Patterns of Genetic Diversity and the Invasion of Commercial Starters in Saccharomyces cerevisiae Vineyard Populations of Santorini Island
Abstract
1. Introduction
2. Materials and Methods
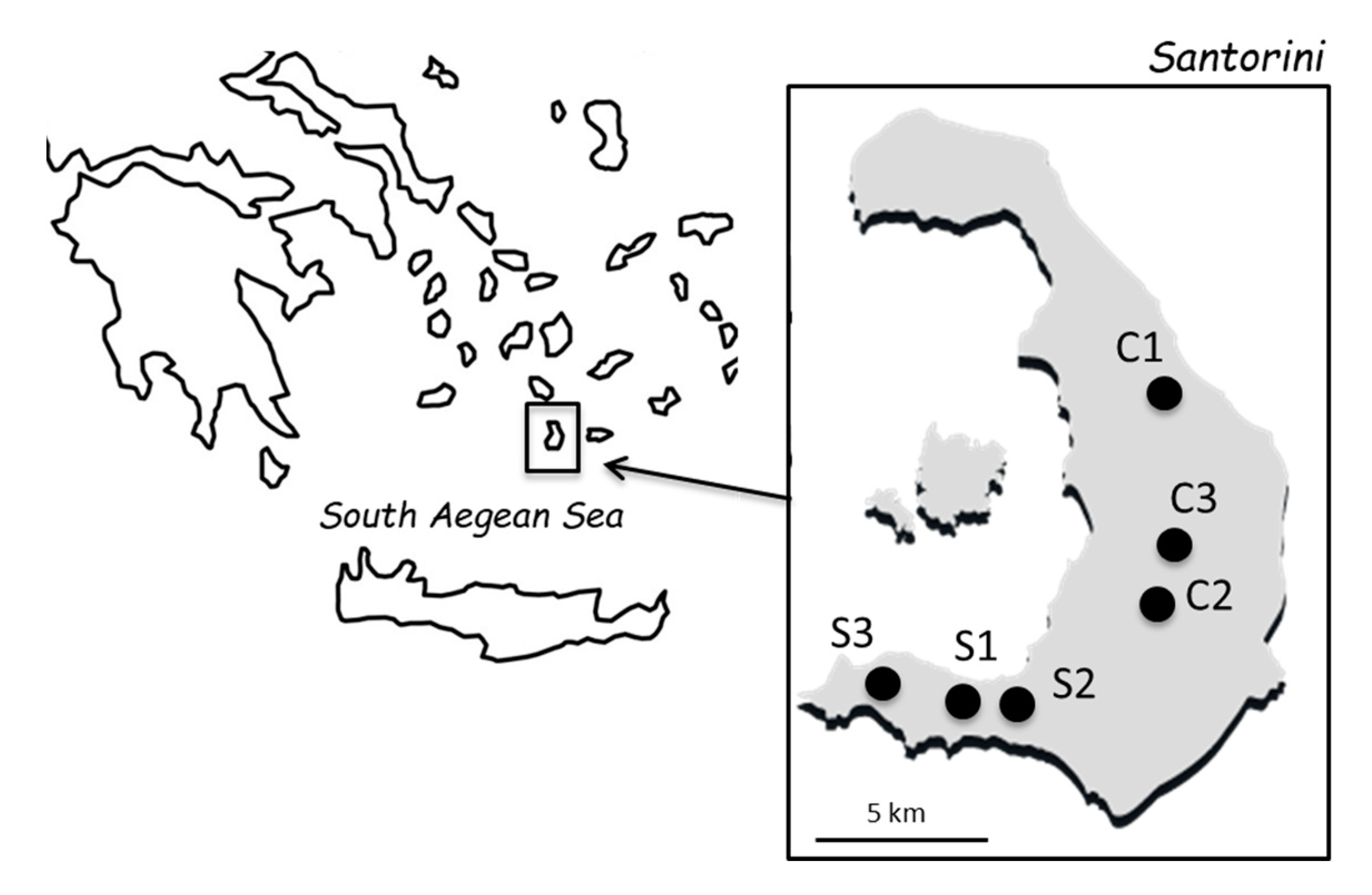
2.1. Grape Samples and Yeast Isolation
2.2. Molecular Typing of Isolates
2.3. Data Analysis
3. Results
3.1. Fermentations and Genotyping
3.2. Biodiversity and Genotype Distribution
3.3. Genetic Similarities of Genotypes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mortimer, R.K. Evolution and variation of the yeast (Saccharomyces) genome. Genome Res. 2000, 10, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.; De Chiara, M.; Friedrich, A.; Yue, J.-X.; Pflieger, D.; Bergström, A.; Sigwalt, A.; Barre, B.P.; Freel, K.; Llored, A.; et al. Genome evolution across 1,011 Saccharomyces cerevisiae isolates. Nature 2018, 556, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Fay, J.C.; Benavides, J.A. Evidence for Domesticated and Wild Populations of Saccharomyces cerevisiae. PLoS Genet. 2005, 1, e5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-M.; Liu, W.-Q.; Liti, G.; Wang, S.-A.; Bai, F.-Y. Surprisingly diverged populations of Saccharomyces cerevisiae in natural environments remote from human activity. Mol. Ecol. 2012, 21, 5404–5417. [Google Scholar] [CrossRef]
- Tofalo, R.; Perpetuini, G.; Schirone, M.; Fasoli, G.; Aguzzi, I.; Corsetti, A.; Suzzi, G. Biogeographical characterization of Saccharomyces cerevisiae wine yeast by molecular methods. Front. Microbiol. 2013, 4, 166. [Google Scholar] [CrossRef]
- Liti, G.; Carter, D.M.; Moses, A.M.; Warringer, J.; Parts, L.; James, S.A.; Davey, R.P.; Roberts, I.N.; Burt, A.; Koufopanou, V.; et al. Population genomics of domestic and wild yeasts. Nature 2009, 458, 337–341. [Google Scholar] [CrossRef]
- Schacherer, J.; Shapiro, J.; Ruderfer, D.M.; Kruglyak, L. Comprehensive polymorphism survey elucidates population structure of Saccharomyces cerevisiae. Nature 2009, 458, 342–345. [Google Scholar] [CrossRef]
- Clowers, K.J.; Heilberger, J.; Piotrowski, J.S.; Will, J.L.; Gasch, A.P. Ecological and Genetic Barriers Differentiate Natural Populations of Saccharomyces cerevisiae. Mol. Boil. Evol. 2015, 32, 2317–2327. [Google Scholar] [CrossRef]
- Gayevskiy, V.; Goddard, M.R. Geographic delineations of yeast communities and populations associated with vines and wines in New Zealand. ISME J. 2011, 6, 1281–1290. [Google Scholar] [CrossRef]
- Nisiotou, A.A.; Spiropoulos, A.E.; Nychas, G.-J.E. Yeast Community Structures and Dynamics in Healthy and Botrytis-Affected Grape Must Fermentations. Appl. Environ. Microbiol. 2007, 73, 6705–6713. [Google Scholar] [CrossRef]
- Chavan, P.; Mane, S.; Kulkarni, G.; Shaikh, S.; Ghormade, V.; Nerkar, D.P.; Shouche, Y.; Deshpande, M.V. Natural yeast flora of different varieties of grapes used for wine making in India. Food Microbiol. 2009, 26, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Cheng, C.; Li, Z.; Chen, J.; Yan, B.; Han, B.-Z.; Reeves, M. Yeast species associated with wine grapes in China. Int. J. Food Microbiol. 2010, 138, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Bueso, G.; Teresa, A.; Serrano, A.; Tello, J.; Aporta, I.; Vélez, M.D.; Valero, E. Influence of the farming system and vine variety on yeast communities associated with grape berries. Int. J. Food Microbiol. 2011, 145, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Drumonde-Neves, J.; Franco-Duarte, R.; Lima, T.; Schuller, D.; Pais, C. Association between Grape Yeast Communities and the Vineyard Ecosystems. PLoS ONE 2017, 12, e0169883. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. USA 2013, 111, E139–E148. [Google Scholar] [CrossRef] [PubMed]
- Banilas, G.; Sgouros, G.; Nisiotou, A. Development of microsatellite markers for Lachancea thermotolerans typing and population structure of wine-associated isolates. Microbiol. Res. 2016, 193, 1–10. [Google Scholar] [CrossRef]
- Schuller, D.; Casal, M. The genetic structure of fermentative vineyard-associated Saccharomyces cerevisiae populations revealed by microsatellite analysis. Antonie Van Leeuwenhoek 2006, 91, 137–150. [Google Scholar] [CrossRef]
- Vigentini, I.; De Lorenzis, G.; Fabrizio, V.; Valdetara, F.; Faccincani, M.; Panont, C.A.; Picozzi, C.; Imazio, S.; Failla, O.; Foschino, R. The vintage effect overcomes the terroir effect: A three year survey on the wine yeast biodiversity in Franciacorta and Oltrepò Pavese, two northern Italian vine-growing areas. Microbiology 2015, 161, 362–373. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Wine and Must Analysis; International Organisation of Vine and Wine (OIV): Paris, France, 2015. [Google Scholar]
- Esteve-Zarzoso, B.; Belloch, C.; Uruburu, F.; Querol, A. Identification of yeasts by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int. J. Syst. Evol. Microbiol. 1999, 49, 329–337. [Google Scholar] [CrossRef]
- Nisiotou, A.A.; Nychas, G.-J.E. Yeast Populations Residing on Healthy or Botrytis-Infected Grapes from a Vineyard in Attica, Greece. Appl. Environ. Microbiol. 2007, 73, 2765–2768. [Google Scholar] [CrossRef]
- Legras, J.L.; Karst, F. Optimisation of interdelta analysis for Saccharomyces cerevisiae strain characterization. FEMS Microbiol. Lett. 2003, 221, 249–255. [Google Scholar] [CrossRef]
- Schuller, D.; Alves, H.; Dequin, S.; Casal, M. Ecological survey of Saccharomyces cerevisiae strains from vineyards in the Vinho Verde Region of Portugal. FEMS Microbiol. Ecol. 2005, 51, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Valero, E.; Cambon, B.; Schuller, D.; Casal, M.; Dequin, S. Biodiversity of Saccharomyces yeast strains from grape berries of wine-producing areas using starter commercial yeasts. FEMS Yeast Res. 2007, 7, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.R. Chi-squared and Fisher–Irwin tests of two-by-two tables with small sample recommendations. Stat. Med. 2007, 26, 3661–3675. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.T. The analysis of 2 × 2 contingency tables—Yet again. Stat. Med. 2011, 30, 890. [Google Scholar] [CrossRef] [PubMed]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Peakall, R.; Smouse, P.E. Genalex 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Knight, S.J.; Karon, O.; Goddard, M.R. Small scale fungal community differentiation in a vineyard system. Food Microbiol. 2019, 87, 103358. [Google Scholar] [CrossRef]
- Tofalo, R.; Perpetuini, G.; Fasoli, G.; Schirone, M.; Corsetti, A.; Suzzi, G. Biodiversity study of wine yeasts belonging to the “terroir” of Montepulciano d’Abruzzo “Colline Teramane” revealed Saccharomyces cerevisiae strains exhibiting atypical and unique 5.8S-ITS restriction patterns. Food Microbiol. 2014, 39, 7–12. [Google Scholar] [CrossRef]
- Viel, A.; Legras, J.-L.; Nadai, C.; Carlot, M.; Lombardi, A.; Crespan, M.; Migliaro, D.; Giacomini, A.; Corich, V. The Geographic Distribution of Saccharomyces cerevisiae Isolates within three Italian Neighboring Winemaking Regions Reveals Strong Differences in Yeast Abundance, Genetic Diversity and Industrial Strain Dissemination. Front. Microbiol. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, P.; Chen, D.; Howell, K. From the Vineyard to the Winery: How Microbial Ecology Drives Regional Distinctiveness of Wine. Front. Microbiol. 2019, 10, 2679. [Google Scholar] [CrossRef] [PubMed]
- Versavaud, A.; Courcoux, P.; Roulland, C.; Dulau, L.; Hallet, J.N. Genetic diversity and geographical distribution of wild Saccharomyces cerevisiae strains from the wine-producing area of Charentes, France. Appl. Environ. Microbiol. 1995, 61, 3521–3529. [Google Scholar] [CrossRef] [PubMed]
- Beltran, G.; Torija, M.-J.; Novo, M.; Ferrer, N.; Poblet, M.; Guillamón, J.M.; Rozès, N.; Mas, A. Analysis of yeast populations during alcoholic fermentation: A six year follow-up study. Syst. Appl. Microbiol. 2002, 25, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, J.F.; Franco, L.; Cools, T.L.; De Meester, L.; Michiels, J.; Wenseleers, T.; Hassan, B.A.; Yaksi, E.; Verstrepen, K.J. The Fungal Aroma Gene ATF1 Promotes Dispersal of Yeast Cells through Insect Vectors. Cell Rep. 2014, 9, 425–432. [Google Scholar] [CrossRef]
- Stefanini, I.; Dapporto, L.; Berna, L.; Polsinelli, M.; Turillazzi, S.; Cavalieri, D. Social wasps are a Saccharomyces mating nest. Proc. Natl. Acad. Sci. USA 2016, 113, 2247–2251. [Google Scholar] [CrossRef]
- Valero, E.; Schuller, D.; Cambon, B.; Casal, M.; Dequin, S. Dissemination and survival of commercial wine yeast in the vineyard: A large-scale, three-years study. FEMS Yeast Res. 2005, 5, 959–969. [Google Scholar] [CrossRef]
- Cubillos, F.A.; Faugeron, S.; Ganga, A.; Vasquez, C.; Martínez, C. Self-fertilization is the main sexual reproduction mechanism in native wine yeast populations. FEMS Microbiol. Ecol. 2009, 67, 162–170. [Google Scholar] [CrossRef]


| Vintage I | Vintage II | ||
|---|---|---|---|
| Genotype Code | Vineyards (%) | Genotype Code | Vineyards (%) |
| G2 | 100 | G28 | 83.3 |
| G1 | 100 | G1, G23 | 50 |
| G3 | 50 | G26 | 50 |
| G4 | 50 | G25 | 50 |
| G5 | 50 | G2 | 33.3 |
| G6, G7 | 50 | G29, G31–G34, G41, G48 | 33.3 |
| G10 | 33.3 | G37 | 16.7 |
| G8–G9 | 16.7 | G3, G11, G24, G27, G30, G35–G36, G38–G40, G42–G47, G49–G72 | 16.7 |
| G11-G22 | 16.7 | ||
| Source of Variation | df | Variance Components | Variation (%) | PhiPT | p-Value |
|---|---|---|---|---|---|
| Between populations | 1 | 0.151 | 0 | −0.013 | 0.544 |
| Within populations | 75 | 19.018 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalvantzi, I.; Banilas, G.; Tassou, C.; Nisiotou, A. Patterns of Genetic Diversity and the Invasion of Commercial Starters in Saccharomyces cerevisiae Vineyard Populations of Santorini Island. Foods 2020, 9, 561. https://doi.org/10.3390/foods9050561
Chalvantzi I, Banilas G, Tassou C, Nisiotou A. Patterns of Genetic Diversity and the Invasion of Commercial Starters in Saccharomyces cerevisiae Vineyard Populations of Santorini Island. Foods. 2020; 9(5):561. https://doi.org/10.3390/foods9050561
Chicago/Turabian StyleChalvantzi, Ioanna, Georgios Banilas, Chrysoula Tassou, and Aspasia Nisiotou. 2020. "Patterns of Genetic Diversity and the Invasion of Commercial Starters in Saccharomyces cerevisiae Vineyard Populations of Santorini Island" Foods 9, no. 5: 561. https://doi.org/10.3390/foods9050561
APA StyleChalvantzi, I., Banilas, G., Tassou, C., & Nisiotou, A. (2020). Patterns of Genetic Diversity and the Invasion of Commercial Starters in Saccharomyces cerevisiae Vineyard Populations of Santorini Island. Foods, 9(5), 561. https://doi.org/10.3390/foods9050561






