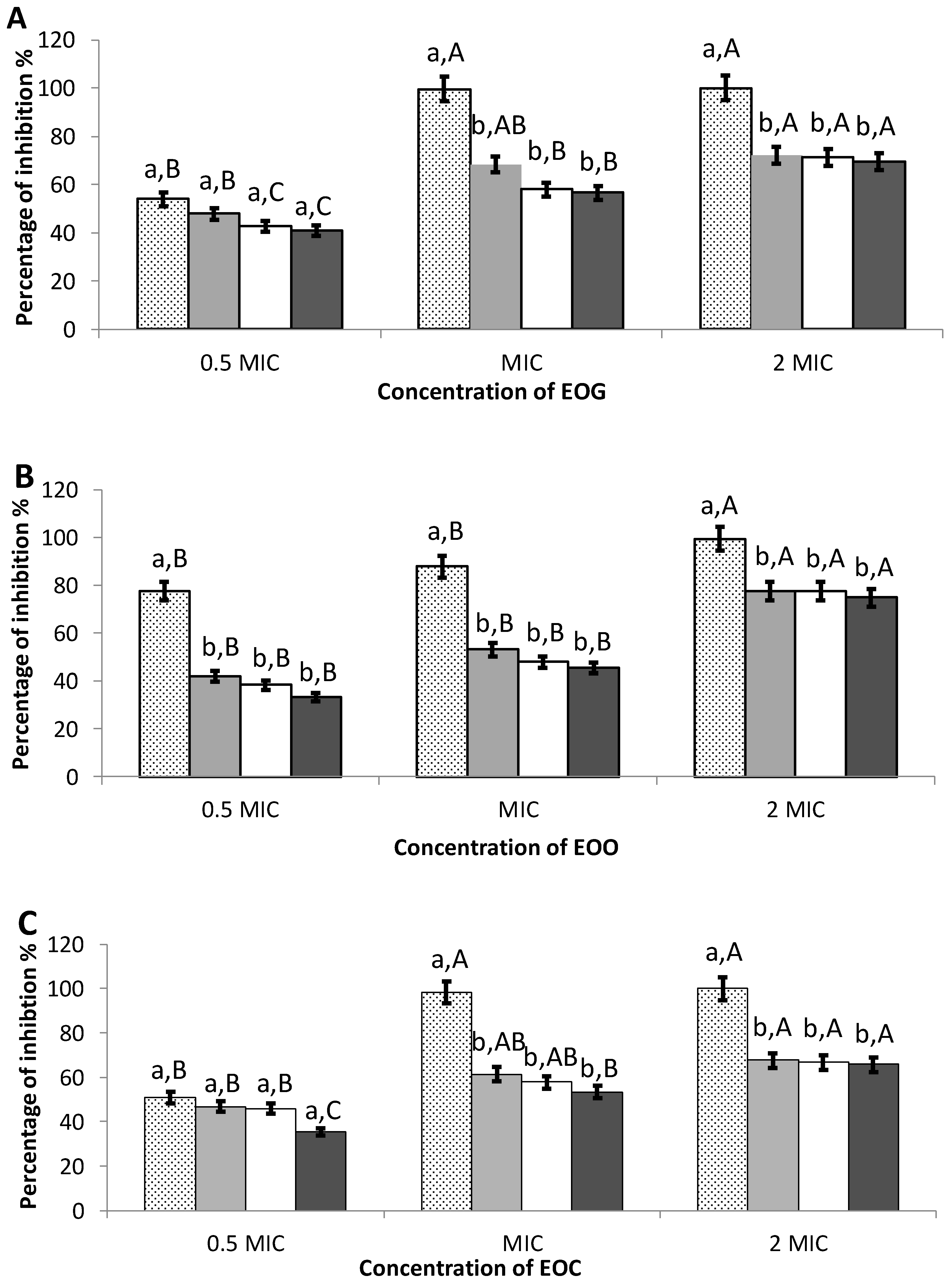
3.3. Inhibition of Initial Cell Attachment
3.4. Preformed Biofilms
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Galie, S.; Garcia-Gutierrez, C.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, 262. [Google Scholar]
- Hansen, L.T.; Vogel, B.F. Desiccation of adhering and biofilm Listeria monocytogenes on stainless steel: Survival and transfer to salmon products. Int. J. Food Microbiol. 2011, 146, 88–93. [Google Scholar] [CrossRef]
- Harvey, J.; Keenan, K.P.; Gilmour, A. Assessing biofilm formation by Listeria monocytogenes strains. Food Microbiol. 2007, 24, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Colagiorgi, A.; Bruini, I.; Di Ciccio, P.A.; Zanardi, E.; Ghidini, S.; Ianieri, A. Listeria monocytogenes biofilms in the wonderland of food industry. Pathogens 2017, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhu, X. Biofilm formation and food safety in food industries. Trends Food Sci. Technol. 2009, 20, 407–413. [Google Scholar] [CrossRef]
- Carpentier, B.; Cerf, O. Review—Persistence of Listeria monocytogenes in food industry equipment and premises. Int. J. Food Microbiol. 2011, 145, 1–8. [Google Scholar] [CrossRef]
- Beer, D.; Srinivasan, R.; Stewart, P.S. Direct measurement of chlorine penetration into biofilms during disinfection. Appl. Environ. Microbiol. 1994, 60, 4339–4344. [Google Scholar] [CrossRef]
- Aryal, M.; Muriana, P.M. Efficacy of commercial sanitizers used in food processing facilities for inactivation of Listeria monocytogenes, E. coli O157:H7, and Samonella biofilms. Foods 2019, 8, 639. [Google Scholar] [CrossRef]
- Brewer, M.S.; Rojas, M. Consumer attitudes toward issues in food safety. J. Food Saf. 2008, 28, 1–22. [Google Scholar] [CrossRef]
- Govaert, M.; Smet, C.; Graeffe, A.; Walsh, J.L.; Van Impe, J.F.M. Inactivation of L. monocytogenes and S. typhimurium biofilms by means of an air-based cold atmospheric plasma (CAP) system. Foods 2020, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- FDA (Food and Drug Administration). Part 582. Substances Generally Recognised as Safe. Sec. 582.20. Essential Oils, Oleoresins (Solvent-Free), and Natural Extractives (Including Distillates). Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=582.20 (accessed on 24 March 2020).
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Jeyaratnam, N.; Nour, A.H.; Kanthasamy, R.; Nour, A.H.; Yuvaraj, A.R.; Akindoyo, J.O. Essential oil from Cinnamomum cassia bark through hydrodistillation and advanced microwave assisted hydrodistillation. Ind. Crops Prod. 2016, 92, 57–66. [Google Scholar] [CrossRef]
- Kumar, S.; Kumari, R. Cinnamomum: Review article of essential oil compounds, ethnobotany, antifungal and antibacterial effects. Open Access J. Sci. 2019, 3, 13–16. [Google Scholar] [CrossRef]
- Benkeblia, N. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium satvum). LWT Food Sci. Technol. 2004, 37, 263–268. [Google Scholar] [CrossRef]
- Djenane, D.; Yangüela, J.; Montañés, L.; Djerbal, M.; Roncalés, P. Antimicrobial activity of Pistacia lentiscus and Satureja montana essential oils against Listeria monocytogenes CECT 935 using laboratory media: Efficacy and synergistic potential in minced beef. Food Control 2011, 22, 1046–1053. [Google Scholar] [CrossRef]
- Rohani, S.M.R.; Moradi, M.; Mehdizadeh, T.; Saei-Dehkordi, S.S.; Griffiths, W.M. The effect of nisin and garlic (Allium sativum L.) essential oil separately and in combination on the growth of Listeria monocytogenes. Food Sci. Technol. 2011, 44, 2260–2265. [Google Scholar]
- Mello, S.; Cunha, A.; Neudí, G.; Luiz, F.; Werneck, C. Chemical composition and antimicrobial activity of essential oils from selected herbs cultivated in the south of Brazil against food spoilage and foodborne pathogens. Ciencia Rural 2012, 42, 1300–1306. [Google Scholar]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antimicrobial mechanism of clove oil on Listeria monocytogenes. Food Control 2018, 94, 140–146. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, D.; Galvão, J.A.; Ambrosio, C.M.S.; Gloria, E.M.; Oetterer, M. Single and binary applications of essential oils effectively control Listeria monocytogenes biofilms. Ind. Crop Prod. 2018, 121, 452–460. [Google Scholar] [CrossRef]
- EUCAST (European Committee on Antimicrobial Susceptibility Testing). EUCAST Disk Diffusion Method Test Methodology v 8.0. Available online: http://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology/ (accessed on 24 March 2020).
- Balouiri, M.; Sadiki, M.; Koraichi, I.S.S. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Sandasi, M.; Leonard, C.M.; Viljoen, A.M. The in vitro antibiofilm activity of selected culinary herbs and medicinal plants against Listeria monocytogenes. Lett. Appl. Microbiol. 2010, 50, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Sandasi, M.; Leonard, C.M.; Viljoen, A.M. The effect of five common essential oil components on Listeria monocytogenes biofilms. Food Control 2008, 19, 1070–1075. [Google Scholar] [CrossRef]
- Djordjevic, D.; Wiedmann, M.; McLandsborough, L.A. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 2002, 68, 2950–2958. [Google Scholar] [CrossRef]
- El-Sayed, H.S.; Chizzola, R.; Ramadan, A.A.; Edris, A.E. Chemical composition and antimicrobial activity of garlic essential oils evaluated in organic solvent, emulsifying, and self-microemulsifying water based delivery systems. Food Chem. 2017, 221, 196–204. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, P.; Zhanga, Z.; Daib, Y.; Hao, L. Physicochemical characteristics of complexes between amylose and garlic bioactive components generated by milling activating method. Food Res. Int. 2018, 105, 499–506. [Google Scholar] [CrossRef]
- Martins, N.; Petropoulos, S.; Ferreira, I.C.F.R. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: A review. Food Chem. 2016, 211, 41–50. [Google Scholar] [CrossRef]
- Sun, L.; Zong, S.B.; Li, J.C.; Lv, Y.Z.; Liu, L.N.; Wang, Z.Z.; Zhou, J.; Cao, L.; Kou, J.P.; Xiao, W. The essential oil from the twigs of Cinnamomum cassia Presl alleviates pain and inflammation in mice. J. Ethnopharmacol. 2016, 194, 904–912. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Vazquez-Armenta, F.J.; Ayala-Zaval, J.F.; Olivas, G.I.; Molina-Corral, F.J.; Espinoza, B.A. Antibrowning and antimicrobial effects of onion essential oil to preserve the quality of cut potatoes. Acta Aliment. 2014, 43, 640–649. [Google Scholar] [CrossRef]
- Mith, H.; Duré, R.; Delcenserie, V.; Zhiri, A.; Daube, G.; Clinquer, A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci. Nutr. 2014, 2, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Bouhdid, S.; Abrini, J.; Amensour, M.; Zhiri, A.; Espuny, M.; Manresa, A. Functional and ultrastructural changes in Pseudomonas aeruginosa and Staphylococcus aureus induced by Cinnamon verum essential oil. J. Appl. Microbiol. 2010, 109, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef]
- Golus, J.; Sawicki, R.; Widelski, J.; Ginalska, G. The agar microdilution method—A new method for antimicrobial susceptibility testing for essential oils and plant extracts. J. Appl. Microbiol. 2016, 121, 1291–1299. [Google Scholar] [CrossRef]
- Jadhav, S.; Shah, S.; Bhave, M.; Palombo, E.A. Inhibitory activity of yarrow essential oil on Listeria planktonic cells and biofilms. Food Control 2013, 29, 125–130. [Google Scholar] [CrossRef]
- Mohammadi-Bazargani, M.; Rohloff, J. Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control 2016, 61, 156–164. [Google Scholar] [CrossRef]
- Lang, M.; Rodrigues, S.; Boulho, R.; Duteil, E.; Bazire, A.; Bedoux, G. An essential oil blend prevents Pseudomonas aeruginosa PA01 from forming biofilms. J. Bacteriol. Parasitol. 2016, 7, 268. [Google Scholar]
- Kačániová, M.; Galovičová, L.; Ivanišová, E.; Vokovic, N.L.; Štefaniková, J.; Valková, V.; Borotová, P.; Žiarovská, J.; Terentjeva, M.; Felšöciová, S.; et al. Antioxidant, antimicrobial and antibiofilm activity of coriander (Coriandrum sativum L.) essential oil for its application in foods. Foods 2020, 9, 282. [Google Scholar] [CrossRef]
- Crawford, R.J.; Webb, H.K.; Truong, V.K.; Hasan, J.; Ivanova, E.P. Surface topographical factors influencing bacterial attachment. Adv. Colloid Interface Sci. 2012, 179, 142–149. [Google Scholar] [CrossRef]
- Simões, M.; Simões, L.C.; Vieira, M.J. Species association increases biofilm resistance to chemical and mechanical treatments. Water Res. 2009, 43, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Albano, M.; Crulhas, B.P.; Alves, F.C.B.; Pereira, A.F.M.; Andrade, B.F.M.T.; Barbosa, L.N.; Furlanetto, A.; Lyra, L.P.D.S.; Rall, V.L.M.; Júnior, A.F. Antibacterial and anti-biofilm activities of cinnamaldehyde against S. epidermidis. Microb. Pathog. 2019, 126, 231–238. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.M.M.; Brugnera, D.F.; Do Nascimento, J.A.; Piccoli, R.H. Control of planktonic and sessile bacterial cells by essential oils. Food Bioprod. Process. 2012, 90, 809–818. [Google Scholar] [CrossRef]
- Driffield, K.; Miller, K.; Bostock, J.M.; O’Neill, A.J.; Chopra, I. Increased mutability of Pseudomonas aeruginosa in biofilms. J. Antimicrob. Chemother. 2008, 61, 1053–1056. [Google Scholar] [CrossRef]
- Sadekuzzamana, M.; Mizana, M.F.R.; Kim, H.S.; Yang, S.; Ha, S.D. Activity of thyme and tea tree essential oils against selected foodborne pathogens in biofilms on abiotic surfaces. Food Sci. Technol. 2018, 89, 134–139. [Google Scholar] [CrossRef]
- Leonard, C.M.; Virijevic, S.; Regnier, T.; Combrinck, S. Bioactivity of selected essential oils and some components on Listeria monocytogenes biofilms. S. Afr. J. Bot. 2010, 76, 676–680. [Google Scholar] [CrossRef]
- Szczepanski, S.; Lipski, A. Essential oils show specific inhibiting effects on bacterial biofilm formation. Food Control 2014, 36, 224–229. [Google Scholar] [CrossRef]
- De Oliveira, M.M.M.; Brugnera, D.F.; Do Nascimento, J.A.; Batista, N.N.; Piccoli, R.H. Cinnamon essential oil and cinnamaldehyde in the control of bacterial biofilms formed on stainless steel surfaces. Eur. Food Res. Technol. 2012, 234, 821–832. [Google Scholar] [CrossRef]
- Mohsenipour, Z.; Hassanshahian, M. The effects of Allium sativum extracts on biofilm formation and activities of six pathogenic bacteria. Jundishapur J. Microbiol. 2015, 8, 1897. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Lee, Y.R. Systematic study on active compounds as antibacterial and antibiofilm agent in aging onions. J. Food Drug Anal. 2018, 26, 518–528. [Google Scholar] [CrossRef]
- Zhang, D.; Gan, R.Y.; Zhang, J.R.; Farha, A.K.; Li, H.B.; Zhu, F.; Wang, X.H.; Corke, H. Antivirulence properties and related mechanisms of spice essential oils: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 1–38. [Google Scholar] [CrossRef]
- Dufour, D.; Leung, V.; Levesque, C.M. Bacterial biofilm: Structure, function, and antimicrobial resistance. J. Endod. 2012, 22, 2–16. [Google Scholar] [CrossRef]
- Bridier, A.; Briandet, R.; Thomas, V.; Dubois-Brissonnet, F. Resistance of bacterial biofilms to disinfectants: A review. Biofouling 2011, 27, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
| Concentration (% Peak Area) | ||||
|---|---|---|---|---|
| No. | Compound | EOG | EOO | EOC |
| 1 | sulfide allyl mehtyl | 1.41 | - | - |
| 2 | 1,2-dithiolane | 0.30 | - | - |
| 3 | diallyl sulfide | 20.89 | - | - |
| 4 | allyl methyl disulfide | 1.12 | - | - |
| 5 | diallyl disulfide | 22.74 | - | - |
| 6 | trisulfide, methyl 2-propenyl | 2.94 | - | - |
| 7 | 4-mehtyl-1,2,3-trithiolane | 0.58 | - | - |
| 8 | 3,vinil-1,2-dithiacyclohex-5-nne | 0.52 | - | - |
| 9 | diallyl trisulfide | 25.13 | - | - |
| 10 | 1-allyl-3-propyl trisulfane | 0.27 | - | - |
| 11 | 5-methyl,1,2,3,4-tetrathiane | 0.43 | - | - |
| 12 | tetrasulfide, di-2-propenyl | 13.54 | - | - |
| 13 | 6,htyl-4,5,7-trithia-2,8-decadiene | 0.54 | - | - |
| 14 | 1,allyl-3-(2 allylthio)propyl)trisulfane | 0.93 | - | - |
| 15 | dimethyl disulfide | - | 0.07 | - |
| 16 | ropyl ydrodisulfide | - | 0.09 | - |
| 17 | propyl sulfide | - | 0.33 | - |
| 18 | disulfide, mehtyl propyl | - | 5.11 | - |
| 19 | dimehtyl trisulfide | - | 0.25 | - |
| 20 | dipropyl disulfide | - | 31.11 | - |
| 21 | trisulfide, mehtyl propyl | - | 6.69 | - |
| 22 | trisulfide, dipropyl | - | 35.46 | - |
| 23 | tetrasulfide, dipropyl | - | 17.65 | - |
| 24 | benzaldehyde | - | - | 0.97 |
| 25 | benzaldehyde, 2-hydroxy | - | - | 0.21 |
| 26 | phenyl ethyl alcohol | - | - | 0.93 |
| 27 | benzenepropanal | - | - | 0.49 |
| 28 | benzofuran, 2-mehtyl- | - | - | 0.17 |
| 29 | octanoic acid, ethyl ester | - | - | 0.05 |
| 30 | (Z)-cinnamaldehyde | - | - | 0.37 |
| 31 | 3-phenylpropanol | - | - | 0.07 |
| 32 | benzaldehyde,2-methoxy- | - | - | 0.22 |
| 33 | acetic acid, 2-phenylethyl ester | - | - | 0.08 |
| 34 | E-cinnamaldehyde | - | - | 76.54 |
| 35 | cinnamyl alcohol | - | - | 0.30 |
| 36 | copaene | 0.30 | ||
| 37 | coumarin | - | - | 2.59 |
| 38 | acetic acid, cinnamyl eeter | - | - | 4.21 |
| 39 | (Z)-2-mehtoxy cinnamaldehyde | - | - | 10.30 |
| Essential Oil | MIC | Inhibition Zone |
|---|---|---|
| Garlic | 0.100 | 31.0 ± 1.7 b |
| Cinnamon | 0.100 | 12.3 ± 0.5 a |
| Onion | 0.025 | 37.8 ± 0.4 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somrani, M.; Inglés, M.-C.; Debbabi, H.; Abidi, F.; Palop, A. Garlic, Onion, and Cinnamon Essential Oil Anti-Biofilms’ Effect against Listeria monocytogenes. Foods 2020, 9, 567. https://doi.org/10.3390/foods9050567
Somrani M, Inglés M-C, Debbabi H, Abidi F, Palop A. Garlic, Onion, and Cinnamon Essential Oil Anti-Biofilms’ Effect against Listeria monocytogenes. Foods. 2020; 9(5):567. https://doi.org/10.3390/foods9050567
Chicago/Turabian StyleSomrani, Mariem, María-Carmen Inglés, Hajer Debbabi, Ferid Abidi, and Alfredo Palop. 2020. "Garlic, Onion, and Cinnamon Essential Oil Anti-Biofilms’ Effect against Listeria monocytogenes" Foods 9, no. 5: 567. https://doi.org/10.3390/foods9050567
APA StyleSomrani, M., Inglés, M.-C., Debbabi, H., Abidi, F., & Palop, A. (2020). Garlic, Onion, and Cinnamon Essential Oil Anti-Biofilms’ Effect against Listeria monocytogenes. Foods, 9(5), 567. https://doi.org/10.3390/foods9050567






